Abstract
Amine functionalized soluble single walled carbon nanotubes (SWNTs) were derivatized with cisplatin prodrug conjugates as a delivery system by which to internalize multiple prodrug centers. The platinum(IV) complex, c,c,t-[Pt(NH3)2Cl2(OEt)(O2CCH2CH2CO2H)], was tethered to the surface of the carbon nanotubes through peptide linkages formed by the reaction of the SWNT-tethered amines with the carboxylate moiety. The SWNTs are taken into testicular cancer cells by endocytosis where the drop in pH facilitates reductive release of the platinum(II) core complex, which then readily diffuses throughout the cell, as determined by platinum atomic absorption spectroscopy. The entrapment of the SWNTs within the endosomes was confirmed by fluorescence microscopy of SWNTs containing both tethered fluorescein and platinum units. The cytotoxicity of the free platinum(IV) complex increases by greater than 100-fold when attached to the surface of the functionalized SWNTs.
Attempts to devise platinum drugs that surpass the anticancer properties of cisplatin have produced many compounds that display biological activity, but only a handful of these have shown any real promise in clinical trials.1 This loss of activity in the body can be associated with poor circulation and delivery to the tumor as well as deactivation mechanisms that irreversibly alter the chemistry of these molecules, particularly those of platinum(II), rendering them ineffective.2 We can circumvent many pathways that deactivate platinum(II) drug candidates by utilizing substitutionally more inert platinum(IV) compounds as prodrugs or by using carrier molecules as delivery systems.1,3 We recently demonstrated the utility of this approach by attaching cell-sensitizing estradiol units to platinum(IV) compounds which, upon entry into the cell, were reduced to release the cytotoxic compound cis-[Pt(NH3)2Cl2].4 The axial ligands of platinum(IV) complexes, which dissociate upon reduction to platinum(II), can be used to adjust properties such as redox potential, aqueous solubility, and lipophilicity that can affect the method and rate of uptake of a drug.5
Another delivery system that has emerged as a highly effective means of transporting cargos of various sizes and types across the cell membrane is the functionalized soluble single-walled carbon nanotube (SWNT), which carries smaller molecules into cells through clathrin-dependent endocytosis.6 SWNTs tethered to substrates by disulfide linkages use the reducing environment of endosomes into which they are taken to selectively release their cargo only following cellular internalization.7 By combining the ability of platinum(IV) complexes that resist ligand substitution with the proven capacity of SWNTs to act as a longboat, shuttling smaller molecules across cell membranes, we have constructed a SWNT-tethered platinum(IV) conjugate that effectively delivers a lethal dose of cis-[Pt(NH3)2Cl2] upon reduction inside the cell.
We first prepared c,c,t-[Pt(NH3)2Cl2(OEt)(O2CCH2CH2CO2H)], 2 (Figure 1a), a platinum(IV) construct capable of being tethered through one of its axial ligands to an amine-functionalized SWNT surface. Compound 2 was chosen as the longboat cargo in order to allow us to regenerate and release the toxic molecule cis-[Pt(NH3)2Cl2] upon intracellular reduction. The precursor complex, c,c,t-[Pt(NH3)2Cl2(OH)(OEt)], 1, was synthesized in a manner similar to other trans-alkoxohydroxoplatinum(IV) complexes by the oxidation of cis-[Pt(NH3)2Cl2] in dilute ethanolic hydrogen peroxide.8 The axial hydroxide is sufficiently nucleophilic to attack succinic anhydride upon mild heating to afford the asymmetrically substituted trans-alkoxocarboxylato complex 2. The X-ray structure of 2 is described in the Supporting Information.
Figure 1.
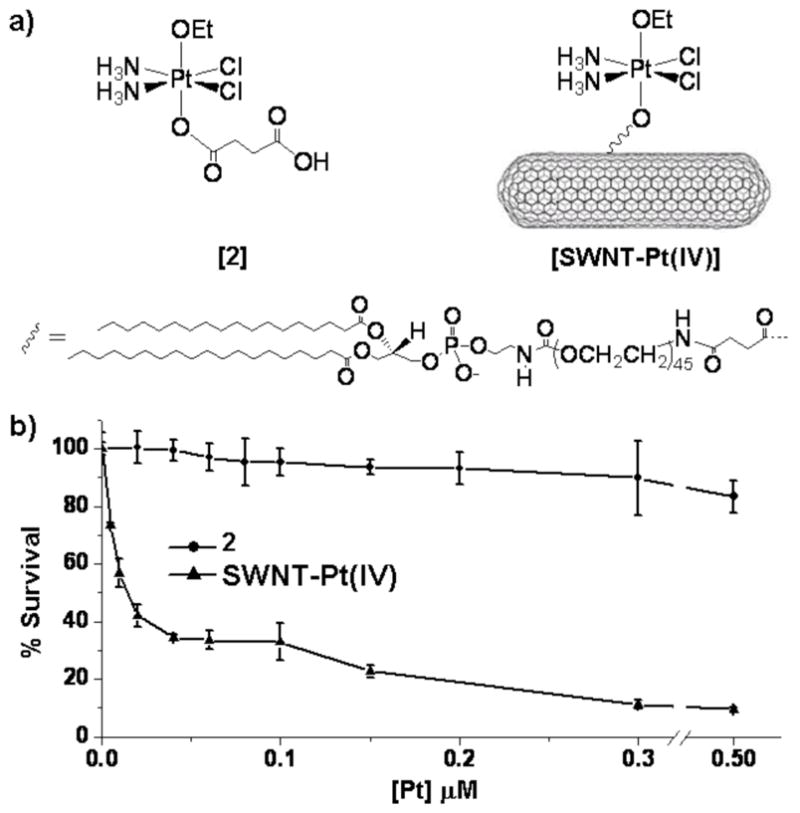
(a) c,c,t-[Pt(NH3)2Cl2(OEt)(O2CCH2CH2CO2H)], 2, and the SWNT-tethered conjugate. (b) Cytotoxicity of free 2 and SWNT-tethered 2 in NTera-2 cells.
Electrochemical studies of 1 and 2 revealed the expected irreversible reduction maxima in their cyclic voltammograms corresponding to loss of the axial ligands (Figs. S1–S8, Supporting Information). At pH 7.4 the reduction potentials extrapolated to 0.0 mV s−1 scan rate (see Supporting Information) for 1 and 2 are −0.604 and −0.724 V, respectively. At pH 6.0, a value similar to that reported for endosomes and lysosomes,7a,7b there is a positive shift in the reduction potentials of both complexes by greater than 100 mV, the respective reduction peaks occurring at −0.499 and −0.623 V. The more facile reduction reflects protonation of the axial ligands as they dissociate upon reduction of the platinum atom. This property will significantly affect the activity of these complexes in the low pH environment of many solid tumors and inside the endosomes.
The SWNTs used in this study were functionalized by nonspecific binding of phospholipid tethered amines to the nanotube surface.6a A polyethyleneglycol chain between the amine and the anchoring phospholipid serves to solubilize the SWNTs and extend the functional group away from the nanotube surface. Treating c,c,t-[Pt(NH3)2Cl2(OEt)(O2CCH2CH2CO2H)] with 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) creates the N-succinimidyl ester, which readily forms amide linkages with the PEG-tethered primary amines on the SWNT surface. After the tethering reaction, dialysis through a 3500 MW cutoff membrane separates any free platinum from the much larger SWNT-Pt(IV) conjugates. The result is a SWNT longboat that carries a cargo average of 65 platinum(IV) centers per nanotube as determined by atomic absorption spectroscopy (AAS). Formation of the amide bond is not expected to significantly alter the reduction potential of the platinum(IV) center since the electronic interaction of the two positions is insulated by two methylene groups. In order to evaluate the feasibility of this method for delivering a toxic dose of platinum we conducted cytotoxicity assays of SWNT-Pt(IV) using the testicular carcinoma cell line NTera-2. We applied the MTT assay to determine cell viability following treatment for four days with either the free platinum(IV) complex 2 or with the SWNT-tethered analog (Figure 1b). Compared to cis-[Pt(NH3)2Cl2] (IC50 = 0.05 μM; Figs. S9, S10) the cytotoxicity of the asymmetrically substituted platinum(IV) compound 2 is insignificant (IC50 ≫ 0.5 μM). The amine-functionalized SWNTs also have negligible cytotoxicity. The SWNT-Pt(IV) conjugate, however, shows a substantial increase in toxic character with respect to the free complex 2 (IC50 = 0.02 μM) and surpasses that of cis-[Pt(NH3)2Cl2] when compared on a per platinum basis.
In order to investigate the mechanism by which the SWNTs transport Pt(IV) compounds into cells and release them once inside we devised a strategy to track their intracellular location. To follow the movement of the SWNTs we cotethered a fluorescein-based fluorophore (6-carboxy-2′,7′-dichloro-fluorescein-3′,6′-diacetatesuccinimidyl ester) to our SWNT-Pt(IV) conjugates.9 After a 1.5 h incubation with the SWNT-Pt(IV)/Fl conjugates the now fluorescent SWNTs were readily located within small (~2 μm) vesicles in the cell (Figure 2). This finding supports previous reports that SWNTs are taken up by cells through endocytosis.6c,6f To determine the locale of platinum once inside the cells and to learn whether or not it was released from the SWNT delivery vessel, extracts were taken from the cytosol and nucleus of cells incubated with SWNT-Pt(IV). AAS analysis of both fractions showed considerable amounts of platinum (23 ng Pt/mg protein in the cytosol and 36 ng Pt/mg protein in the nucleus) in cells that were incubated for 3 h with platinum concentrations of 1.0 μM. These levels were 6–8 times higher in platinum concentration than that found in cells incubated with the untethered complex 2 and also twice the platinum concentration than measured in cells incubated with cis-[Pt(NH3)2Cl2]. This intracellular accumulation and distribution of platinum reveals that functionalized SWNTs are an effective tool for transport and delivering small molecule platinum prodrugs.
Figure 2.
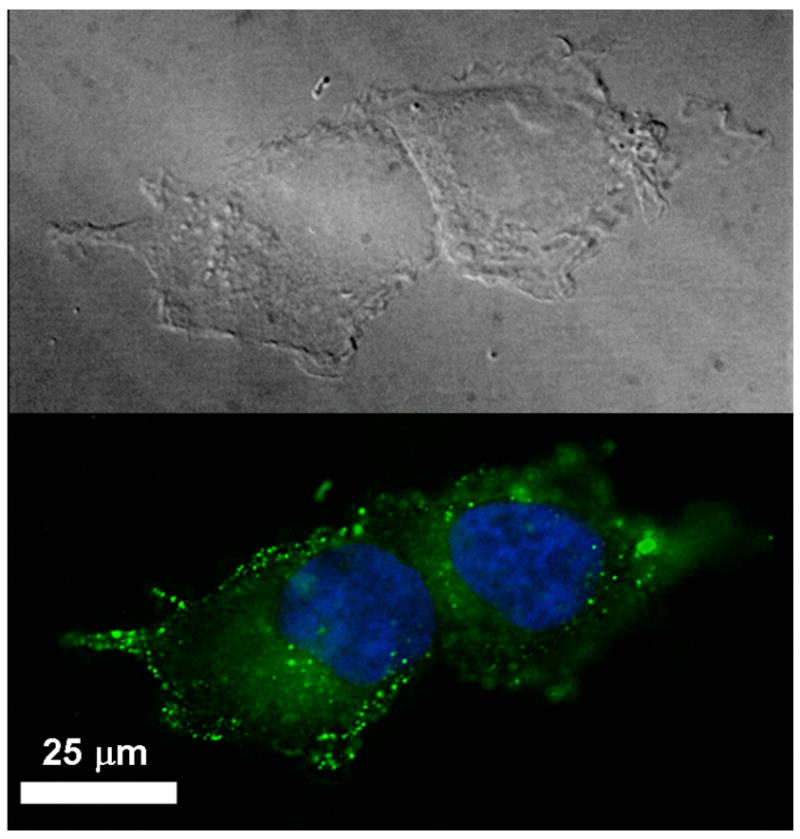
Fluorescence of SWNT-Pt(IV)/Fl in NTera-2 cells. The blue dye is Hoechst (H33258) nuclear stain.
We have shown in this study that the platinum(IV) complex c,c,t-[Pt(NH3)2Cl2(OEt)(O2CCH2CH2CO2H)], which is nearly nontoxic to testicular cancer cells, displays a significantly enhanced cytotoxicity profile when attached to the surface of amine-functionalized soluble SWNTs. The soluble nanotubes internalized the platinum(IV) compound through endocytosis, providing six times the concentration that the untethered complex is able to achieve on its own. Once confined within the endosomes, the lower pH environment facilitates release of platinum as its core compound, cis-[Pt(NH3)2Cl2], by reduction and concomitant loss of the axial ligands by which it is tethered to the SWNT surface. Atomic absorption spectroscopy confirms the distribution of platinum throughout the cell interior, while fluorescence microscopy verifies the trapped nature of the SWNTs. These results demonstrate the value of SWNTs for transporting platinum(IV) prodrugs across cell membranes where they can be reductively released as active platinum(II) species. The tethering and endosomal release of platinum(IV) prodrugs can be generally applied to any platinum(IV) compound that has a functional group on its axial ligands capable of being coupled to one of the many types of functionalized SWNTs. By linking additional groups, such as cancer-cell targeting moieties, to the platinated SWNTs as long boat passengers it may be possible to achieve highly selective constructs for use in clinical trials.
Supplementary Material
Supporting Information Available: Experimental details for preparation of all complexes and cell work. Crystallographic data for 2 in cif format. This material is available free of charge via the internet at http://pubs.acs.org.
Acknowledgments
This work was supported by the National Cancer Institute under grant CA34992 at MIT and NCI-NIH-CCNE-TR at Stanford. We thank Dr. Elizabeth Nolan for the preparation of the fluorophore used herein, Dr. Shanta Dhar for valuable discussions concerning electrochemistry and Zhuang Liu for information about the synthesis and properties of SWNTs.
References
- 1.(a) Galanski M, Jakupec MA, Keppler BK. Curr Med Chem. 2005;12:2075–2094. doi: 10.2174/0929867054637626. [DOI] [PubMed] [Google Scholar]; (b) Wong E, Giandomenico CM. Chem Rev. 1999;99:2451–2466. doi: 10.1021/cr980420v. [DOI] [PubMed] [Google Scholar]
- 2.Wang D, Lippard SJ. Nat Rev Drug Discovery. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 3.Xu P, VanKirk EA, Murdoch WJ, Zhan Y, Isaak DD, Radosz M, Shen Y. Biomacromolecules. 2006;7:829–835. doi: 10.1021/bm050902y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes KR, Kutikov A, Lippard SJ. Chem Biol. 2004;11:557–564. doi: 10.1016/j.chembiol.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 5.Hall MD, Hambley TW. Coord Chem Rev. 2002;232:49–67. [Google Scholar]
- 6.(a) Bottini M, Cerignoli F, Dawson MI, Magrini A, Rosato N, Mustelin T. Biomacromolecules. 2006;7:2259–2263. doi: 10.1021/bm0602031. [DOI] [PubMed] [Google Scholar]; (b) Gao L, Ni L, Wang T, Qin Y, Guo Z, Yang D, Yan X. ChemBioChem. 2006;7:239–242. doi: 10.1002/cbic.200500227. [DOI] [PubMed] [Google Scholar]; (c) Kam NWS, Dai H. J Am Chem Soc. 2005;127:6021–6026. doi: 10.1021/ja050062v. [DOI] [PubMed] [Google Scholar]; (d) Kam NWS, Jessop TC, Wender PA, Dai H. J Am Chem Soc. 2004;126:6850–6851. doi: 10.1021/ja0486059. [DOI] [PubMed] [Google Scholar]; (e) Nadine Wong Shi Kam ZLHD. Angew Chem, Int Ed. 2006;45:577–581. doi: 10.1002/anie.200503389. [DOI] [PubMed] [Google Scholar]; (g) Shi Kam NW, O’Connell M, Wisdom JA, Dai H. Proc Natl Acad Sci U S A. 2005;102:11600–11605. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Liu Z, Cai W, He L, Nakayama N, Chen K, Sun X, Chen X, Dai H. Nat Nano. 2007;2:47–52. doi: 10.1038/nnano.2006.170. [DOI] [PubMed] [Google Scholar]; (f) Nakayama-Ratchford N, Bangsaruntip S, Sun X, Welsher K, Dai H. J Am Chem Soc. 2007;129:2448–2449. doi: 10.1021/ja068684j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Arunachalam B, Phan UT, Geuze HJ, Cresswell P. Proc Natl Acad Sci U S A. 2000;97:745–750. doi: 10.1073/pnas.97.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kam NWS, Dai H. Phys Status Solidi B. 2006;243:3561–3566. [Google Scholar]; (c) Kam NWS, Liu Z, Dai H. J Am Chem Soc. 2005;127:12492–12493. doi: 10.1021/ja053962k. [DOI] [PubMed] [Google Scholar]; (d) Zhuang Liu MWMHHD. Angew Chem, Int Ed. 2007;46:2023–2027. doi: 10.1002/anie.200604295. [DOI] [PubMed] [Google Scholar]
- 8.(a) Giandomenico CM, Abrams MJ, Murrer BA, Vollano JF, Rheinheimer MI, Wyer SB, Bossard GE, Higgins JD. Inorg Chem. 1995;34:1015–1021. doi: 10.1021/ic00109a004. [DOI] [PubMed] [Google Scholar]; (b) Lee Y-A, Ho Yoo K, Jung O-S. Inorg Chem Commun. 2003;6:249–251. [Google Scholar]; (c) Lee YA, Jung OS. Bull Chem Soc Jpn. 2002;75:1533–1537. [Google Scholar]; (d) Young A, Lee O-S. J Angew Chem, Int Ed. 2001;40:3868–3870. [Google Scholar]
- 9.Woodroofe CC, Won AC, Lippard SJ. Inorg Chem. 2005;44:3112–3120. doi: 10.1021/ic048789s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available: Experimental details for preparation of all complexes and cell work. Crystallographic data for 2 in cif format. This material is available free of charge via the internet at http://pubs.acs.org.


