Abstract
Modern metal-on-metal hip resurfacing was introduced as a bone-preserving method of joint reconstruction for young and active patients; however, the large diameter of the bearing surfaces is of concern for potential increased metal ion release. We hypothesized there were no differences in serum concentrations of chromium, cobalt, and molybdenum between patients who had metal-on-metal hip resurfacing (Group A; average head diameter, 48 mm; median followup, 24 months) and patients who had 28-mm metal-on-metal THA (Group B; median followup, 25 months). Serum concentrations also were compared with concentrations in healthy subjects. We identified no differences in ion levels between Groups A and B. A distinction was made according to gender. Women showed a higher chromium release in Group A whereas men had a higher cobalt release in Group B. Values obtained from Group A were higher than those of the control subjects. Our data suggest metal-on-metal bearings for THA should not be rejected because of concern regarding potential increased metal ion release; however, patients with elevated ion levels, even without loosening or toxicity, could be at higher risk and should be followed up periodically.
Level of Evidence: Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Total hip arthroplasty leads to marked improvement in the quality of life of patients who suffer from severe forms of hip diseases. Young and/or active patients are challenging for orthopaedic surgeons because conventional joint arthroplasty does not provide a lasting solution for their needs [9, 21, 39]. In addition, patient expectations have changed during the past decade in that modern prosthetic designs must address the low-demand requirements of elderly patients and work and leisure aspirations of younger patients.
Metal-on-metal (MOM) total hip resurfacing offers a suitable solution for young and/or active patients affected by osteoarthritis, osteonecrosis, epiphysiolysis, congenital hip dysplasia, and rheumatoid arthritis, but the large surface area of large diameter implants raises concerns regarding postoperative metal ion release [5]. Potential effects of chronic increases in serum Co and Cr levels include local tissue toxicity, inflammation, bone loss, renal function alteration, immune modulation, hypersensitivity, chromosomal damage, and malignant cellular transformation [20, 27, 36, 41]. Although there is no evidence prolonged exposure to elevated metal ion release results in any negative biologic effect, the indications for MOM hip resurfacing are limited by concerns related to increased metal ion release [24].
Metal-on-metal bearings have been used extensively in 28-mm THAs. Corrosion is partly influenced by the surface area but fluid film lubrication ostensibly is associated with large diameters which should reduce wear. We therefore suggest the volume of wear debris produced from MOM bearings in hip resurfacing would not be substantially different from that in 28-mm THAs.
We hypothesized there would be no differences in serum concentrations of Cr, Co, and Mo between patients who had a large diameter MOM hip resurfacing and a small diameter MOM THA. We also asked whether patients with large diameter head resurfacings had greater Cr, Co, Mo, and Ni levels than healthy subjects. Finally, we asked whether implant size, hip score, and length of followup influenced ion release.
Materials and Methods
We prospectively followed two groups of patients who were recruited for this study: Group A had large diameter MOM heads (average head diameter, 48 mm) and Group B had a 28-mm MOM head (Table 1). We included patients with a followup longer than 13 months, a good clinical outcome as shown by a Harris hip score (HHS) [18] higher than 80, the ability to walk more than 3 km per day, and no radiographic evidence of implant loosening. Because our interest was systemic release of metal ions from the bearings, we paid particular attention to other potential sources of metal ion release. Thus, we excluded two patients with the presence of other metal foreign bodies (n = 1) and occupational exposure to Co and/or Cr (n = 1). Ingestion of prescription or nonprescription drugs containing metal ions, such as vitamin B12, and renal impairment were considered exclusion criteria, but no patients had these characteristics. The patients were matched for age, HHS [18], and length of followup, as verified by the Mann-Whitney U test, and for gender, as evaluated by the Fisher exact test. We did not consider the surgical indication a potentially confounding variable. Forty-eight clinically normal volunteers (blood donors), who in the last months had not taken any medication and were without metal implants, were recruited to obtain normal reference values (Group C). The clinical study was approved by the Institutional Ethics Committee on human research and the subjects signed informed consent forms to participate in the study.
Table 1.
Profiles of patients (Groups A and B) and control subjects (Group C)
| Variable | Group A (n = 20) | Group B (n = 26) | Group C (n = 48) | p Value* |
|---|---|---|---|---|
| Gender | 0.90 | |||
| Males | 10 | 12 | 37 | |
| Females | 10 | 14 | 11 | |
| Age (years) | 0.69 | |||
| Mean ± SEM (median) | 49 ± 3 (44) | 48 ± 2 (47) | 42 ± 2 (40) | |
| Range | 26–73 | 30–67 | 20–71 | |
| Diagnosis | ||||
| Osteoarthritis | 10 | 21 | ||
| Congenital hip dysplasia | 7 | 5 | ||
| Rheumatoid arthritis | 2 | |||
| Trauma | 1 | |||
| Followup (months) | 0.1 | |||
| Mean ± SEM (median) | 24 ± 1 (24) | 26.3 ± 1.0 (25) | ||
| Range | 15–33 | 14–38 | ||
| Harris hip score [18] | 0.08 | |||
| Mean ± SEM (median) | 94 ± 1.4 (96) | 91 ± 1.1 (90) | ||
| Range | 83.5–100 | 80–100 |
* Group A versus Group B; SEM = standard error of the mean.
We performed a pretest power analysis to ensure the study was not underpowered: as effect size, we used one standard deviation of the previously analyzed MOM implants for each element, ie, 1.7, 1.3, and 0.27 for Cr, Co, and Mo, respectively, because a biologically or clinically meaningful difference in outcome is not known. We found 80% power for all the elements corresponded to a sample size of 16 subjects for each group. As a secondary research question, we questioned if Cr, Co, Mo, and Ni in patients who had resurfacing were increased in comparison with amounts in control subjects. The different ratio of women to men in control subjects in comparison with Group A was not considered a bias because no difference in ion content was found between healthy men and women. Pretest power analysis, performed by using one standard deviation of the control subjects, indicated a sample size of 16 had an 80% power to detect a difference between groups for Cr, Co, and Mo. A sample size of 17 was necessary for Ni.
Group A included 20 selected patients (10 women, 10 men) who had MOM Birmingham Hip Resurfacing™ (BHR; Midland Medical Technologies, Bromsgrove, UK). Fifteen patients had unilateral and five had bilateral total hip resurfacing (Fig. 1). The bearing was fabricated from a cast high-carbon Co-Cr alloy. The average head diameter of the femoral component was 48 mm (52 mm and 44 mm in men and women, respectively). In the bilateral cases, the second operations were performed after 7, 5, 6, 11, and 9 months.
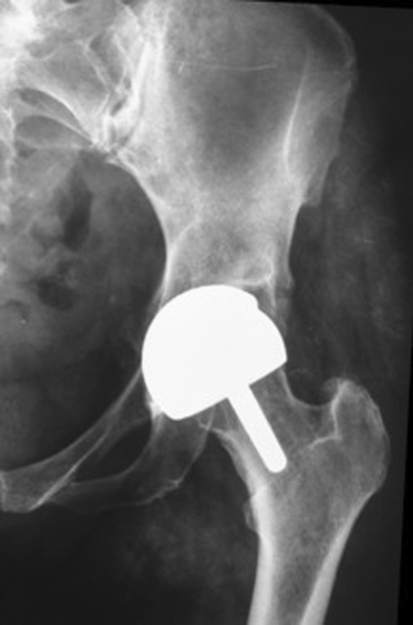
Fig. 1.
A radiograph taken at the 31-month followup of the outlier patient in Group A shows the MOM BHR implant is well positioned.
Group B consisted of 26 selected patients (14 women, 12 men) who had 28-mm Metasul® MOM THAs (Sulzer Orthopedics Ltd, Winterthur, Switzerland). Twenty-five patients had unilateral and one had bilateral THAs. In these implants, the acetabular component consisted of a titanium shell with a noncross-linked ultrahigh-molecular-weight polyethylene (UHMWPE) insert, containing a Co-Cr alloy liner. The UHMWPE was sterilized in nitrogen. The head, which was fabricated from a forged high-carbon Co-Cr alloy, was connected to an uncemented Ti alloy femoral stem (Fig. 2). Results of Group B were published previously [32]. Surgical time ranged from 45 to 90 minutes for all the operations.
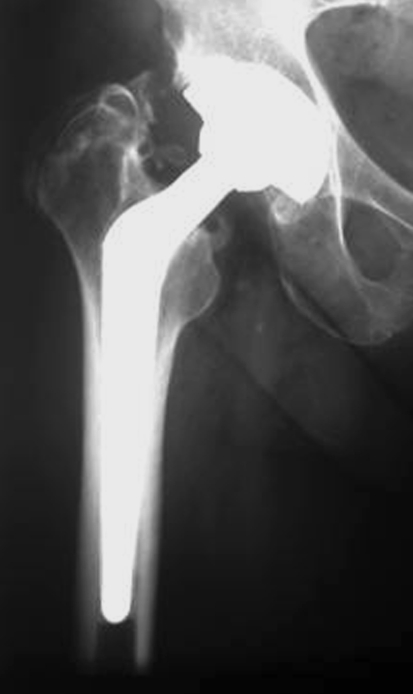
Fig. 2.
A radiograph taken at the 34-month followup of a patient in Group B shows the 28-mm Metasul® MOM THA.
At the time of the metal ion analysis, all patients had resumed the activity level they were accustomed to before the onset of the disease that required hip surgery. No patient in Group A or Group B was involved in strenuous activities. Two patients in Group A were involved in low-impact sport activities (jogging and weight lifting, once a week). The activity level was not considered a confounding variable based on a previous study [19].
Environmental contamination was avoided by using a dedicated room for furnace testing with efficient fume extraction. Moreover, personnel could not enter the room to access other parts of the laboratory. A clean bench area was reserved for solution preparation. All volumetric and storage wares used for the analysis were kept separate from the apparatus used for conventional laboratory work. During sampling and sample preparation, we paid particular attention to avoid contamination: polystyrene, polypropylene, or polyethylene disposable material was used after soaking for 1 day in 2 vol % HNO3 solution in double-deionized water and then rinsed thoroughly in double-distilled and deionized water.
Every procedure used from the time of sampling until analysis was regarded as a potential source of contamination and therefore was checked using a nitric acid leach test to ensure it did not contain detectable amounts of any of the trace elements under study. The use of reagents for sample preparation was kept to a minimum and all of them were checked to ensure they did not contain any ions of interest. Blood samples were obtained from antecubital veins of fasting subjects using a disposable intravenous cannula and collected in metal-free Vacutainers® (Becton Dickinson and Co, Meylan, France). To avoid contamination from the needle, the first 5 mL of blood withdrawn was discarded. Serum was separated by centrifugation at 400 × g for 10 minutes at 4° C. Ion content was measured using a graphite furnace atomic absorption spectrometer equipped with double background correction deuterium/Zeeman, autosampler, and pyrolytic carbon-coated graphite tubes (Unicam Model Solaar 939 QZ; Unicam, Cambridge, UK). Calibration of the spectrometer was performed by applying the Standard Addition Method [4] and by using certified standard solutions at three concentrations for each element (National Institute of Standards and Technology [NIST]). Specimens were diluted with 0.1 vol % HNO3 and 0.05 vol % Triton® X100 and analyzed as 15-μL aliquots in triplicate. For Cr and Co analysis, magnesium nitrate was added as a matrix modifier. The accuracy and precision of the method were validated using SRM 1598 NIST human serum for all the elements and normal trace elements and high-range trace elements UTAK® for Cr. Rejection of results with a related deviation standard % (RDS%) greater than 10% assured test repeatability.
All results were expressed as nanograms per milliliter (equivalent to micrograms per liter and parts per billion). The sensitivity of the method was established by using detection limits for sample matrix, ie, 0.06 ng/mL for Cr, 0.08 ng/mL for Co, 0.83 ng/mL for Mo, and 0.10 ng/mL for Ni. All ion levels from samples taken from patients that were below the detection levels were assigned the detection level values.
Ion values were reported as mean ± standard error of the mean (SEM), minimum to maximum range, and the median value. Cr, Co, and Mo values in Group A were compared with values in Group B by applying the Mann-Whitney U test. Furthermore, we stratified the groups by gender, which was a confounding factor as shown by the multivariate analysis; the comparison between the subgroups was made by the Mann-Whitney test, calculated with the Monte Carlo method; corrections were made for multiple comparisons. The Mann-Whitney U test also was applied to verify if Cr, Co, Mo, and Ni contents in patients who had resurfacing were increased in comparison with control subjects. The correlation between ion values and other parameters, ie, implant size, length of followup, age of patient, and HHS, also was calculated in the resurfacing group by using the Spearman r coefficient. A multivariate analysis, considering implant type (resurfacing versus MOM THA), presence of unilateral-bilateral implant, gender, and length of followup, was performed by using the General Linear Model. Implant type in association with gender affected Cr (p = 0.009) and Co release (p = 0.088) whereas neither the presence of unilateral versus bilateral implant nor length of followup had an effect on ion release. Data were analyzed with StatView® 5.0.1.0 software (SAS Institute, Inc, Cary, NC). SPSS software (SPSS Inc, Chicago, IL) was used to perform the General Linear Model and to apply the Monte Carlo method. The probability values are provided in the Results section.
Results
We found no differences in the Co, Cr, and Mo levels of patients in Group A and Group B (Table 2). We excluded one outlier from the comparison between groups because the ion levels were higher than the means. When the subjects in each population were divided according to gender, women showed a higher (p = 0.008) Cr release in Group A whereas men had a higher (p = 0.037) Co release in Group B (Table 3).
Table 2.
Ion values in patients (Groups A and B) and control subjects (Group C)
| Ion (ng/mL) | Group A | Group B [12] | Group C | p Value* | p Value† |
|---|---|---|---|---|---|
| Chromium | < 0.001 | 0.06 | |||
| Mean ± SEM (median) | 2.30 ± 1.72 (1.73) | 1.73 ± 0.33 (1.66) | 0.25 ± 0.03 (0.28) | ||
| Range | 0.69–7.24 | 0.22–6.65 | 0.06–0.67 | ||
| Cobalt | < 0.001 | 0.30 | |||
| Mean ± SEM (median) | 1.40 ± 0.47 (0.75) | 1.33 ± 0.25 (0.97) | 0.29 ± 0.03 (0.27) | ||
| Range | 0.08–8.96 | 0.34–5.32 | 0.08–0.86 | ||
| Nickel | 0.80 | ||||
| Mean ± SEM (median) | 0.70 ± 0.06 (0.69) | ‡ | 0.68 ± 0.07 (0.71) | ||
| Range | 0.1–1.31 | 0.10–1.69 | |||
| Molybdenum | 0.005 | 0.78 | |||
| Mean ± SEM (median) | 0.90 ± 0.03 (0.83) | 0.96 ± 0.07 (0.83) | Less than detection limit | ||
| Range | 0.83–1.30 | 0.83–1.73 |
* Group A versus Group C; †Group A versus Group B; ‡insufficient serum was available from Group B patients for nickel testing; SEM = standard error of the mean.
Table 3.
Ion values in patients separated by gender
| Ion (ng/mL) | Males | p Value | Females | p Value | ||
|---|---|---|---|---|---|---|
| Group A | Group B | Group A | Group B | |||
| Chromium | 0.97 | 0.008 | ||||
| Mean ± SEM (median) | 1.59 ± 0.34 (1.16) | 1.98 ± 0.56 (1.16) | 3.07 ± 0.67 (2.36) | 1.51 ± 0.39 (1.07) | ||
| Range | 0.69–4.04 | 0.22–6.65 | 1.40–7.24 | 0.33–5.63 | ||
| 95% CI | 0.82–2.38 | 0.75–3.21 | 1.53–4.62 | 0.67–2.35 | ||
| Cobalt | 0.037 | 0.68 | ||||
| Mean ± SEM (median) | 0.95 ± 0.41 (0.49) | 1.38 ± 0.38 (0.99) | 1.89 ± 0.90 (0.96) | 1.29 ± 0.33 (0.91) | ||
| Range | 0.08–4.18 | 0.34–4.98 | 0.41–8.96 | 0.41–5.32 | ||
| 95% CI | 0.03–1.87 | 0.55–2.22 | −0.17–3.96 | 0.56–2.01 | ||
| Molybdenum | 0.54 | 0.67 | ||||
| Mean ± SEM (median) | 0.86 ± 0.02 (0.83) | 0.99 ± 0.10 (0.83) | 0.94 ± 0.06 (0.83) | 0.90 ± 0.07 (0.83) | ||
| Range | 0.83–1.04 | 0.83–1.73 | 0.83–1.30 | 0.83–1.19 | ||
| 95% CI | 0.81–0.91 | 0.76–1.24 | 0.80–1.09 | 0.71–1.10 | ||
SEM = standard error of the mean; 95% CI = 95% confidence interval for the mean.
The serum concentrations of Cr, Co, and Mo were higher in patients in Group A compared with concentrations of control subjects, whereas Ni levels were similar (Table 2). Cr and Co levels correlated (r = 0.77, p = 0.001) in Group A. Head diameter correlated with (r = 0.52, p = 0.04) the HHS, and inverse correlated with Cr, Co (r = −0.64, p = 0.002 for Cr; r = −0.51, p = 0.01 for Co). Length of followup and age of patient did not correlate with any ion concentrations. No other correlation was found.
Discussion
Although the clinical use of MOM THA is increasing in popularity, concerns remain regarding wear and corrosion of the bearing surfaces and the consequent increase in metal ion serum levels [10, 26, 31, 32, 35]. It is certain that metal ions are released because of the combined effect of corrosion of the implant surface and wear particles. Metal wear particles are in the nanometer size range and therefore have a high surface area to volume ratio and are capable of releasing metal ions [8]. Because the amount of wear and corrosion is considered related to the area of the bearing surfaces, the introduction of large-diameter MOM bearings, as used in hip resurfacing, has prompted even more concern. However, classic elastohydrodynamic theory suggests fluid film lubrication, which is believed responsible for reduced wear, is more likely to occur with large-diameter MOM bearings [38]. Considering that the potentially negative effect of metal ion release resulting from corrosion associated with large-diameter bearings could be balanced by the potentially positive effect of increased fluid film lubrication, we expected no differences in metal ion release between large- and small-diameter metal bearings. We tested the hypothesis that there were no differences in serum concentrations of Cr, Co, and Mo between two groups of patients who had either hip resurfacing with a mean head diameter of 48 mm or 28-mm MOM THA. Serum concentrations of Cr, Co, Mo, and Ni in the patients who had resurfacing also were compared with those observed in control subjects. The relationship between levels of metal ions, age, length of followup, implant size, and HHS also was investigated in patients who had resurfacing.
The major limitation of our study was the low number of subjects enrolled: although the pretest analysis showed 16 subjects provided an 80% power, we found gender in association with implant type, ie, resurfacing versus MOM THA, was a confounding factor for Cr and Co. Consequently, we divided patients by gender, and the post hoc power was reduced to 70 and 60 for Cr and Co, respectively. In the univariate model, we found no difference in ion release between patients who had MOM THA and patients who had resurfacing; however, in the multivariate model, gender affected ion levels. Men showed a better performance with hip resurfacing than with MOM THA whereas the opposite was observed in women. In one patient in Group A, ion levels were substantially higher than the averages (33.75 and 36.0 ng/mL for Cr and Co, respectively). Radiographs taken at the time of surgery and at 31 months’ followup showed correct positioning of both components and there was no radiographic sign of component loosening (Fig. 1). We do not have an explanation for the results observed in this patient; a previous study [19] suggested it was not a variable in ion release.
Clinical interpretation of increased ion levels is difficult because the in vivo threshold limit is still unknown. International and national working groups are discussing only the reference values to be set for hazardous occupational toxicants in body fluids, ie, “exposure equivalents for carcinogenic substances” (EKA values) and “biological tolerance values for occupational exposure” (BAT values) of the Deutsche Forschungsgemeinschaft Commission [16] and “Biological Exposure Indices” (BEI) of the American Conference of Governmental Industrial Hygienists [29]. Researchers aimed to identify markers to detect the local or systemic damage caused by metal ion elevation. The results suggest a possible toxic/sensitizing effect of Cr and Co ions [11–13, 15, 17, 22, 33, 34, 40]. However, no evidence of a correlation between ion concentrations and exposure effects has been shown [14].
Several studies evaluated ion release in the presence of metallic implants in the hip [8, 30] and the knee [23]. There are many complex issues associated with analysis of metal ions, including collection technique, analysis, statistical methodology, and reporting of the results. Published data suggest much variability in all these factors. Following the recommendation of MacDonald et al. [25] regarding the use of serum analysis for comparison purposes, we measured ion levels in the serum. With whole blood analysis, matrix digestion protocols are needed, but these can introduce contaminants [24]. The large amount of iron contained in the erythrocytes could be another confounding factor. Another possible confounding factor associated with whole blood analysis is the Cr presence in the erythrocytes. These disadvantages can be avoided by using serum analysis.
Our data are consistent with those reported by Vendittoli et al. [42] who evaluated blood concentrations of Cr and Co in patients who had MOM Durom™ Hip Resurfacing (Zimmer, Winterthur, Switzerland) and found a trend toward smaller component size and female gender as factors that increased metal ion levels in resurfacing implants. They hypothesized the difference in ion levels between genders may be secondary to differences in metal ion metabolism, such as different lean body mass, cellular or extracellular storage, or renal excretion. Another explanation could be limitation in precision of implant manufacturing. Variations may occur with specific component clearances and smaller components may be more sensitive to such variations. Our results, however, are consistent with those reported by Skipor et al. [37] who did not find any differences in Co and Cr levels between patients who had either hip resurfacing or MOM THA. The trend in our results also agrees with that reported by Daniel et al. [6] for the same followup in a study of BHR with bearing diameters of 50 mm and 54 mm versus 28-mm Metasul® MOM THA, in which whole blood samples were analyzed. In contrast, Clarke et al. [5], at a median of 16 months, reported higher median Co and Cr serum levels in patients who had hip resurfacing arthroplasty compared with patients who had MOM THAs. Unlike our study, Clarke et al. [5] used two different prosthetic designs. One was the double heat-treated Cormet 2000™ (Corin Group PLC, Cirencester, UK) (carbide volume fraction, 2.3%) and the other was the as-cast BHR (carbide volume fraction, 5%). The benefits or otherwise of carbides in CoCrMo bearing surfaces is controversial. In general, the greater the carbide content, the greater is the hardness and thus potential for resistance to wear, although the relationship is not straightforward [5]. However, a problem with enhanced carbide levels is reduction in corrosion resistance [28]. With the Cormet 2000™, the lower carbide content of the alloy, which is caused by the heat treatment, seemed to be a potential for increased wear. This assumption is confirmed by the results because the median plasma Co and Cr values obtained from patients implanted with the Cormet 2000™ were 44% and 60% higher, respectively, compared with those obtained from patients who received BHR implants. Furthermore, the median followup of our patients was 24.5 months whereas it was 16 months for patients in the study reported by Clarke et al. [5]. There is evidence that there is an initial running-in period of MOM implants during which there is a sharp increase in metal ion serum levels and after which these levels decrease [1, 6]. Thus, length of followup is an important variable in studies of the wear of MOM bearings. A study conducted by Witzleb et al. [43] confirmed the results reported by Clarke et al. [5]. A greater increase in serum levels of Co and Cr was found in hip resurfacing arthroplasty when compared with 28-mm MOM THA. Furthermore, in contrast to our results, they reported that serum concentrations hardly changed with time in either group and were not different from the concentrations observed in the control subjects. Considering that gender may influence the metal ion concentrations, the higher values of Co and Cr observed in patients who had hip resurfacing may be attributable to different rates in the women in the two groups, 0.3 and 0.6 in the MOM THA and hip resurfacing, respectively.
As expected, the Co, Cr, and Mo serum levels in patients in Group A were higher than in the subjects of the control group. We report results for Ni ions, information that is lacking in previous reports [5, 37, 42, 43]. Unlike Cr, Co, and Mo, we found no difference with controls for Ni ions. There are two possible explanations for this finding. First, these ions are present in the bearing alloy in very small quantities (maximum 1% of the alloy). Second, these ions are rapidly transported to the urine and eliminated from the body. Consequently, they are not considered a concern in the biologic response to metal implants [27]. It is well known that a larger diameter femoral head provides better range of motion [7]. We found a correlation between HHS and head diameter in resurfacing implants. An inverse correlation between HHS and ion release also was found. A possible explanation for this result may be that a well-functioning hip resurfacing often reflects a well-positioned implant, avoiding impingement phenomena that may lead to higher ion release.
Metal-on-metal bearings are being used as a strategy for reducing the revision rate associated with metal-UHMWPE bearings. In our study, at a mean followup of 24 months, no differences were found between the Co, Cr, and Mo serum levels of patients with resurfacing implants and those of patients with 28-mm MOM THA. However, contradictory results are reported in the literature; data available are insufficient to define systemic metal concentrations in patients with hip resurfacing devices, and additional research is necessary. If, in the future, lower exposure to the perioperative risks of revision surgery with MOM bearings is confirmed, this will outweigh the theoretical risks of increased metal ions. From this perspective, if MOM bearings are acceptable for use in THA, we believe they should not be rejected for use in hip resurfacing. Nevertheless, as with any other surgical indication, patient selection is critical. It has been confirmed that gender has a considerable impact on a patient’s metal ion levels and should be considered for the choice of implant design. Metal-on-metal bearings for THA should not be rejected because of concerns regarding potential increased metal ion release. However, patients with elevated ion levels, even without evident signs of loosening or toxicity, could be at higher risk and warrant medium- and long-term studies and heightened surveillance. Moreover, we are of the opinion that MOM bearings should be contraindicated in patients with renal malfunction, as very high levels of Co are associated with chronic renal failure [2].
Acknowledgments
We thank Michelina Greco for her contribution to the serum ion measurement.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
References
- 1.Back DL, Young DA, Shimmin AJ. How do serum cobalt and chromium levels change after metal-on-metal hip resurfacing? Clin Orthop Relat Res. 2005;438:177–181. [DOI] [PubMed]
- 2.Brodner W, Bitzan P, Meisinger V, Kaider A, Gottsauner-Wolf F, Kotz R. Serum cobalt levels after metal-on-metal total hip arthroplasty. J Bone Joint Surg Am. 2003;85:2168–2173. [DOI] [PubMed]
- 3.Brodner W, Grübl A, Jankovsky R, Meisinger V, Lehr S, Gottsauner-Wolf F. Cup inclination and serum concentration of cobalt and chromium after metal-on-metal total hip arthroplasty. J Arthroplasty. 2004;19(8 suppl 3):66–70. [DOI] [PubMed]
- 4.Butcher DJ, Sneddon J. A practical guide to graphite furnace atomic absorption spectrometry. In: Winefordner JD, ed. Chemical Analysis: A Series of Monographs on Analytical Chemistry and Its Applications. New York, NY: Wiley; 1998.
- 5.Clarke MT, Lee PT, Arora A, Villar RN. Levels of metal ions after small- and large-diameter metal-on-metal hip arthroplasty. J Bone Joint Surg Br. 2003;85:913–917. [PubMed]
- 6.Daniel J, Ziaee H, Salama A, Pradhan C, McMinn DJ. The effect of the diameter of metal-on-metal bearings on systemic exposure to cobalt and chromium. J Bone Joint Surg Br. 2006;88:443–448. [DOI] [PubMed]
- 7.dela Rosa MA, Silva M, Heisel C, Reich M, Schmalzried TP. Range of motion after total hip resurfacing. Orthopedics. 2007;30:352–357. [DOI] [PubMed]
- 8.Doorn PF, Campbell PA, Worrall J, Benya PD, McKellop HA, Amstutz HC. Metal wear particle characterization from metal on metal total hip replacements: transmission electron microscopy study of periprosthetic tissues and isolated particles. J Biomed Mater Res. 1998;42:103–111. [DOI] [PubMed]
- 9.Dorr LD, Kane TJ 3rd, Conaty JP. Long-term results of cemented total hip arthroplasty in patients 45 years old or younger: a 16 year follow-up study. J Arthroplasty. 1994;9:453–456. [DOI] [PubMed]
- 10.Dumbleton JH, Manley MT. Metal-on-metal total hip replacement: what does the literature say? J Arthroplasty. 2005;20:174–188. [DOI] [PubMed]
- 11.Granchi D, Cenni E, Ciapetti G, Savarino L, Stea S, Gamberini S, Gori A, Pizzoferrato A. Cell death induced by metal ions: necrosis or apoptosis? J Mater Sci Mater Med. 1998;9:31–37. [DOI] [PubMed]
- 12.Granchi D, Ciapetti G, Savarino L, Stea S, Filippini F, Sudanese A, Rotini R, Giunti A. Expression of the CD69 activation antigen on lymphocytes of patients with hip prosthesis. Biomaterials. 2000;21:2059–2065. [DOI] [PubMed]
- 13.Granchi D, Ciapetti G, Stea S, Savarino L, Filippini F, Sudanese A, Zinghi GF, Montanaro L. Cytokine release in mononuclear cells of patients with Co-Cr hip prosthesis. Biomaterials. 1999;20:1079–1086. [DOI] [PubMed]
- 14.Granchi D, Savarino L, Ciapetti G, Cenni E, Rotini R, Mieti M, Baldini N, Giunti A. Immunological changes in patients with total joint replacement following idiopathic osteoarthritis of the hip. J Bone Joint Surg Br. 2003;85:758–764. [PubMed]
- 15.Granchi D, Verri E, Ciapetti G, Stea S, Savarino L, Sudanese A, Mieti M, Rotini R, Dallari D, Zinghi G, Montanaro L. Bone resorbing cytokines in serum of patients with aseptic loosening of hip prosthesis. J Bone Joint Surg Br. 1998;80:912–917. [DOI] [PubMed]
- 16.Greim H, Lehnet G. Critical Data Evaluation for BAT and EKA Values, Series: Biological Exposure Values for Occupational Toxicants and Carcinogens H. Vol 2. Weinheim, Germany: Wiley-VCH; 1995.
- 17.Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83:428–436. [DOI] [PubMed]
- 18.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed]
- 19.Heisel C, Silva M, Skipor AK, Jacobs JJ, Schmalzried TP. The relationship between activity and ions in patients with metal-on-metal bearing hip prostheses. J Bone Joint Surg Am. 2005;87:781–787. [DOI] [PubMed]
- 20.Jacobs JJ, Roebuck KA, Archibeck M, Hallab NJ, Glant TT. Osteolysis: basic science. Clin Orthop Relat Res. 2001;393:71–77. [DOI] [PubMed]
- 21.Joshi AB, Porter ML, Trail IA, Hunt LP, Murphy JC, Hardinge K. Long term results of Charnley low-friction arthroplasty in young patients. J Bone Joint Surg Br. 1993;75:616–623. [DOI] [PubMed]
- 22.Lee SH, Brennan FR, Jacobs JJ, Urban RM, Ragasa DR, Glant TT. Human monocyte/macrophage response to cobalt-chromium corrosion products and titanium particles in patients with total joint replacements. J Orthop Res. 1997;15:40–49. [DOI] [PubMed]
- 23.Luetzner J, Krummenauer F, Lengel AM, Ziegler J, Witzleb WC. Serum metal ion exposure after total knee arthroplasty. Clin Orthop Relat Res. 2007;461:136–142. [DOI] [PubMed]
- 24.MacDonald SJ. Metal-on-metal total hip arthroplasty: the concerns. Clin Orthop Relat Res. 2004;429:86–93. [DOI] [PubMed]
- 25.MacDonald SJ, Brodner W, Jacobs JJ. A consensus paper on metal ions in metal-on-metal hip arthroplasties. J Arthroplasty. 2004;19(8 suppl 3):12–16. [DOI] [PubMed]
- 26.Maezawa K, Nozawa M, Hirose T, Matsuda K, Yasuma M, Shitoto K, Kurosawa H. Cobalt and chromium concentrations in patients with metal-on-metal and other cementless total hip arthroplasty. Arch Orthop Trauma Surg. 2002;122:283–287. [DOI] [PubMed]
- 27.Merritt K, Brown SA. Distribution of cobalt chromium wear and corrosion products and biologic reactions. Clin Orthop Relat Res. 1996;329(suppl):S233–S243. [DOI] [PubMed]
- 28.Montero-Ocampo C, Lopez H, Salinas Rodriguez A. Effect of compressive straining on corrosion resistance of a shape memory Ni-Ti alloy in Ringer’s solution. J Biomed Mater Res. 1996;32:583–591. [DOI] [PubMed]
- 29.Morgan MS. The biological exposure indices: a key component in protecting workers from toxic chemicals. Environ Health Perspect. 1997;105(suppl 1):105–115. [DOI] [PMC free article] [PubMed]
- 30.Sargeant A, Goswami T, Swank M. Ion concentrations from hip implants. J Surg Orthop Adv. 2006;15:113–114. [PubMed]
- 31.Savarino L, Granchi D, Ciapetti G, Cenni E, Greco M, Rotini R, Veronesi CA, Baldini N, Giunti A. Ion release in stable hip arthroplasties using metal-on-metal articulating surfaces: a comparison between short- and medium-term results. J Biomed Mater Res. 2003;66:450–456. [DOI] [PubMed]
- 32.Savarino L, Granchi D, Ciapetti G, Cenni E, Nardi Pantoli A, Rotini R, Veronesi CA, Baldini N, Giunti A. Ion release in patients with metal-on-metal hip bearings in total joint replacement: a comparison with metal-on-polyethylene bearings. J Biomed Mater Res. 2002;63:467–474. [DOI] [PubMed]
- 33.Savarino L, Granchi D, Ciapetti G, Stea S, Donati ME, Zinghi G, Fontanesi G, Rotini R, Montanaro L. Effects of metal ions on white blood cells of patients with failed total joint arthroplasties. J Biomed Mater Res. 1999;47:543–550. [DOI] [PubMed]
- 34.Savarino L, Stea S, Granchi D, Visentin M, Ciapetti G, Donati ME, Rollo G, Zinghi G, Pizzoferrato A, Montanaro L, Toni A. Sister chromatid exchanges and ion release in patients wearing fracture fixation devices. J Biomed Mater Res. 2000;50:21–26. [DOI] [PubMed]
- 35.Schaffer AW, Pilger A, Engelhardt C, Zweymueller K, Ruediger HW. Increased blood cobalt and chromium after total hip replacement. J Toxicol Clin Toxicol. 1999;37:839–844. [DOI] [PubMed]
- 36.Signorello LB, Ye W, Fryzek JP, Lipworth L, Fraumeni JF Jr, Blot WJ, McLaughlin JK, Nyren O. Nationwide study of cancer risk among hip replacement patients in Sweden. J Natl Cancer Inst. 2001;93:1405–1410. [DOI] [PubMed]
- 37.Skipor AK, Campbell PA, Patterson LM, Amstutz HC, Schmalzried TP, Jacobs JJ. Serum and urine metal levels in patients with metal-on-metal surface arthroplasty. J Mater Sci Mater Med. 2002;13:1227–1234. [DOI] [PubMed]
- 38.Smith SL, Dowson D, Goldsmith AA. The effect of femoral head diameter upon lubrication and wear of metal-on-metal total hip replacement. Proc Inst Mech Eng H. 2001;215:161–170. [DOI] [PubMed]
- 39.Soderman P, Malchau H, Herberts P. Outcome after total hip arthroplasty: Part I. General health evaluation in relation to definition of failure in the Swedish National Total Hip Arthroplasty register. Acta Orthop Scand. 2000;71:354–359. [DOI] [PubMed]
- 40.Stea S, Visentin M, Granchi D, Savarino L, Dallari D, Gualtieri G, Rollo G, Toni A, Pizzoferrato A, Montanaro L. Sister chromatid exchange in patients with joint prostheses. J Arthroplasty. 2000;15:772–777. [DOI] [PubMed]
- 41.Tharani R, Dorey FJ, Schmalzried TP. The risk of cancer following total hip or knee arthroplasty. J Bone Joint Surg Am. 2001;83:774–780. [DOI] [PubMed]
- 42.Vendittoli PA, Mottard S, Roy AG, Dupont C, Lavigne M. Chromium and cobalt ion release following the Durom high carbon content, forged metal-on-metal surface replacement of the hip. J Bone Joint Surg Br. 2007;89:441–448. [DOI] [PubMed]
- 43.Witzleb WC, Ziegler J, Krummenauer F, Neumeister V, Guenther KP. Exposure to chromium, cobalt and molybdenum from metal-on-metal total hip replacement and hip resurfacing arthroplasty. Acta Orthop. 2006;77:697–705. [DOI] [PubMed]