Abstract
Aneurysmal bone cysts are associated with a high rate of recurrence. Many aneurysmal bone cysts arise near open physes or articular cartilage in skeletally immature patients. Fear of damaging these structures could cause surgeons to curette the tumors less aggressively. We hypothesized location of an aneurysmal bone cyst in a periarticular or juxtaphyseal location would increase the risk of recurrence. We retrospectively studied 53 patients with aneurysmal bone cysts treated between 1989 and 2004. All patients had primary disease, and all patients underwent curettage of the lesion. Ten patients (18.9%) had local recurrence. Gender, race, and size did not predict recurrence; however 12 years of age or younger was associated with recurrence. Of the 19 juxtaphyseal cysts directly adjacent to an open physis, eight developed recurrence. Of the five periarticular cysts, two developed recurrence. The data suggest the risk of recurrence is highest in pediatric patients with juxtaphyseal or periarticular aneurysmal bone cysts. Meticulous treatment of these cysts is necessary, but we believe an overly aggressive approach that destroys the physis or articular cartilage is not warranted. Preservation of these structures remains a high priority of treatment.
Level of Evidence: Level IV, case series. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
The aneurysmal bone cyst (ABC) is a true neoplasm. It is not simply a developmental abnormality or a secondary finding in the setting of another bone tumor. The identification of a consistent t(16;17) chromosomal translocation in primary cases suggests the lesion is a tumor in its own right, characterized by a specific mutational event [16].
Of the benign bone tumors, the ABC is one of the more aggressive and difficult to treat. The reported rate of recurrence varies widely, but in most studies, approximately 12% to 30% of patients experience recurrence after initial treatment [3, 4, 11–15]. Young age has been associated with a higher risk of recurrence [1, 2, 10, 18, 19]. However, this remains controversial because at least one recent study suggests age does not predict recurrence [8].
One of the unique features of pediatric patients is they have open physes. Similar to unicameral bone cysts, ABCs occur near open physes. In skeletal sites where a secondary ossification center is not present, such as the periacetabular region of the pelvis, the ABC may rest directly against articular cartilage. The location of the ABC in these sensitive sites could affect the ability of the surgeon to remove the lesion without damaging the articular cartilage. Although the use of a high-speed burr can reduce the recurrence rate [10], this tool cannot be used with abandon near the physis or articular cartilage.
In addition to the technical difficulty of removing tumor near the physis, there may be biologic factors related to the environment of the physis that could influence recurrence of ABCs. Although direct experimental evidence has not been reported for this concept, it seems plausible that the rich production of growth factors and the unique vascular anatomy of the region potentially could be important to the formation and persistence of neoplasms such as ABCs. The biologic factors could work in concert with surgical obstacles to promote recurrence of disease near the physis, and these processes are not mutually exclusive.
Relatively little has been written regarding ABCs arising near growth plates. In one study from the Rizzoli Institute, a juxtaepiphyseal location was associated with an increased risk of subsequent growth arrest and skeletal deformities [5]. However, the effect on recurrence was not reported, and in the classification scheme of Capanna et al. [4], the juxtaphyseal position was not used to stratify patients.
We hypothesized patients with ABCs in juxtaphyseal or periarticular locations would have an increased risk of recurrence. In addition to this primary hypothesis, we also determined whether age of the patient, gender, race, size, skeletal site, and the use of a high-speed burr influenced the rate of local recurrence.
Materials and Methods
The study design consisted of a retrospective cohort study of 53 patients with primary ABCs treated between 1989 and 2004. The location of cysts with regard to the physis was determined by review of radiographs. Using Kaplan-Meier analysis and the log-rank test, the effect of the location of cysts and other factors on local recurrence was determined. The relative importance of these factors was assessed further by multivariate analysis. Clinical records, radiographs, and pathology reports were reviewed after the study was approved by the Institutional Review Board.
We initially reviewed the records of 96 consecutive patients surgically treated for ABCs between 1989 and 2004. Eighteen secondary ABCs, which involved an underlying primary tumor, were excluded from the series. These included chondroblastoma, giant cell tumor, nonossifying fibroma, and other tumors. We excluded six patients treated with a surgical adjuvant (methylmethacrylate, phenol, or liquid nitrogen). The use of surgical adjuvant was made at the discretion of the surgeon, and the exact reason could not be determined retrospectively. Minimum followup was 24 months (mean, 35 months; range, 24–112 months). Nineteen patients with inadequate followup were excluded, leaving 53 patients in the study cohort (Table 1). There were 28 females and 25 males, and the median age was 14 years (range, 4–65 years) (Fig. 1).
Table 1.
Demographic data
| Characteristic | Number |
|---|---|
| Total number of patients | 53 |
| Gender | |
| Female | 28 |
| Male | 25 |
| Race | |
| Black | 5 |
| Hispanic | 18 |
| White | 30 |
| Age (years) | |
| Median | 14 |
| Range | 4–65 |
| Followup (months) | |
| Mean | 37 |
| Range | 24–99 |
| Size of cyst (cm) | |
| Mean | 5.2 |
| Range | 1.2–13.1 |
| Site of cyst | |
| Central (n = 9) | |
| Pelvis (periacetabular) | 2 |
| Sacroiliac | 1 |
| Scapula (glenoid) | 1 |
| Spine | 5 |
| Extremity (n = 44) | |
| Clavicle | 5 |
| Humerus (proximal) | 4 |
| Humerus (distal) | 1 |
| Forearm (distal radius) | 2 |
| Forearm (other bones) | 3 |
| Hand | 2 |
| Femur (proximal) | 2 |
| Femur (distal) | 7 |
| Tibia (proximal) | 8 |
| Tibia (distal) | 4 |
| Fibula | 5 |
| Foot | 1 |
Fig. 1.
The distribution of cases according to age group is shown. The median age was 14 years.
A sample size power analysis was performed for the primary research question, which pertained to the effect of the juxtaphyseal location of ABCs on local recurrence. Cysts were categorized as juxtaphyseal or nonjuxtaphyseal (see below). When the sample size in each group was 26, with a total number of events required of 40, a 0.050 level two-sided log-rank test for equality of survival curves will have 61% power to detect the difference in survival between two groups with proportions of progression at the end of the study as 27% and 7% for juxtaphyseal and nonjuxtaphyseal cysts, respectively. This assumes no dropouts before the end of the study.
We (PPL, CB) reviewed the initial and followup radiographs. To determine whether the position of a cyst relative to the physis affected recurrence, each cyst was categorized as being juxtaphyseal or nonjuxtaphyseal. A juxtaphyseal cyst was defined as one directly abutting the physis. Any separation of the cyst from the physis was considered a nonjuxtaphyseal cyst. On the initial radiographs, we assessed size of lesion, location in the bone, sclerotic margin, permeative features, and destruction of overlying cortex. Computed tomography (CT) or MRI scans were available for 34 of 53 patients. These were reviewed to determine exact size of lesion and distance from physis. We also determined whether the physis was open or closed on radiographs; an open physis was defined as a physis with two parallel sclerotic lines, whereas a closed physis was defined as a physis with a single fused line throughout the entire length of the physis. Erosion through the physis was noted. The relationship to the physis was categorized as being directly adjacent to the physis or away from the physis, depending on the presence of normal trabecular bone between the physis and the cyst.
Likewise, the relationship to the articular surface was determined in areas such as the periacetabular region, where no secondary ossification center and physis are present. The lesions were categorized as periarticular or nonperiarticular, depending on whether the cyst was directly adjacent to the subchondral bone.
All patients were treated with meticulous curettage. A high-speed burr was used in 35 patients (67%). It could not be determined in this analysis whether the burr was applied uniformly throughout the entire cavity in cases in which it was used. The defect in bone was filled with allograft bone in 45 patients (85%) and autograft in eight patients (15%), and the choice of graft was made at the discretion of the surgeon. During the study period, there were five attending surgeons.
All tumor specimens were examined by pathologists specializing in bone tumors and inspected for evidence of an underlying primary tumor other than ABC. Areas that had an unusual appearance, particularly solid areas and areas with calcified or ossified matrix, were submitted as samples for microscopic examination. We used the following histopathologic criteria for the diagnosis of primary ABC: (1) presence of blood-filled spaces; (2) membranes composed of spindle-shaped mononuclear stromal cells; (3) lack of organization in stromal cells; (4) osteoclastlike giant cells scattered throughout the lesion; (5) focal concentrated aggregates of giant cells; and (6) absence of a chondroid matrix and the plump mononuclear cells typical of giant cell tumors.
Patients were followed every 3 to 6 months with clinical examination and radiographs during the first 2 years and then yearly thereafter. The radiographs were assessed for recurrence of the lesion, which was determined by the presence of an expanding lytic region on serial radiographs. We noted whether the physis remained open or whether there was premature closure. Skeletal deformity resulting from partial fusion of the physis was noted.
Local recurrence-free survival was calculated by Kaplan-Meier analysis. The log-rank test was used to compare survival curves for univariate analysis. Analysis of variance was used to compare means of the size of tumors and the age of patients. Multivariate analysis was performed by a Cox proportional hazards model using the forward stepwise (conditional likelihood ratio) method. The categorical variables used in the multivariate regression included age of 12 years or younger, race, gender, site, size of lesion, surgeon, use of high-speed burr, open physis, juxtaphyseal location, and periarticular location. Significance was defined as p ≤ 0.05. The SPSS 12.0 (SPSS Inc, Chicago, IL) computer program was used to perform statistical calculations.
Results
Ten of 53 patients (18.9%) had local recurrence after surgical curettage. The local recurrence-free survival was 80% at 5 years.
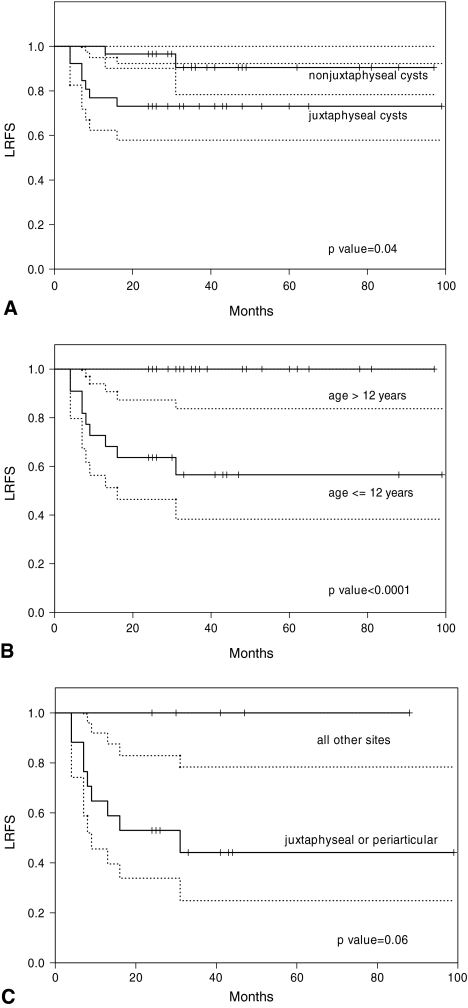
Location of the ABC in a juxtaphyseal site predicted recurrence (Fig. 2A). Eight recurrences developed in the 19 juxtaphyseal cysts, which were directly adjacent to an open physis. The local recurrence-free survival at 5 years was lower (p = 0.04) for patients with juxtaphyseal cysts than for patients with nonjuxtaphyseal cysts (73% vs 93%, respectively). There were three cysts less than 1 cm away from the physis (the closest being 3 mm) but considered not on the physis and therefore nonjuxtaphyseal. None of these recurred. Of the five periarticular cysts where no secondary ossification center was present, two patients had recurrence. In each of these cases, the tumor was directly adjacent to the subchondral bone of the articular surface.
Fig. 2A–C.
(A) The local recurrence-free survival was 93% for nonjuxtaphyseal cysts and 73% for juxtaphyseal cysts (p = 0.04). The 95% confidence intervals are indicated by the dotted lines. (B) The local recurrence-free survival at 5 years was 100% for patients older than 12 years and 53% for patients 12 years or younger (p < 0.0001). (C) The local recurrence-free survival was analyzed for the group of patients 12 years or younger. The local recurrence-free survival at 5 years was 39% for ABCs located in a juxtaphyseal or periarticular location. For all other cysts located away from these sites, the local recurrence-free survival was 100% (p = 0.06).
Age of 12 years or younger was associated with recurrence (Fig. 2B). The local recurrence-free survival was higher (p < 0.0001) for patients older than 12 years than for patients 12 years or younger (100% vs 53%, respectively). There was a higher rate of recurrence associated with younger age group, and all three patients 5 years and younger had recurrence (Table 2). The mean age of patients with recurrences was lower (p = 0.006) than the mean age of patients without recurrences was (7.6 years vs 20.5 years, respectively). Only one factor, age of 12 years or younger independently predicted recurrence (Table 3).
Table 2.
Age dependence of recurrence
| Age group (years) | Number of cases | Juxtaphyseal cysts | Number of recurrences |
|---|---|---|---|
| 1–5 | 3 | 2 | 3 (100%) |
| 6–10 | 12 | 9 | 4 (33%) |
| 11–15 | 19 | 8 | 3 (15%) |
| > 15 | 19 | 0 | 0 |
p < 0.0001.
Table 3.
Multivariate analysis
| Factor | Wald statistic | Regression coefficient (B) | Hazards ratio* | 95% Confidence interval for hazards ratio |
|---|---|---|---|---|
| Age (≤ 12 years) | 3.07 | 5.00 | 148 | 0.55–4090 |
Variables not in the equation include race, gender, site, size of lesion, surgeon, use of high-speed burr, open physis, juxtaphyseal location, and periarticular location; *hazards ratio = eB.
Gender, race, size, axial skeletal site, and use of a high-speed burr were not associated with recurrence (Table 4). The mean size of recurrent cysts was similar to (p = 0.58) the mean size of nonrecurrent cysts (5.6 cm vs 5.0 cm, respectively). The high-speed burr was used in 35 patients, but these patients had a similar (p = 0.34) 5–year local recurrence-free survival as the 18 patients not treated with a high-speed burr (82% vs 74%, respectively).
Table 4.
Effect of factors on local recurrence
| Category | Group | 5–year LRFS | p Value |
|---|---|---|---|
| Age | ≤ 12 years | 52% | < 0.0001 |
| > 12 years | 100% | ||
| Physis | Open | 68% | 0.003 |
| Closed | 100% | ||
| Physis | Adjacent to physis | 73% | 0.04 |
| Not adjacent | 93% | ||
| Size | ≤ 5 cm | 89% | 0.21 |
| > 5 cm | 72% | ||
| Burr | Yes | 82% | 0.34 |
| No | 74% | ||
| Periarticular | Yes | 53% | 0.28 |
| No | 83% | ||
| Race | White | 80% | 0.58 |
| Nonwhite | 78% | ||
| Site | Extremity | 87% | 0.65 |
| Central | 78% | ||
| Gender | Male | 84% | 0.68 |
| Female | 75% |
LRFS = local recurrence-free survival.
All of the recurrences developed in pediatric patients with ABCs located in either a juxtaphyseal location (eight cases) or a periarticular position (two cases). There were no recurrences in pediatric patients whose tumors were separated from the physis or the articular surface of joints (Fig. 2C). This included four patients with periosteal ABCs that arose on the surface of long bones. None of these patients had recurrence. The three patients 5 years and younger all had juxtaphyseal (two cases) or periarticular (one case) disease, and all three of these patients had recurrences.
Four of the 10 patients had more than one recurrence (range, 2–4). These recurrences were managed with further curettage. Wide excision was not performed to control disease in any patient (followup 22–120 months after recurrence).
Most patients, including those with juxtaphyseal disease, had continued growth (Fig. 3). Three patients had some disturbance of growth. Two of these patients had more than one recurrence. One patient presented with a distal femoral ABC at the age of 6 years and had shortening of the limb despite continued growth at the physis. The second patient presented at the age of 7 years with a proximal humeral lesion. This patient had varus deformity at the surgical neck and shortening of the arm but did not undergo surgical correction. The third patient had partial physeal arrest at the radius and subsequent deformity at the wrist.
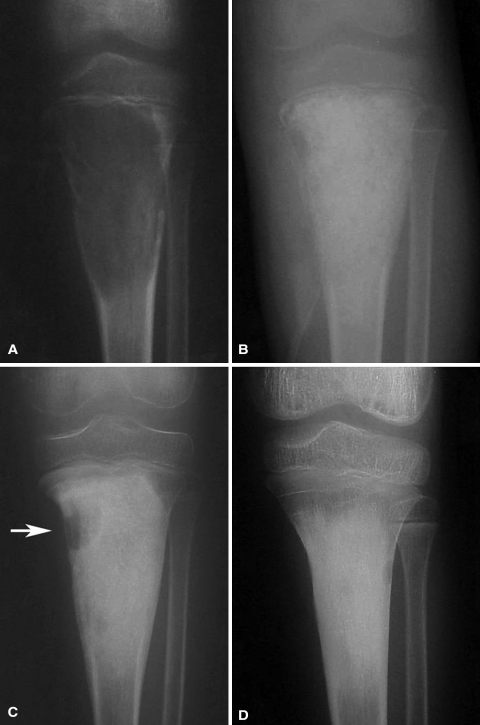
Fig. 3A–D.
(A) A large juvenile juxtaphyseal ABC is present in the proximal tibia of a 7-year-old boy. (B) A postoperative radiograph shows packing of the defect with allograft bone. (C) A small recurrence developed in the metaphysis at 7 months (arrow), and repeat curettage was done in this area. (D) At the 24-month followup, longitudinal bone growth can be observed by growth of the physis away from the graft. Clinically, the patient has no leg length discrepancy.
Discussion
ABC is one of the more aggressive benign bone tumors. The reported rate of recurrence varies, but in most studies, approximately 12% to 30% of patients experience recurrence after initial treatment [3, 4, 11–15]. Several studies suggest young age is associated with a higher risk of recurrence [1, 2, 10, 18, 19], but at least one study suggests age does not predict recurrence [8]. This may be related to aggressiveness in curettage near an epiphysis. We hypothesized the location of an ABC near a physis would increase the risk recurrence. The technical difficulties and the biologic environment potentially could contribute to an increased recurrence rate in this region. In addition to determining the effect of juxtaphyseal position on recurrence, we sought to analyze the effect of various other factors on local recurrence, including age and the use of the high-speed burr.
The major limitations of this study include its being retrospective and the limited sample size. Because the study was retrospective, there could have been selection bias in choices of treatment (eg, use of a high-speed burr) or degree of aggressiveness of curettage for lesions near the physis. Although the number of patients in the study is comparable to others, it nevertheless is small from a statistical perspective, as seen by the sample size power analysis. A greater number of patients would provide greater power, but given the fact that the disease is uncommon, a considerable amount of time would be needed to accrue more patients. It is possible, with a larger sample size, other factors besides age, such as location of cyst, also might independently predict recurrence.
The failure of juxtaphyseal position to emerge as an independent predictor may be attributable in part to the fact that there is age dependency for juxtaphyseal position of cysts. These must by definition develop in skeletally immature patients with open physes. As patients grow and become more skeletally mature, the physis grows away from the cysts. We found skeletally immature patients whose cysts were not directly adjacent to the physis had a local recurrence rate of 0%. This was similar to the rate for skeletally mature patients.
Age and use of a high-speed burr have been associated with recurrence risk. Biesecker et al. [1] reported a higher rate of recurrence in patients 15 years and younger, whereas other studies report patients younger than 10 years have an increased risk of recurrence [9, 10]. In another study, the risk of recurrence was correlated with decreasing age group, and the highest rate of recurrence (75%) was found in children 5 years or younger [17]. Our results corroborate the observation that there is a trend toward an increasing rate of recurrence with young age. In our study, all of the recurrences developed in patients 12 years or younger. Furthermore, the risk of recurrence increased as age decreased, and all patients 5 years or younger had recurrence.
In addition to young age, it has been suggested the use of a high-speed burr reduces the rate of recurrence. Gibbs et al. [10] reported a recurrence rate of 12% in 34 patients treated with curettage and a high-speed burr, which compared favorably with published rates. We detected no difference in recurrence rate with or without use of the high-speed burr. There are several potential explanations for this. First, the number of patients may have resulted in inadequate power. Second, the patients were not randomized, and there may have been some selection bias or differences in the patient groups. Third, the presence of an open physis may have been a confounding factor. Fourth, it may have been the thoroughness of the curettage and not the use of a burr per se that determined whether a recurrence developed. The high-speed burr cannot be used indiscriminately at the open physis, as such use could destroy the growth plate and cause cessation of growth [17]. We believe meticulous curettage with small curettes is preferable for the physeal border. It is possible a contributing factor to recurrence in these areas is the inability to eradicate disease on the growth plate or the periarticular surface. The surgeon may be justifiably cautious in these areas and less likely to be aggressive in the curettage or use of the high-speed burr on the physis. In nonphyseal locations, we do favor the use of the high-speed burr. None of the patients with periosteal ABCs had recurrence develop. The lesions on the surface of the diaphyseal cortex are surgically more accessible and more amenable to aggressive treatment with the high-speed burr.
Although four patients had multiple recurrences, we do not believe it is necessary to perform wide excision to eradicate the disease in most cases. Others have expressed a similar opinion [6, 7]. All four of our patients with multiple recurrences had eventual control of disease with repeat curettage of tumor. We believe it is preferable to preserve growth of the limb, especially in a very young patient, even at the risk of a higher recurrence rate.
Recurrence of ABCs may not be strictly a matter of surgical technique and incomplete excision. There may be an inherent propensity toward recurrence for ABCs in the juxtaphyseal and periarticular locations. Numerous as-yet-undefined factors may contribute to the growth and recurrence of the disease. A followup study to examine whether there are any molecular, cellular, or histologic differences in juxtaphyseal ABCs may be worthwhile.
Our data suggest the risk of recurrence of ABCs may be a function of not just patient age but also location of the cyst. Young patients whose lesions reside in a juxtaphyseal or periarticular location may be more apt to have recurrences. Surgical treatment of ABCs is technically demanding. Great care must be exercised to remove the tumor meticulously while minimizing damage to the articular and physeal cartilage. Despite the high recurrence rate, we believe curettage remains an acceptable means of treatment. Control of the disease and maintenance of skeletal growth can be achieved in the majority of patients. We recommend avoiding radical measures that obliterate the growth plate and instead making every effort to preserve the integrity of the physis and articular cartilage.
Acknowledgments
We thank Terri Robinson for assistance in preparation of the manuscript.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
References
- 1.Biesecker JL, Marcove RC, Huvos AG, Miké V. Aneurysmal bone cysts: a clinicopathologic study of 66 cases. Cancer. 1970;26:615–625. [DOI] [PubMed]
- 2.Bollini G, Jouve JL, Cottalorda J, Petit P, Panuel M, Jacquemier M. Aneurysmal bone cyst in children: analysis of twenty-seven patients. J Pediatr Orthop B. 1998;7:274–285. [DOI] [PubMed]
- 3.Campanacci M, Capanna R, Picci P. Unicameral and aneurysmal bone cysts. Clin Orthop Relat Res. 1986;204:25–36. [PubMed]
- 4.Capanna R, Bettelli G, Biagini R, Ruggieri P, Bertoni F, Campanacci M. Aneurysmal cysts of long bones. Ital J Orthop Traumatol. 1985;11:409–417. [PubMed]
- 5.Capanna R, Springfield DS, Biagini R, Ruggieri P, Giunti A. Juxtaepiphyseal aneurysmal bone cyst. Skeletal Radiol. 1985;13:21–25. [DOI] [PubMed]
- 6.Cottalorda J, Chotel F, Kohler R, de Gauzy JS, Louahem D, Lefort G, Dimeglio A, Bourelle S. Aneurysmal bone cysts of the pelvis in children: a multicenter study and literature review. J Pediatr Orthop. 2005;25:471–475. [DOI] [PubMed]
- 7.Cottalorda J, Kohler R, Chotel F, de Gauzy JS, Lefort G, Louahem D, Bourelle S, Diméglio A. Recurrence of aneurysmal bone cysts in young children: a multicentre study. J Pediatr Orthop B. 2005;14:212–218. [DOI] [PubMed]
- 8.Dormans JP, Hanna BG, Johnston DR, Khurana JS. Surgical treatment and recurrence rate of aneurysmal bone cysts in children. Clin Orthop Relat Res. 2004;421:205–211. [DOI] [PubMed]
- 9.Freiberg AA, Loder RT, Heidelberger KP, Hensinger RN. Aneurysmal bone cysts in young children. J Pediatr Orthop. 1994;14:86–91. [DOI] [PubMed]
- 10.Gibbs CP Jr, Hefele MC, Peabody TD, Montag AG, Aithal V, Simon MA. Aneurysmal bone cyst of the extremities: factors related to local recurrence after curettage with a high-speed burr. J Bone Joint Surg Am. 1999;81:1671–1678. [DOI] [PubMed]
- 11.Koskinen EV, Visuri TI, Holmström T, Roukkula MA. Aneurysmal bone cyst: evaluation of resection and of curettage in 20 cases. Clin Orthop Relat Res. 1976;118:136–146. [PubMed]
- 12.Kransdorf MJ, Sweet DE. Aneurysmal bone cyst: concept, controversy, clinical presentation, and imaging. AJR Am J Roentgenol. 1995;164:573–580. [DOI] [PubMed]
- 13.Leithner A, Windhager R, Lang S, Haas OA, Kainberger F, Kotz R. Aneurysmal bone cyst: a population based epidemiologic study and literature review. Clin Orthop Relat Res. 1999;363:176–179. [DOI] [PubMed]
- 14.Martinez V, Sissons HA. Aneurysmal bone cyst: a review of 123 cases including primary lesions and those secondary to other bone pathology. Cancer. 1988;61:2291–2304. [DOI] [PubMed]
- 15.Mirra MJ. Aneurysmal bone cyst. In: Mirra MJ, Picci P, Gold RH, eds. Bone Tumors: Clinical, Radiologic and Pathologic Correlations. Philadelphia, PA: Lea and Febiger; 1989:1267–1307.
- 16.Oliveira AM, Hsi BL, Weremowicz S, Rosenberg AE, Dal Cin P, Joseph N, Bridge JA, Perez-Atayde AR, Fletcher JA. USP6 (Tre2) fusion oncogenes in aneurysmal bone cyst. Cancer Res. 2004;64:1920–1923. [DOI] [PubMed]
- 17.Ramírez AR, Stanton RP. Aneurysmal bone cyst in 29 children. J Pediatr Orthop. 2002;22:533–539. [DOI] [PubMed]
- 18.Vergel De Dios AM, Bond JR, Shives TC, McLeod RA, Unni KK. Aneurysmal bone cyst: a clinicopathologic study of 238 cases. Cancer. 1992;69:2921–2931. [DOI] [PubMed]
- 19.Zehetgruber H, Bittner B, Gruber D, Krepler P, Trieb K, Kotz R, Dominkus M. Prevalence of aneurysmal and solitary bone cysts in young patients. Clin Orthop Relat Res. 2005;439:136–143. [DOI] [PubMed]