Abstract
The prognosis of patients with bone metastasis from lung cancer has not been well documented. We assessed the survival rates after bone metastasis and prognostic factors in 118 patients with bone metastases from lung cancer. The cumulative survival rates after bone metastasis from lung cancer were 59.9% at 6 months, 31.6% at 1 year, and 11.3% at 2 years. The mean survival was 9.7 months (median, 7.2 months; range, 0.1–74.5 months). A favorable prognosis was more likely in women and patients with adenocarcinoma, solitary bone metastasis, no metastases to the appendicular bone, no pathologic fractures, performance status 1 or less, use of systemic chemotherapy, and use of an epithelial growth factor receptor inhibitor. Analyses of single and multiple variables indicated better prognoses for patients with adenocarcinoma, no evidence of appendicular bone metastases, and treatment with an epithelial growth factor receptor inhibitor. The mean survival period was longer in a small group treated with an epithelial growth factor receptor inhibitor than in the larger untreated group. The data preliminarily suggest treatment with an epithelial growth factor receptor inhibitor may improve survival after bone metastasis.
Level of Evidence: Level IV, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Metastatic bone tumors occur at particularly high rates in cancers of the breast, prostate, lung, and kidney, accounting for 75% of all patients [16]. Many patients with lung cancer are in advanced stages of the disease at the time of diagnosis. The 5-year survival rate for patients with lung cancer is 10% to 20%, as reported by Stanley [15] and Freise et al. [4], indicating a poor prognosis. Although it is reported bone metastasis from lung cancer occurs in 14% to 40% of patients, its clinical features have not been clearly described [9].
When treating skeletal metastasis, it is important to know the prognostic factors and prognosis after bone metastasis. Tokuhashi et al. [17] proposed six factors that predicted survival for tumors metastatic to the spine: general condition, number of extraspinal bone metastases, number of metastases in the vertebral body, metastases to major internal organs, primary site of the cancer, and severity of spinal cord palsy. The grade of malignancy of the primary tumors, visceral metastasis to vital organs, and number of bone metastases are reportedly important prognostic factors [18, 19]. In a report of 350 patients with bone metastasis, the primary site, performance status (PS), number of bone metastases, metastasis to organs, and previous chemotherapy were important prognostic factors, with lung cancer being the poorest [10]. The Scandinavian Sarcoma Group [5] examined prognostic factors in 460 patients undergoing surgery for bone metastasis and reported poor prognoses in patients with lung cancer as the primary site, pathologic fracture, and metastasis to organs. In another report of 342 patients with vertebral metastasis, the important prognostic factors included PS, metastasis to organs, and the primary site [20]. Prognosis in bone metastasis from lung cancer also was reported as poor. However, these studies [5, 10, 17–20] reported on bone metastasis from various cancers and did not focus on lung cancer alone. Therefore, the prognostic factors and survival rates of patients with bone metastasis from lung cancer remain unclear.
Several recent reports suggest an epithelial growth factor receptor (EGFR) inhibitor has been effective in treating lung cancer [6, 11, 12, 21]. The EGFR inhibitor is a new molecule-targeted agent for lung cancer that is reported to have a considerable effect on females and nonsmokers, especially those with adenocarcinoma [6, 12]. However, its effectiveness in patients with bone metastasis from lung cancer is unknown.
We first assessed the survival rates and explored various prognostic factors of 118 patients with bone metastasis from lung cancer. We then preliminarily ascertained in a small group of patients whether treatment with an EGFR inhibitor had the potential to influence survival.
Materials and Methods
We retrospectively reviewed 1157 patients with lung cancer treated at Aichi Cancer Center Hospital between January 1, 1999, and December 31, 2002. Of these, 121 patients (10.4%) were treated for lung cancer that had metastasized to bone. We excluded three patients because of incomplete information; this left 118 patients (77 men, 41 women) who had bone metastasis from lung cancer (Table 1). Fifty-two of the 118 patients met criteria (see below) for administering an oral selective EGFR inhibitor and it was administered to 14 of the 52 patients. It was not administered to the remaining 38 patients because the use of EGFR inhibitor was not available before June 2002 in Japan. Apart from determining survival, our primary outcome was survival in patients receiving an EGFR inhibitor. Based on survival in our hospital [12], the power would be approximately 70% using a two-side type I error of 5% to detect a 30% difference in 1–year survival among the 52 patients who met the criteria for administering EGFR inhibitor.
Table 1.
Distribution of patients with skeletal metastases of lung cancer (n = 118)
| Prognostic factor | Subgroups | Number of patients |
|---|---|---|
| Age (years) | ≥ 60 | 67 (57%) |
| < 60 | 51 (43%) | |
| Gender | Female | 41 (35%) |
| Male | 77 (65%) | |
| Performance status | 0,1 | 67 (57%) |
| 2, 3, 4 | 51 (43%) | |
| Subtype | Adenocarcinoma | 83 (70%) |
| Nonadenocarcinoma | 35 (30%) | |
| Surgery for lung cancer | Yes | 36 (31%) |
| No | 82 (69%) | |
| Number of bone metastases | Solitary | 19 (16%) |
| Multiple | 99 (84%) | |
| Appendicular bone metastasis | Yes | 21 (18%) |
| No | 97 (82%) | |
| Pathologic fracture | Yes | 15 (13%) |
| No | 103 (87%) | |
| Brain metastasis | Yes | 48 (41%) |
| No | 70 (59%) | |
| Liver metastasis | Yes | 16 (14%) |
| No | 102 (86%) | |
| Chemotherapy | Yes | 67 (57%) |
| No | 51 (43%) | |
| Radiation | Yes | 61(52%) |
| No | 57 (48%) | |
| Gefitinib | Yes | 14 (12%) |
| No | 104 (88%) |
The mean age of the 118 patients at the time of bone metastasis was 59.6 years (standard deviation [SD], 10.2 years; range, 28–85 years). Lung cancer diagnosis was confirmed by computed tomography, fiberscope examination, and biopsy. Presence or absence of bone metastasis was confirmed by radiography or bone scintigraphy. All patients provided informed consent for participation in this study.
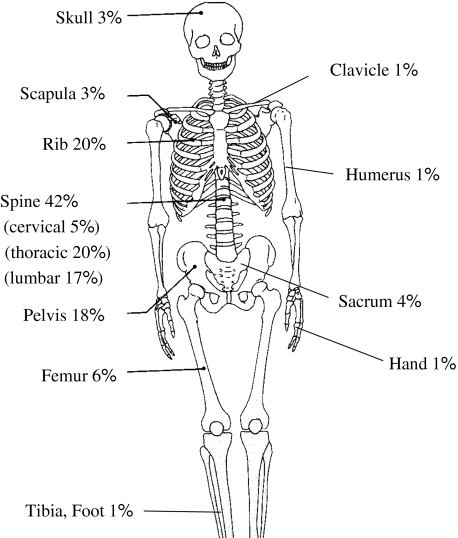
Among the 118 patients, 308 sites with bone metastasis were determined. Sites with high incidence included the rib, vertebra, and pelvis, where there is a high concentration of red marrow (Fig. 1). When bone metastasis was first confirmed by radiography or scintigraphy, 19 patients (16%) had a solitary site of metastasis and 99 patients (84%) had multiple sites. Eight (42%) of the 19 patients had a solitary bone metastasis that developed in other new sites. The remaining 11 patients (58%) remained with a solitary site of metastasis at followup. The minimum followup was 0.2 months (mean ± SD, 12.8 ± 14.6 months; range, 0.2–54.0 months)
Fig. 1.
Anatomic localization of skeletal metastases from lung cancer is shown (n = 118).
The time from lung cancer diagnosis to bone metastasis was less than 1 month in 54 patients (46%), of which 12 patients initially had been diagnosed with an unknown primary cancer. For the remaining patients, the time from diagnosis to bone metastasis was 1 to 6 months in 23 patients, 6 months to 1 year in 11 patients, and 1 to 2 years in 10 patients. There were 20 patients (17%) whose lung cancer metastasized to the bone longer than 2 years after diagnosis, among whom the primary site was excised in 19 cases.
The major histologic type of the primary site was adenocarcinoma (83 patients), followed by squamous cell carcinoma (17 patients), small cell and large cell carcinoma (seven patients), and adenosquamous carcinoma (four patients). The primary site already had been excised at the time of bone metastasis in 36 patients (31%), but not in the remaining 82 patients (69%). After bone metastasis, 44 patients (37%) had brain metastasis, 12 patients (10%) had liver metastasis, and four patients (4%) had metastasis to the brain and liver. Approximately 50% of the patients had brain or liver metastasis.
Performance status was evaluated using the method devised by the Eastern Cooperative Oncology Group [14]. Patients with PS 0 are fully active and have no limitation in daily life; patients with PS 1 are restricted in physically strenuous activity but are ambulatory and able to do work of a light or sedentary nature; patients with PS 2 are ambulatory and capable of all self-care but are unable to do work activities; patients with PS 3 are capable of only limited self-care, are confined to bed or chair for greater than 50% of working hours; and patients with PS 4 are completely disabled, cannot do any self-care, and are totally confined to a bed or chair. Seventeen patients had PS 0, 50 patients had PS 1, 17 patients had PS 2, 17 patients had PS 3, and 17 patients had PS 4. Pathologic fractures during the course occurred in 15 patients, among which five underwent surgery for femoral pathologic fractures. Three patients were treated by intralesional resection with prosthesis implantation and two patients were treated with compound plate osteosynthesis. The remaining 10 patients had spinal compression fractures, of which two patients had complete paralysis of the lower extremities after pathologic fracture.
Regarding treatment of the primary site, radiotherapy was performed in 61 patients and systemic chemotherapy was administered to 67 patients. The administered regimens varied among patients, which included gemcitabine hydrochloride and vinorelbin ditartrate (11 patients), cisplatin and vinorelbin ditartrate (seven patients), carboplatin and vinorelbin ditartrate (six patients), carboplatin and paclitaxel (six patients), carboplatin and etoposide (four patients), carboplatin and etoposide (four patients), cisplatin and paclitaxel (four patients), cisplatin and etoposide (two patients), cisplatin and irinotecan hydrochloride (two patients), cisplatin and gemcitabine hydrochloride (one patient), carboplatin and docetaxel hydrate (one patient), and unknown (19 patients). Systemic chemotherapy was not given to the remaining 51 patients.
We examined the cumulative survival rate after bone metastasis and prognostic factors for patients with bone metastasis from lung cancer (Table 1) and then calculated overall survival based on absence or presence of an EGFR inhibitor (Tables 2, 3).
Table 2.
Data for patients with adenocarcinoma and performance status 1 or less (n = 14) with user of gefitinib
| Patient number | Age (years) | Gender | Performance status | Metastasis to appendicular bone | Pathologic fracture | Solitary or multiple | Radiation | Chemo therapy | Outcome | Survival period (days) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 42 | Female | 0 | − | − | Multiple | − | + | Dead | 898 |
| 2 | 72 | Female | 1 | − | − | Solitary | − | + | Alive | 736 |
| 3 | 57 | Male | 0 | − | − | Multiple | + | + | Dead | 467 |
| 4 | 68 | Female | 0 | − | − | Multiple | − | + | Alive | 903 |
| 5 | 56 | Male | 1 | − | − | Solitary | − | + | Alive | 251 |
| 6 | 72 | Male | 1 | + | − | Multiple | + | + | Dead | 531 |
| 7 | 66 | Male | 0 | − | – | Multiple | − | + | Dead | 427 |
| 8 | 65 | Male | 0 | − | − | Multiple | + | + | Dead | 387 |
| 9 | 72 | Female | 1 | − | − | Solitary | + | + | Dead | 431 |
| 10 | 69 | Male | 1 | − | − | Multiple | + | + | Alive | 491 |
| 11 | 62 | Female | 1 | + | − | Solitary | + | + | Dead | 448 |
| 12 | 66 | Female | 1 | − | − | Multiple | − | + | Dead | 310 |
| 13 | 66 | Male | 1 | − | − | Multiple | − | + | Alive | 621 |
| 14 | 54 | Female | 1 | − | − | Solitary | + | − | Alive | 577 |
Table 3.
Data for patients among patients with adenocarcinoma and performance status 1 or less (n = 38) without use of gefitinib
| Patient number | Age (years) | Gender | Performance status | Metastasis to appendicular bone | Pathologic fracture | Solitary or multiple | Radiation | Chemotherapy | Outcome | Survival period (days) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | Male | 0 | − | − | Multiple | + | + | Dead | 460 |
| 2 | 61 | Male | 1 | − | − | Multiple | − | − | Alive | 48 |
| 3 | 59 | Male | 0 | − | − | Multiple | − | + | Dead | 294 |
| 4 | 39 | Female | 0 | − | − | Multiple | + | + | Dead | 365 |
| 5 | 28 | Female | 0 | − | − | Multiple | − | + | Dead | 336 |
| 6 | 56 | Male | 0 | − | − | Multiple | − | + | Dead | 369 |
| 7 | 51 | Female | 0 | − | − | Multiple | + | + | Dead | 201 |
| 8 | 61 | Male | 1 | − | − | Solitary | + | − | Dead | 188 |
| 9 | 57 | Male | 1 | − | − | Multiple | − | + | Dead | 28 |
| 10 | 49 | Female | 1 | − | − | Multiple | + | + | Alive | 303 |
| 11 | 49 | Male | 1 | − | − | Multiple | + | + | Dead | 243 |
| 12 | 63 | Male | 1 | − | − | Solitary | − | + | Alive | 390 |
| 13 | 59 | Male | 1 | − | − | Multiple | − | + | Dead | 144 |
| 14 | 54 | Female | 0 | − | − | Multiple | + | + | Alive | 285 |
| 15 | 51 | Female | 1 | + | − | Multiple | + | − | Dead | 61 |
| 16 | 57 | Female | 1 | − | − | Multiple | + | − | Dead | 53 |
| 17 | 63 | Female | 1 | − | + | Multiple | − | + | Dead | 244 |
| 18 | 65 | Male | 1 | − | − | Multiple | + | + | Dead | 166 |
| 19 | 62 | Male | 0 | − | − | Multiple | − | + | Alive | 470 |
| 20 | 55 | Female | 1 | − | − | Solitary | + | + | Alive | 207 |
| 21 | 42 | Male | 0 | − | − | Multiple | + | − | Dead | 36 |
| 22 | 56 | Male | 1 | + | − | Multiple | + | + | Alive | 316 |
| 23 | 28 | Female | 0 | − | − | Multiple | − | + | Alive | 308 |
| 24 | 56 | Female | 1 | − | − | Multiple | + | + | Dead | 351 |
| 25 | 60 | Female | 1 | + | − | Multiple | + | + | Dead | 196 |
| 26 | 63 | Female | 1 | − | − | Multiple | − | − | Dead | 68 |
| 27 | 47 | Female | 1 | + | − | Multiple | + | + | Dead | 163 |
| 28 | 66 | Female | 1 | − | − | Multiple | − | + | Dead | 393 |
| 29 | 45 | Female | 1 | − | − | Multiple | − | + | Dead | 345 |
| 30 | 41 | Male | 1 | + | − | Multiple | + | + | Dead | 306 |
| 31 | 55 | Male | 1 | − | − | Solitary | − | + | Alive | 1619 |
| 32 | 69 | Male | 1 | − | − | Multiple | + | − | Dead | 164 |
| 33 | 50 | Male | 1 | − | + | Multiple | − | − | Dead | 18 |
| 34 | 57 | Female | 1 | − | − | Multiple | − | + | Alive | 855 |
| 35 | 42 | Female | 1 | − | − | Multiple | + | + | Dead | 366 |
| 36 | 60 | Female | 1 | − | − | Multiple | − | − | Dead | 156 |
| 37 | 51 | Male | 1 | − | − | Multiple | + | + | Dead | 387 |
| 38 | 59 | Female | 1 | − | − | Solitary | + | + | Alive | 1416 |
Gefitinib (Irresa®; AstraZeneca, Osaka, Japan), an oral selective inhibitor of EGFR, was administered to patients with adenocarcinoma and PS 1 or less. In this study, there were 52 patients with adenocarcinoma and PS 1 or less. Gefitinib was administered to 14 of these patients (seven men, seven women; mean age ± SD, 63.4 ± 8.5 years; range, 42–72 years) (Table 2) and not administered to the remaining 38 patients (18 men, 20 women; mean age ± SD, 53.6 ± 9.5 years; range, 28–69 years) (Table 3).
We estimated patient survival using the Kaplan–Meier survival method, considering the relevant time scale for analysis to begin at the time of bone metastasis. Patients were censored on the basis of whether they were alive. The univariate log rank test was used to evaluate the prognostic importance of age, gender, PS, histologic type, condition of the primary site, number of bone metastases, site of bone metastasis, pathologic fractures, metastasis to the brain or liver, chemotherapy or radiotherapy for the primary site, and use of an EGFR inhibitor (gefitinib). Subsequent multivariate analysis was performed to detect factors independently associated with survival using a Cox proportional hazard survival model [4]. Multivariate regression analysis was performed by including all clinical characteristics that independently predicted 1–year survival. The results are reported as a hazard ratio and 95% confidence interval. As for the influence of gefitinib on survival, we used the Kaplan–Meier curve of overall survival based on absence or presence of gefitinib treatment among patients with adenocarcinoma and PS 1 or less. The log rank test was used to evaluate a difference. For all analyses, a p value of 0.05 or less was considered significant. We used SPSS 11.0 (SPSS Inc, Chicago, IL) software to conduct Kaplan–Meier survival analysis and the Cox proportional hazard survival model.
Results
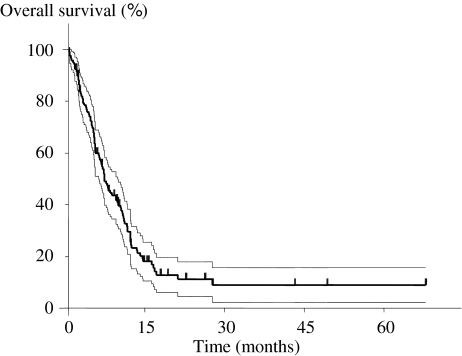
The overall cumulative survival rate after bone metastasis for all 118 patients was 59.9% for 6-month survival, 31.6% for 1 year, and 11.3% for 2 years. The mean survival period was 9.7 months (SD, 10.3 months; median, 7.2 months; range, 0.1–74.5 months) (Fig. 2). Although the prognosis in patients with bone metastasis was generally poor, seven patients survived for at least 2 years (6%).
Fig. 2.
A Kaplan–Meier curve of overall survival for all patients is illustrated. The dotted lines indicate the 95% confidence interval. The overall cumulative survival rates after bone metastasis for all 118 patients are 59.9% for 6 months, 31.6% for 1 year, and 11.3% for 2 years.
We identified eight prognostic factors: gender, PS, histologic type, number of bone metastases, site of bone metastases (bone metastasis to the appendicular bone), pathologic fracture, systemic chemotherapy, and gefitinib use (Table 4). A favorable prognosis was more likely in women and in patients with PS 1 or less, adenocarcinoma, solitary bone metastasis, no metastases to the appendicular bone, no pathologic fractures, use of systemic chemotherapy, and use of gefitinib.
Table 4.
Univariate analysis of 1-year survival rates in patients with skeletal metastases of lung cancer (n = 118)
| Prognostic factor | Subgroup | Survival (months) | 1-year survival rate (%) | p Value |
|---|---|---|---|---|
| Age (years) | ≥ 60 | 9.1 (1.3) | 27.1 (5.6) | 0.38 |
| < 60 | 10.4 (1.5) | 32.6 (7.2) | ||
| Gender | Female | 13 (2.1) | 39.3 (8.1) | 0.02 |
| Male | 7.9 (0.9) | 25.8 (5.2) | ||
| Performance status | 0,1 | 11.6 (1.2) | 44.8 (6.4) | < 0.0001 |
| 2, 3, 4 | 7.1 (1.5) | 13.3 (5.0) | ||
| Subtype | Adenocarcinoma | 11.3 (1.3) | 41.6 (5.7) | < 0.0001 |
| Nonadenocarcinoma | 5.8 (0.8) | 8.9 (4.9) | ||
| Surgery for lung cancer | Yes | 11.0 (2.0) | 27.8 (7.5) | 0.89 |
| No | 9.1 (1.1) | 32.0 (5.6) | ||
| Number of bone metastases | Solitary | 14.0 (3.3) | 54.3 (12.2) | 0.02 |
| Multiple | 8.9 (0.9) | 27.3 (4.7) | ||
| Appendicular bone metastasis | Yes | 6.5 (1.1) | 12.6 (8.0) | 0.03 |
| No | 10.4 (1.1) | 35.6 (5.1) | ||
| Pathologic fracture | Yes | 6.4 (1.2) | 6.7 (6.4) | 0.04 |
| No | 10.2 (1.1) | 35.7 (5.0) | ||
| Brain metastasis | Yes | 9.9 (1.4) | 32.7 (7.3) | 0.65 |
| No | 9.5 (1.3) | 28.9 (5.6) | ||
| Liver metastasis | Yes | 7.0 (1.3) | 13.4 (8.8) | 0.1 |
| No | 10.1 (1.1) | 33.3 (4.9) | ||
| Chemotherapy | Yes | 11.4 (1.2) | 45.3 (6.3) | 0.0009 |
| No | 7.5 (1.5) | 13.0 (4.9) | ||
| Radiation | Yes | 9.7 (1.4) | 30.0 (6.2) | 0.49 |
| No | 9.6 (1.3) | 31.2 (6.5) | ||
| Gefitinib | Yes | 17.8 (1.8) | 84.6 (10.0) | 0.0001 |
| No | 8.6 (1.0) | 22.7 (4.4) |
Values are expressed as mean, with standard error in parentheses.
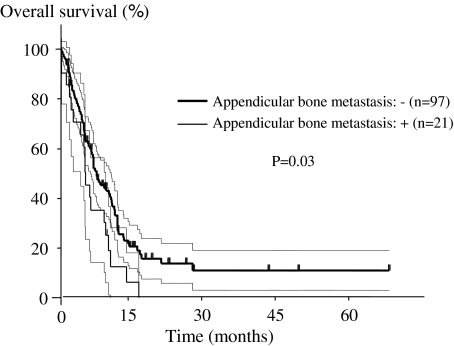
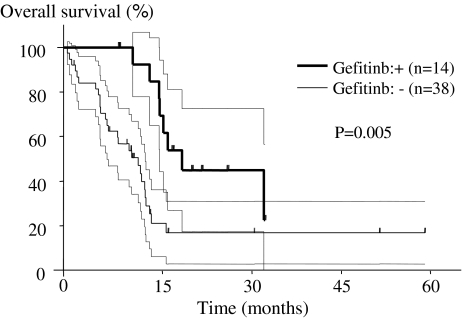
The presence of adenocarcinoma, evidence of appendicular bone metastases, and use of gefitinib independently predicted survival (Table 5). The prognosis was poorer (p = 0.03) in patients with metastasis to the appendicular bone (mean, 6.5 months; range, 0.1–17.7 months) than in patients without metastasis (mean, 10.4 months; range, 0.2–74.5 months) (Fig. 3). The mean survival was longer (p = 0.005) in the group treated with gefitinib (17.8 months; range, 8.4–30.1 months) than in the group without gefitinib (10.8 months; range, 0.6–54.0 months) among 52 patients with adenocarcinoma and PS 1 or less (Fig. 4).
Table 5.
Multivariate analysis of selected clinical factors in patients with skeletal metastasis of lung cancer
| Prognostic factor | p Value | Hazard ratio (95% confidence interval) |
|---|---|---|
| Positive | ||
| Gender (female) | 0.63 | 1.13 (0.68–1.88) |
| Performance status (0, 1) | 0.09 | 1.69 (0.93–3.08) |
| Adenocarcinoma | < 0.01 | 2.17 (1.30–3.62) |
| Pathologic fracture | 0.33 | 1.39 (0.71–2.73) |
| Chemotherapy | 0.53 | 1.20 (0.68–2.11) |
| Gefitinib | 0.03 | 2.42 (1.09–5.32) |
| Negative | ||
| Multiple bone metastasis | 0.14 | 1.68 (0.84–3.34) |
| Appendicular bone metastasis | 0.01 | 2.05 (1.18–3.56) |
Fig. 3.
A Kaplan–Meier curve of overall survival based on absence or presence of metastasis of the appendicular bone is illustrated. The dotted lines indicate the 95% confidence interval. The prognosis is poorer (p = 0.03) in patients with metastasis to the appendicular bone than in patients without metastasis.
Fig. 4.
A Kaplan–Meier curve of overall survival based on absence or presence of gefitinib treatment is illustrated. The dotted lines indicate the 95% confidence interval. The mean survival is longer (p = 0.005) in the group treated with gefitinib than in the untreated group among 52 patients with adenocarcinoma and PS 1 or less.
Discussion
It is important to know the prognosis after bone metastasis when treating bone metastasis from lung cancer. Primary site, PS, presence or absence of metastasis to organs, and number of bone metastases have been reported as important prognostic indicators in patients with bone metastasis from various cancers [5, 10, 20]. However, we are unaware of any previous reports regarding the prognostic factors of bone metastasis specifically from lung cancer. We examined the survival rates and prognostic indicators after bone metastasis from lung cancer.
The major limitations of our study included the lack of control subjects for comparison. Furthermore, there was a wide range of chemotherapy regimens and a selection bias of gefitinib use among the individual patients. Therefore, we compared the survival based on absence or presence of EGFR inhibitor treatment among patients with adenocarcinoma and PS 1 or less to exclude selection bias. The numbers of patients receiving EGFR was small (14) and therefore the power of the study is limited and must be considered preliminary. However, our study represents the largest followup study of patients with bone metastasis from lung carcinoma at one institution.
Some reports suggest the mean length of survival in patients with Stage IV disease, including distant metastasis, is approximately 6 months [2, 13]. The mean survival period for patients with lung cancer with bone metastasis has been reported as 5 to 6 months [15]. We found a mean survival period after bone metastasis of 9.7 months, with a median of 7.2 months. Approximately 70% of the patients died within 1 year after bone metastasis. Although the prognosis in patients with lung cancer with bone metastasis was extremely poor, seven of the 118 patients (6%) survived for at least 2 years. Hirano et al. [7] reported two patients with a solitary metastasis site who had extended survival by surgical resection of the metastatic site and chemotherapy. Agarwala and Hanna [1] also reported a patient with a solitary bone metastasis had apparently longer survival with aggressive treatment. Ando et al. [2] reported the grade of PS and the number of metastasized organs were important factors in patients with distal metastasis from lung cancer. In our study, the mean length of survival was substantially longer in patients with solitary-site metastasis than in patients with multiple-site metastases, and the survival rate was longer in patients with PS 1 or less than in patients with PS 2 or greater. It is suggested PS and number of bone metastases are associated with survival after bone metastasis [2].
Based on the primary site, Tofe et al. [16] reported a high incidence of metastasis in the lumbar vertebra, femur, and ilium among patients with prostate cancer; in the pelvis, vertebra, femur, and ribs among patients with breast cancer; and in the skull and vertebra among patients with thyroid cancer. We observed a high incidence of bone metastasis from lung cancer in the vertebra, rib, and pelvis, and metastasis to the femur in only 6%. The prognosis was poorer in patients with metastasis to the appendicular bone, such as the femur, than in patients with metastasis only to an axial bone, such as the vertebra, rib, or pelvis. The vertebral vein system is known as a mechanism for spread of axial bone metastasis [3]. In bone metastasis from lung cancer, metastasis may occur easily at an axial bone through the vertebral vein system [3] at an early stage and then at an appendicular bone in more advanced stages of the disease.
Among our study patients, the mean survival period was longer in the group treated with gefitinib than in the untreated group. Gefitinib, an EGFR inhibitor, is a new molecule-targeting treatment for lung cancer. It is reported to have a considerable effect on females and nonsmokers, especially those with adenocarcinoma [6, 11, 12, 21]. Analyses of single and multiple variables indicated better prognoses for patients with adenocarcinoma and patients treated with gefitinib. These findings suggest treatment with gefitinib may improve survival after bone metastasis. However, interstitial pneumonia remains a serious side effect [8]; furthermore, it is reported gefitinib is less effective in patients without the EGFR gene [12]. Therefore, indications for treatment with gefitinib should be considered carefully before improvement in survival can be expected.
We found a favorable prognosis was more likely in women and in patients with PS 1 or less, adenocarcinoma, solitary bone metastasis, no metastasis to the appendicular bone, no pathologic fracture, use of systemic chemotherapy, and use of gefitinib. Histologic subtype, no evidence of appendicular bone metastases, and use of gefitinib independently predicted survival. Our findings suggest treatment with EGFR inhibitor improves survival after bone metastasis. However, further investigations such as controlled clinical trials are needed to verify the usefulness of EGFR inhibitor.
Acknowledgments
We thank Mitsuko Yokouchi and Kiyonori Kuriki for statistical assistance and Shoko Miyachi for administrative assistance in the preparation of this manuscript.
Footnotes
One or more of the authors (HS) has received funding from the Grant-in-Aid for Cancer Research (14–16) from the Ministry of Health, Labour and Welfare, Japan.
Each author certifies that his or her institution has approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Agarwala AK, Hanna NH. Long-term survival in a patient with stage IV non-small-cell lung carcinoma after bone metastasectomy. Clin Lung Cancer. 2005;6:367–368. [DOI] [PubMed]
- 2.Ando M, Ando Y, Sugiura S, Minami H, Saka H, Sakai S, Shimokata K, Hasegawa Y. Prognostic factors for short-term survival in patients with stage IV non-small cell lung cancer. Jpn J Cancer Res. 1999;90:249–253. [DOI] [PMC free article] [PubMed]
- 3.Batson OV. The function of vertebral veins and their role in spread of metastases. Ann Surg. 1940;112:138–149. [DOI] [PMC free article] [PubMed]
- 4.Freise G, Gabler A, Liebig S. Bronchial carcinoma and long-term survival: retrospective study of 433 patients who underwent resection. Thorax. 1978;33:228–234. [DOI] [PMC free article] [PubMed]
- 5.Hansen BH, Keller J, Laitinen M, Berg P, Skjeldal S, Trovik C, Nilsson J, Walloe A, Kalén A, Wedin R. The Scandinavian Sarcoma Group Skeletal Metastasis Register. Survival after surgery for bone metastases in the pelvis and extremities. Acta Orthop Scand Suppl. 2004;75:11–15. [DOI] [PubMed]
- 6.Herbst RS, Maddox AM, Rothenberg ML, Small EJ, Rubin EH, Baselga J, Rojo F, Hong WK, Swaisland H, Averbuch SD, Ochs J, LoRusso PM. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non-small-cell lung cancer and other solid tumors: results of a phase I trial. J Clin Oncol. 2002;20:3815–3825. [DOI] [PubMed]
- 7.Hirano Y, Oda M, Tsunezuka Y, Ishikawa N, Watanabe G. Long-term survival cases of lung cancer presented as solitary bone metastasis. Ann Thorac Cardiovasc Surg. 2005;11:401–404. [PubMed]
- 8.Inoue A, Saijo Y, Maemondo M, Gomi K, Tokue Y, Kimura Y, Ebina M, Kikuchi T, Moriya T, Nukiwa T. Severe acute interstitial pneumonia and gefitinib. Lancet. 2003;361:137–139. [DOI] [PubMed]
- 9.Johnston AD. Pathology of metastatic tumors in bone. Clin Orthop Relat Res. 1970;73:8–32. [DOI] [PubMed]
- 10.Katagiri H, Takahashi M, Wakai K, Sugiura H, Kataoka T, Nakanishi K. Prognostic factors and a scoring system for patients with skeletal metastasis. J Bone Joint Surg Br. 2005;87:698–703. [DOI] [PubMed]
- 11.Matsuyama W, Yamamoto M, Machida K, Mitsuyama H, Watanabe M, Higashimoto I, Osame M, Arimura K. [Two lung adenocarcinoma patients with multiple brain metastasis treated with gefitinib and surviving more than 2 years] (in Japanese). Nihon Kokyuki Gakkai Zasshi. 2006;44:653–658. [PubMed]
- 12.Mitsudomi T, Kosaka T, Endoh H, Horio Y, Hida T, Mori S, Hatooka S, Shinoda M, Takahashi T, Yatabe Y. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol. 2005;23:2513–2520. [DOI] [PubMed]
- 13.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717 [DOI] [PubMed]
- 14.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [DOI] [PubMed]
- 15.Stanley KE. Prognostic factors for survival in patients with inoperable lung cancer. J Natl Cancer Inst. 1980;65:25–32. [PubMed]
- 16.Tofe AJ, Francis MD, Harvey WJ. Correlation of neoplasms with incidence and localization of skeletal metastases: an analysis of 1,355 diphosphonate bone scans. J Nucl Med. 1975;16:986–989. [PubMed]
- 17.Tokuhashi Y, Matsuzaki H, Toriyama S, Kawano H, Ohsaka S. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine. 1990;15:1110–1113. [DOI] [PubMed]
- 18.Tomita K, Kawahara N, Baba H, Tsuchiya H, Nagata S, Toribatake Y. Total en bloc spondylectomy for solitary spinal metastases. Int Orthop. 1994;18:291–298. [DOI] [PubMed]
- 19.Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine. 2001;26:298–306. [DOI] [PubMed]
- 20.van der Linden YM, Dijkstra SP, Vonk EJ, Marijnen CA, Leer JW. Dutch Bone Metastasis Study Group. Prediction of survival in patients with metastases in the spinal column: results based on a randomized trial of radiotherapy. Cancer. 2005;103:320–328. [DOI] [PubMed]
- 21.Yokouchi H, Yamazaki K, Kinoshita I, Konishi J, Asahina H, Sukoh N, Harada M, Akie K, Ogura S, Ishida T, Munakata M, Dosaka-Akita H, Isobe H, Nishimura M. Clinical benefit of readministration of gefitinib for initial gefitinib-responders with non-small cell lung cancer. BMC Cancer. 2007;7:51. [DOI] [PMC free article] [PubMed]