Abstract
We developed a novel method for analyzing d-limonene levels in adipose tissue. Fat samples were subjected to saponification followed by solvent extraction. d-Limonene in the sample extract was analyzed using gas chromatography-mass spectrometry (GC-MS) with selected ion monitoring. Linear calibration curves were established over the mass range of 79.0-2,529 ng d-limonene per 0.1 grams of adipose tissue. Satisfactory within day precision (RSD 6.7 to 9.6%) and accuracy (% difference of −2.7 to 3.8%) and between day precision (RSD 6.0 to 10.7%) and accuracy (% difference of 1.8 to 2.6%) were achieved. The assay was successfully applied to human fat biopsy samples from a d-limonene feeding trial.
Keywords: d-limonene, GC-MS, adipose
1. Introduction
d-Limonene is a bioactive food component commonly found in the peel of citrus. It comprises 90–95% orange peel oil and 75% of lemon peel oil [1]. d-Limonene has demonstrated strong chemopreventive effects in rodent lymphomas [2], and mammary [3], gastric [4], skin, liver, and lung cancers [5]. In humans, consumption of citrus peels has been shown to be significantly related to lower incidence of squamous cell carcinoma, suggesting a protective effect [6]. Investigations of the in vivo disposition of d-limonene and its metabolites are limited. In rodents, radiolabeled d-limonene and/or its derived metabolites preferentially distribute to anatomical sites rich in fatty tissue such as adipose and mammary tissue rather than remaining in plasma [7]. Accurate assessment of the tissue distribution of d-limonene is a critical step to evaluate the potential of d-limonene as a chemopreventive agent in humans. Further, given that adipose tissue is not merely an inert reservoir for fat soluble substances, but an active organ producing and secreting adipokines and hormones with biological activity[8,9], the specific role of apidose-associated d-limonene is of even greater relevance.
In this research, we developed a gas chromatography-mass spectrometry (GC-MS) assay for quantification of d-limonene in adipose tissue. GC-MS was chosen based on its applicability for measuring trace amounts of volatile, organic molecules within a biological matrix and is considered the “gold standard” for such molecules [10]. A number of sensitive GC-MS methods have been developed for the analysis of d-limonene levels in human or rat plasma [11–13]. However, the applicability of these methods to solid tissues has not been studied. While d-limonene levels in solid tissues have been measured using liquid chromatography-mass spectrometry [14], our preliminary analytical work suggests that GC-MS provides improved sensitivity. In addition, GC-MS allows for the direct injection of the non-polar solvents used for extracting d-limonene from the fat mass.
Here we present a novel, sensitive, accurate and precise assay for quantification of d-limonene in adipose tissue. This assay is highly amenable to human or animal studies assessing nutraceutically or food derived (i.e. citrus peel) d-limonene tissue uptake and distribution.
2. Experimental
2.1 Materials
d-Limonene and perillyl aldehyde were purchased from Sigma Chemicals (St. Louis, MO, USA). HPLC grade dichloromethane and methanol, and 200 proof ethanol were obtained from EM Science (Gibbstown, NJ, USA). HPLC grade hexane was purchased from Honeywell Burdick & Jackson (Morristown, NJ, USA). ACS certified potassium hydroxide was purchased from Fisher Scientific (Pittsburg, PA, USA). Deionized (DI) water was purified by a Barnstead Nanopure Infinity water purifier (Barnstead International, Dubuque, IA, USA). Pork fat was purchased from a local market for assay development and validation.
d-Limonene and the internal standard, perillyl aldehyde, working stock solutions (0.843 mg/mL and 0.967 mg/mL, respectively) were prepared fresh weekly in methanol. The working stocks were stored at 4°C prior to use. The internal standard working solution (0.193 mg/mL) was prepared fresh each day by diluting the perillyl aldehyde working stock in methanol. d-Limonene calibration working standards were prepared fresh daily by serially diluting the working stock in methanol to a concentration range of 2.63 μg/mL to 84.3 μg/mL.
2.2. Sample processing
Adipose tissue (0.1 grams) was spiked with 30 μl of d-limonene calibration working standards and incubated at 37° C in a water bath for 2.5 hr with 200 μl of 30% potassium hydroxide and 1 mL ethanol to induce saponification. After cooling to room temperature, 3 mL hexane, 1 mL purified H20, and 30 μl internal standard working solution were added. Samples were vortexed for 20 s and then centrifuged at 1,400× g for 10 min at room temperature. The hexane layer was then removed and concentrated to 0.3 mL under a stream of nitrogen on a metal block that has been pre-cooled in a −80°C freezer for at least an hour. The remaining concentrate was then transferred to a GC-MS vial from Thermo Electron (San Jose, CA, USA) for injection into the GC-MS system.
2.3. GC-MS conditions
The GC-MS system consisted of a TRACE™ GC with a PVT injector and a Finnigan™ TRACE™ DSQ™ MS featuring a quadrupole mass spectrometer and a curved prefilter to reduce noise (Thermo Electron Corporation, San Jose, CA, USA). Chromatographic separation of d-limonene and internal standard was achieved on a high resolution GC DB5-MS fused silica capillary column (30 m × 0.247 mm, I.D: 0.25 μm) from J&W Scientific (Agilent Technologies, Santa Clara, CA, USA). Data was analyzed using XCalibur Software package, version 1.4 SRI (Thermo Electron Corporation San Jose, CA, USA). Helium was used as the carrier gas at a constant flow rate of 1.5 mL/min.
The GC-MS oven method was modified from that reported by Hakim et al.[15]. The initial oven temperature was held at 70°C for 10 min, ramping 15°C/min up to 300° and held for 5 min. The mass spectrometer source temperature was set at 250°C. One microliter of the sample was injected with the PVT splitless injector at 220°C. Mass spectrometric detection was accomplished with selected ion monitoring (SIM) and was divided into segments in the instrument method so the prominent masses for each analyte could be analyzed separately. Segment one began at 5.45 min and monitored for m/z 67, 68, and 93 which are the prominent d-limonene ions. Segment two began at 11 min and monitored for m/z 67, 68, and 79 which are the prominent perillyl aldehyde ions. The peak identity was confirmed with the XCalibur software library as well as the ion ratios.
2.4 Assay validations
Three curve validations were completed on separate days to determine linearity, accuracy, precision and reproducibility of the assay. Each standard curve was prepared by spiking the pork fat (0.1 grams) with d-limonene masses of 0.0, 79.0, 157.9, 315.8, 631.5, 1,263, and 2,529 ng. The accuracy and precision of the assay were determined at three different amounts of d-limonene using 5 replicates of each of these quality control (QC) samples; 157.9, 631.5, and 2,529 ng. The standards and QCs were prepared by adding 30 μl of the corresponding d-limonene calibration working standard to 0.1 grams of pork fat. These samples were processed and analyzed as described earlier.
2.5 Assay application
The validated assay was applied to the analysis of d-limonene levels in buttocks adipose biopsies obtained during a human volunteer high-limonene feeding trial. This study was reviewed and approved by the University of Arizona Institutional Review Board. In this trial, study participants were required to consume 40 oz of Mediterranean-style lemonade per day for 4 weeks. The lemonade was prepared by blending two whole lemons with the peel in 40 oz of water which is equivalent to approximately 500–600 mg of d-limonene. A needle fat biopsy was collected with a 15 gauge needle from the buttock 6 hr after initial feeding of 40oz of lemonade, and then again 6 hr after the last lemonade feeding following 4 weeks of daily feeding. There were seven matched initial and end of study biopsies available for analysis. These biopsies were weighed and subjected to the saponification and extraction procedure as described above. A calibration curve was prepared and processed as described above for each set of sample analysis.
3. Results and Discussion
3.1. Sample processing
The first step in developing an assay for quantification of d-limonene in the adipose tissue required the development of a sample processing procedure that insured efficient and consistent extraction of d-limonene from the sampled tissue. Pork fat was selected as a surrogate fat sample source for all sample handling and processing development and for preparation of standard curves. The use of pork fat as a surrogate to human tissue proved to be an appropriate alternative in that it reduced analytical costs and also provided a source of d-limonene-free test tissue and a true blank to establish the standard curve. Initially, we homogenized the fat in chloroform or hexane using a mechanical tissue homogenizer. However, there were concerns of incomplete fat tissue homogenization and evaporation of solvents and analytes during the homogenization procedure.
These limitations led to adaptation of a saponification procedure to optimize fat sample processing. This process has been used for the analysis of fat soluble compounds such as vitamin E [16] and carotenoids [17] from adipose tissue for dietary intake studies. The saponification protocol used in our research was adapted primarily from Yeum et al [17]. The fat mass was incubated at 37° C in a water bath for 2.5 hr with 30% KOH and ethanol to induce saponification. Saponification was performed in a sealed test tube, minimizing d-limonene evaporation. Saponification facilitated the complete emulsification of the fat mass into a solution, permitting efficient digestion of the adipose tissue. Following saponification, d-limonene was then extracted from the emulsified liquid phase into hexane in a sealed tube.
3.2 Chromatography and mass spectrometry
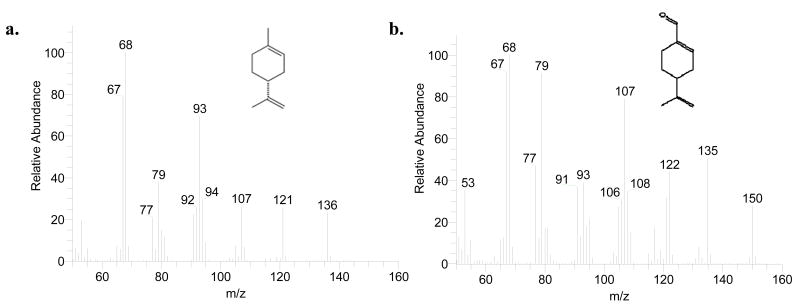
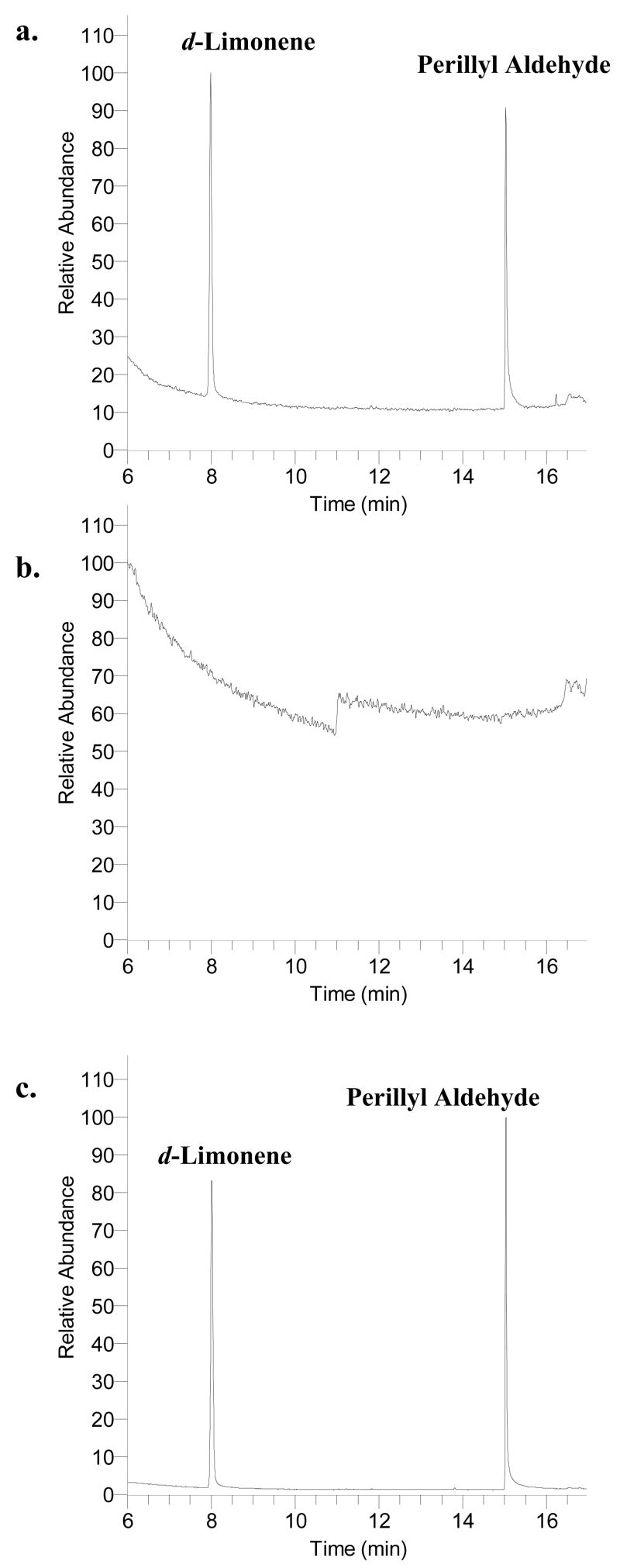
The GC temperature gradient method was optimized during early experimentation. A higher ending temperature, 300° C, held for 5 min was adapted in order to prevent excess long chain fatty acids from accumulating on the GC column. Because fatty acids elute off the column after d-limonene and their peak areas often dwarfed the peaks of interest in the chromatogram, data acquisition was terminated at 18 min. Figure 1 illustrates the mass spectra of d-limonene and perillyl aldehyde. We selected to monitor the sum of three prominent ions of each analyte (m/z 67, 68, and 93 for d-limonene and m/z 67, 68, and 79 for perillyl aldehyde) to help improve assay sensitivity. In addition, the use of selected ion monitoring resulted in a cleaner baseline and thus improved signal-to-noise ratios for the analytes of interest. The detection sensitivity for d-limonene was as low as 0.1 ng injected on column with a signal-to-noise ratio of 11. Figure 2a is a representative chromatogram of an unextracted standard of d-limonene and perillyl aldehyde. d-Limonene and perillyl aldehyde elution times approximated 8 and 15 min, respectively. Figure 2b and 2c are representative chromatograms of extracted blank pork fat and pork fat spiked with d-limonene and perillyl aldehyde, respectively. All chromatograms are plotted using the sum of abundances of m/z 67, 68, and 93 (prominent ions for d-limonene) from 5.45 to 11 min (segment 1) and of m/z 67, 68, and 79 (prominent ions for perillyl aldehyde) from 11 to 18 min (segment 2). There were no interfering peaks in the blank pork fat and the elution times of d-limonene and perillyl aldehyde were not affected by the presence of the pork fat in the sample.
Figure 1.
Mass spectra of d-limonene (a) and perillyl aldehyde (b).
Figure 2.
Representative chromatograms of unextracted standard, blank pork fat sample, and extracted standard; a. Unextracted standard of d-limonene (2,529 ng spiked) and perillyl aldehyde (5,790 ng spiked); b. Extracted blank pork fat sample; c. Extracted standard d-limonene (2,529 ng spiked) and perillyl aldehyde (5,790 ng spiked) from the pork fat.
3.3. Tests for peak response amplification
In order to further improve the sensitivity of the assay, experiments were performed to determine whether the extracting solvent can be concentrated to a smaller volume prior to sample injection. These experiments were performed with pork fat spiked with three pre-selected levels of d-limonene (157.9, 631.5, and 2,529 ng). Samples were then subjected to the previously described saponification procedure and extracted with 3 mL of hexane. A set of samples were injected straight from the 3 mL volume of hexane for GC-MS analysis. Another set of samples were concentrated under a stream of nitrogen from 3 mL to 0.3 mL on a pre-cooled metal block prior to sample injection. Table 1 summarizes the effects of concentrating the extraction solvent on d-limonene peak area response amplification. For the lowest concentration, d-limonene was not detectable in the standard when hexane was not concentrated. After concentrating, the absolute peak areas of d-limonene and perillyl aldehyde were determined to be 6–9 times of those detected in the un-concentrated samples for the middle and high QC standards. Even though the absolute magnitude of d-limonene peak amplification seems to be inconsistent, the peak area ratio of d-limonene/perillyl aldehyde is similar between the concentrated and un-concentrated extracts. This suggests that the amplification differences are most likely due to variations in the ending volume after concentration; the internal standard corrects for this variation. These results indicate that concentrating the hexane layer to 0.3 mL on a pre-cooled block is appropriate and necessary to amplify absolute d-limonene peak area, and thus increase assay sensitivity. Concentration did not change the reported amount of d-limonene measured, when perillyl aldehyde is used as the internal standard. Therefore, all subsequent experiments were performed by concentrating the organic extract.
Table 1.
Amplification of d-limonene peak response by concentration of extraction solvent
| d-limonene amount(ng) | Hexane volume | d-limonene peak area | Magnitude of amplification | d-limonene/I.S. |
|---|---|---|---|---|
| 157.9 | 0.3ml | 105,002 | NA | 0.09 |
| 157.9 | 3ml | ND | ND | |
| 631.5 | 0.3ml | 555,150 | 9.25 | 0.30 |
| 631.5 | 3ml | 60,008 | 0.39 | |
| 2,529 | 0.3ml | 2,021,037 | 6.37 | 2.06 |
| 2,529 | 3ml | 317,066 | 2.11 |
ND: Not detectable
NA: Not applicable
3.4. Analyte stability and recovery during saponification
To completely emulsify the fat, the saponification process is typically performed at an elevated temperature in the presence of an ethanolic base mixture for an extended time period. Experiments were performed to determine the stability of d-limonene during the saponification process. d-Limonene was added before or after the incubation period and then subjected to organic extraction as described above. The test was performed with three different amounts of d-limonene (157.9, 631.5, and 2,529 ng) and the internal standard was added to all test samples immediately after incubation. At each d-limonene level, d-limonene peak response was similar between samples where d-limonene was added before and after the saponification process (data not shown), indicating that d-limonene is stable throughout the saponification procedure.
The stability of perillyl aldehyde was also tested by adding it to the sample before and after the incubation period. Perillyl aldehyde was not consistently detected when it was added before the incubation period indicating that perillyl aldehyde is not stable through the saponication reaction. When the internal standard was added after incubation, it demonstrates consistent peak response (data not shown). Because the saponification step is to emulsify the fat and does not appear to affect the amount of d-limonene in the tissue, we believe that it is appropriate to add the internal standard following the saponification step to serve as an internal control for sample extraction and GC-MS analysis. The assay was subsequently validated with perillyl aldehyde added immediately after the saponification step.
3.5. Analyte extraction recovery
The extraction recovery of d-limonene was determined by comparing the peak response from an extracted standard to unextracted standard prepared in hexane. Unextracted controls for each d-limonene amount were prepared by spiking each of the test amounts of d-limonene into 3 mL of hexane and then concentrating to 0.3 mL on a pre-cooled block. Extraction recovery of d-limonene was 79–82% at the three d-limonene levels (157.9, 631.5, and 2,529 ng) (Table 2). Extraction recovery of perillyl aldehyde was also determined in a similar fashion at a single level (3,900 ng) and was found to be 64%.
Table 2.
Assay accuracy, precision, and extraction recovery of d-limonene in adipose tissue
| Theoretical d-limonene amount added(ng) | |||
|---|---|---|---|
| 157.9 | 631.5 | 2,529 | |
|
|
|||
| Within Day | |||
| Mean Measured Amount1 | 153.6 | 643.7 | 2,623.7 |
| Precision (% RSD)3 | 9.3 % | 9.6 % | 6.8 % |
| Accuracy (% difference)4 | −2.7 % | 1.9 % | 3.8 % |
| Between Day | |||
| Mean Measured Amount2 | 160.8 | 647.8 | 2,578.0 |
| Precision (% RSD)3 | 10.7 | 7.0 | 6.0 |
| Accuracy (% difference)4 | 1.8% | 2.6% | 2.1% |
| Extraction Recovery (%, n = 3) | 80.0 | 79.1 | 82.4 |
Average of five replicates
Average from three validation days
Precision expressed as R.S.D = [(SD/mean) × 100]
Accuracy expressed as % difference = [(concentration found – concentration added)/concentration added] × 100
d-Limonene recovery was also tested with and without the presence of 0.1 grams of fat which is the estimated amount of fat collected and recovered from a single needle biopsy in humans. The presence or absence of 0.1 grams of pork fat did not affect the extraction recovery of d-limonene (data not shown). Similar extraction recovery of d-limonene from 0.1 and 0.4 grams of fat were also found (data not shown).
3.6 Assay validations
The assay for the adipose tissue analysis was validated over three separate analysis days. The calibration curve was constructed using the peak area ratios between d-limonene and the internal standard versus the theoretical d-limonene amount spiked into the adipose tissue. The curve is linear over 79.0 to 2,529 ng of d-limonene spiked into 0.1 grams of pork fat. The R2 value ranged from 0.9987 to 0.9998 over the three test days. On each validation day, the difference in each standard was within 8% of the predicted value of the curve.
Precision and accuracy were calculated based on QC samples chosen at three different amounts of d-limonene. Table 2 summarizes the intra-day assay variation on one of the validation days and the between day assay variation among three validation days. Intra-day assay precision, %RSD, was within 10% for all QC concentrations. The average percent difference, assessment of assay accuracy, was under 5% for all QC sets. Between-day variation was also estimated by averaging the amounts predicted for the QC standards from each validation day. The estimated between-day precision and accuracy were also within an acceptable range [18].
For the second and third validation sets, within-day %RSD was less than 14% at the low end, and was less than 6% for the middle and high QCs. All three validations show good accuracy and precision of the assay demonstrating that this assay should be appropriate for analyzing d-limonene in adipose tissue. The validation shows that the lower limit of quantification (LLOQ) of d-limonene in fat, defined as the lowest concentration of the calibration curve that can be measured with acceptable accuracy and precision, was 79 ng per 0.1 grams of fat. This provides sufficient sensitivity to quantify d-limonene levels that would have biological significance [19–21].
3.7. Assay application
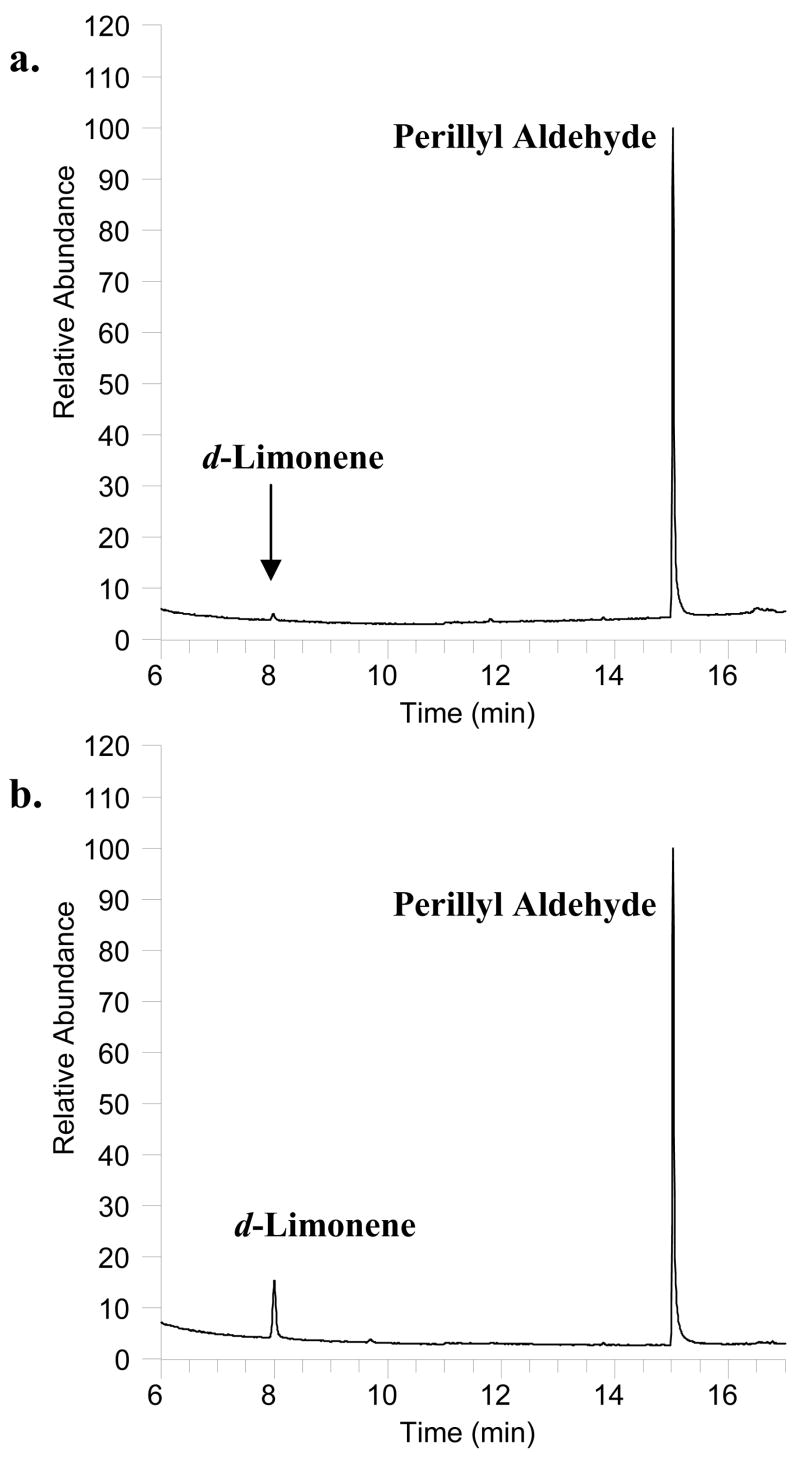
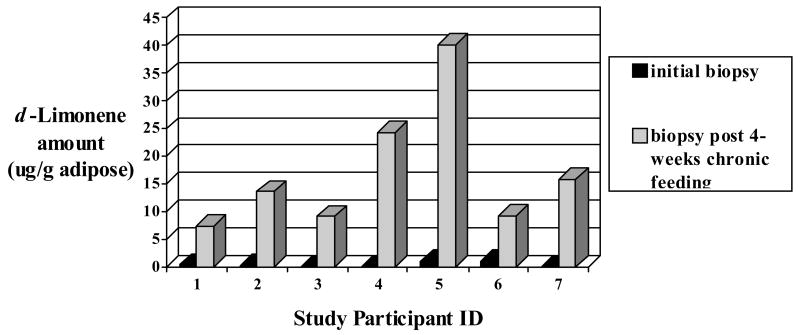
This assay was successfully applied to seven matched (pre-and post chronic high d-limonene exposure) adipose tissue biopsies collected from seven volunteers participating in a high d-limonene lemonade feeding study. The study participants consumed 40 oz of Mediterranean-style lemonade prepared fresh daily with two whole lemons. Figures 3a and 3b are representative chromatograms of the fat biopsy samples collected 6 hr post a single dose of lemonade and 6 hr after the last lemonade feeding following 4 weeks of daily consumption, respectively. Figure 4 illustrates the d-limonene levels in each set of study participant fat biopsy samples. d-Limonene levels in fat biopsy collected 6 hr after the initial lemonade feeding ranged from undetectable to 1 μg/g of adipose tissue. d-Limonene levels in fat biopsy collected after 4 weeks of daily lemonade feeding ranged from 7 – 40 μg/g adipose tissue. This supports the hypothesis that d-limonene would accumulate in adipose tissue. In the future, this assay could be applied to clinical or preclinical studies assessing the biodistribution and accumulation of limonene in the fat or be used as a biomarker for assessing citrus peel consumption.
Figure 3.
Representative chromatograms of the initial biopsy and the post-intervention biopsy collected from a Mediterranean lemonade feeding study; a. Biopsy sample collected 6 hr after consumption of 40oz of Mediterranean lemonade. d-Limonene level was determined to be 42.7 ng in 0.123 g of fat; b. Biopsy sample collected after 4 weeks of daily Mediterranean lemonade consumption. d-Limonene level was determined to be 447 ng in 0.061g of fat.
Figure 4.
d-Limonene levels quantified by GC/MS in study participant fat biopsies collected from a 4-week Mediterranean-style lemonade feeding trial (500-600mg d-limonene daily).
4. Conclusion
A novel, sensitive, accurate, and precise GC-MS assay has been developed for the determination of d-limonene in adipose tissue. This assay has been successfully applied to human samples from a limonene feeding trial and could be used as a biomarker to quantify d-limonene exposure in epidemiological research. This assay could also be applied to clinical or preclinical studies assessing the biodistribution and accumulation of limonene in the fat.
Acknowledgments
This work was partially supported by the Arizona Cancer Center Core Grant (P30-CA-23074) and DOD Idea Award (BC061529).
The abbreviations used are
- GC-MS
gas chromatography/mass spectrometry
- QC
quality control
- HPLC
high performance liquid chromatography
- KOH
potassium hydroxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kesterson JW, Hendrickson R, Braddock RJ. Technical Bulletin. Vol. 749. University of Florida; 1971. p. 3. [Google Scholar]
- 2.Del Toro-Arreola S, Flores-Torales E, Torres-Lozano C, Del Toro-Arreola A, Tostado-Pelayo K, Guadalupe Ramirez-Duenas M, Daneri-Navarro A. Int Immunopharmacol. 2005;5:829. doi: 10.1016/j.intimp.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Haag JD, Lindstrom MJ, Gould MN. Cancer Research. 1992;52:4021. [PubMed] [Google Scholar]
- 4.Lu XG, Zhan LB, Feng BA, Qu MY, Yu LH, Xie JH. World J Gastroenterol. 2004;10:2140. doi: 10.3748/wjg.v10.i14.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowell PL, Gould MN. Crit Rev Oncog. 1994;5:1. doi: 10.1615/critrevoncog.v5.i1.10. [DOI] [PubMed] [Google Scholar]
- 6.Hakim IA, Harris RB, Ritenbaugh C. Nutr Cancer. 2000;37:161. doi: 10.1207/S15327914NC372_7. [DOI] [PubMed] [Google Scholar]
- 7.Crowell PL, Kennan WS, Haag JD, Ahmad S, Vedejs E, Gould MN. Carcinogenesis. 1992;13:1261. doi: 10.1093/carcin/13.7.1261. [DOI] [PubMed] [Google Scholar]
- 8.Fischer-Posovszky P, Wabitsch M, Hochberg Z. Horm Metab Res. 2007;39:314. doi: 10.1055/s-2007-976539. [DOI] [PubMed] [Google Scholar]
- 9.Calabro P, Yeh ET. Subcell Biochem. 2007;42:63. [PubMed] [Google Scholar]
- 10.Medeiros PM, Simoneit BR. J Sep Sci. 2007;30:1516. doi: 10.1002/jssc.200600399. [DOI] [PubMed] [Google Scholar]
- 11.Crowell PL, Elson CE, Bailey HH, Elegbede A, Haag JD, Gould MN. Cancer Chemother Pharmacol. 1994;35:31. doi: 10.1007/BF00686281. [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Chan KK, Budd T. J Pharm Biomed Anal. 1998;17:631. doi: 10.1016/s0731-7085(97)00243-4. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Chen Y, Gao Z, Xiong M, Zhong Z, Ye L. J Pharm Biomed Anal. 2007;44:1095. doi: 10.1016/j.jpba.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Poon GK, Vigushin D, Griggs LJ, Rowlands MG, Coombes RC, Jarman M. Drug Metab Dispos. 1996;24:565. [PubMed] [Google Scholar]
- 15.Hakim IA, McClure T, Liebler D. J Food Compost Anal. 2000;13:329. [Google Scholar]
- 16.Kayden HJ, Hatam LJ, Traber MG. J Lipid Res. 1983;24:652. [PubMed] [Google Scholar]
- 17.Yeum KJ, Ahn SH, Rupp de Paiva SA, Lee-Kim YC, Krinsky NI, Russell RM. J Nutr. 1998;128:1920. doi: 10.1093/jn/128.11.1920. [DOI] [PubMed] [Google Scholar]
- 18.Bressolle F, Bromet-Petit M, Audran M. J Chromatogr B Biomed Appl. 1996;686:3. doi: 10.1016/s0378-4347(96)00088-6. [DOI] [PubMed] [Google Scholar]
- 19.Hardcastle IR, Rowlands MG, Barber AM, Grimshaw RM, Mohan MK, Nutley BP, Jarman M. Biochem Pharmacol. 1999;57:801. doi: 10.1016/s0006-2952(98)00349-9. [DOI] [PubMed] [Google Scholar]
- 20.Bardon S, Picard K, Martel P. Nutr Cancer. 1998;32:1. doi: 10.1080/01635589809514708. [DOI] [PubMed] [Google Scholar]
- 21.Bardon S, Foussard V, Fournel S, Loubat A. Cancer Lett. 2002;181:187. doi: 10.1016/s0304-3835(02)00047-2. [DOI] [PubMed] [Google Scholar]