Duplay (1896) was the first investigator to recognize pathologic disorders of extra-articular tissues as possible factors responsible for stiff and painful shoulders. It was his belief that an inflammatory process of the subacromial bursa was the causative agent producing this syndrome. He designated the entity “scapulohumeral periarthritis.” Soon this term came to include a host of heterogeneous cases which presented similar clinical features, namely, all had pain and stiffness in the shoulder region. Since the original work of Duplay many workers have succeeded in extracting from the all-inclusive term “scapulo-periarthritis” many unequivocal entities. Notably among these observers were Painter, Baer, King and Holmes, Mumford and Martin, Codman, Meyer, Pasteur, Gilcreest, and more recently Lippmann, Abbott, Saunders and Hitchcock and Bechtol.
Meyer [11] was the first observer to focus our attention on the tendon of the long head of the biceps brachii muscle as a possible source of disorders about the shoulder. He noted in anatomical specimens advanced degenerative abnormalities comprising fraying, shredding, fasciculations and tearing of the fibers of the biceps tendon. Some specimens exhibited partial or complete dislocation of the tendon out of the bicipital groove and several specimens revealed absence of the intrascapular portion of the tendon while the extracapsular portion had attained a bony attachment on the shaft of the humerus in the region of the lesser tuberosity. It was Meyer’s belief that the aforementioned changes were the products of several causes: (1) contact of the tendon with a supratubercular ridge when present, (2) irregularities and excrescences in the floor of the intertubercular groove, and (3) contact of the tendon with raised cartilagenous margins of the head of the humerus or with the lesser tuberosity. He further believed that these alterations were the result of using the upper extremity in a position of abduction and external rotation. His observations were made on the shoulder joints of cadavers in whom no clinical data were available prior to death; therefore it was impossible to evaluate the clinical significance of these alterations.
Meyer’s observations were confirmed by the author together with J. B. White and G. Callery in an investigation conducted on shoulder joints which were obtained postmortem from individuals on whom clinical data were available prior to death. It was interesting to note that many of the degenerative and attritional changes described by Meyer were compatible with good, painless function of the shoulder joint.
In 1932 Pasteur [13] described a new clinical syndrome “tenobursite” which he believed was responsible for frozen shoulder. He was the first to correlate bicipital tenosynovitis with frozen shoulder. This was followed by the work of Schrager who, for the first time in American literature, described tenosynovitis as an unequivocal entity.
Many other workers soon began to recognize the significant role that the tendon of the long head of the biceps brachii muscle played in stiff and painful shoulders. Lippmann [9] in 1943 described adhesive tenosynovitis as the causative agent of frozen shoulder, a concept with which the author is not in total agreement.
On the other hand, Codman [3], one of the greatest authorities on lesions of the shoulder joint, wrote “Personally, I believe that the sheath of the biceps tendon is less apt to be involved than are other structures. I have never proved its involvement in a single case. I think that the substance of the tendon of the supraspinatus is the most often involved.” Codman was of the opinion that tendinitis of the rotator tendons was the underlying pathologic disorder responsible for frozen shoulder.
In the following study an attempt will be made to show that frozen shoulder is a diffuse inflammatory process implicating all the soft tissue components of the scapulohumeral joint including the biceps tendon and not any one component or an isolated region of the joint; also, that bicipital tenosynovitis is a concomitant finding in all frozen shoulder, that it may be primary or secondary in origin, and that it is responsible for the pain factor which is the most distressing feature of this syndrome. Furthermore, it will be pointed out that once the pain element in frozen shoulders is eliminated, restoration of function can begin and continues until optimum function is regained and all tissues are returned to normal. The observations herein recorded were made on a clinical study of 38 cases, of which 32 were investigated surgically. Correlation of these pathologic findings with those recorded in a previously reported investigation on degenerative lesions of the shoulder joint allows one to reach some logical conclusions concerning the causes and pathogenesis of frozen shoulders.
Clinical Characteristics
Frozen shoulder is a malady usually encountered in individuals past 40 years of age; in this series only one case was under 40. The greatest incidence is found between 50 and 60 years and women are more frequently affected than men (68.3 per cent of the cases in this study were in women). It is a relatively common disorder in patients afflicted with cardiovascular, pulmonary, or metabolic disorders.
The syndrome exhibits a characteristic cycle of events. It may be initiated by trauma to the region of the shoulder; on the other hand its onset may be insidious without injury. Early in the disease pain and stiffness in the shoulder are the outstanding symptoms. Pain is accentuated by activity and specific motions at the scapulohumeral joint such as abduction and external rotation or dorsal flexion and internal rotation. Also, in most instances the pain is projected to the anterolateral aspect of the shoulder and to the anterior aspect and middle of the upper arm, occasionally to the flexor surface of the forearm. Pain during the night is often severe and interferes with sleep. Pressure over the intertubercular sulcus and rolling the biceps tendon under the examiner’s thumb elicits tenderness in all instances. The severity of the symptoms slowly and steadily increase while the arcs of painless motion at the scapulohumeral joint become progressively smaller until little or no motion is demonstrable in the joint (Fig. 1). At this stage of the disease the extremity is held at the side in a position of internal rotation. All shoulder motions are guarded and painful and atrophy of deltoid and spinatii muscles is discernible. As a rule, a few degrees of painless motion are demonstrable in the antero-posterior plane. Occasionally, all the cardinal signs and symptoms of a scalenus anticus syndrome may confuse the clinical picture.
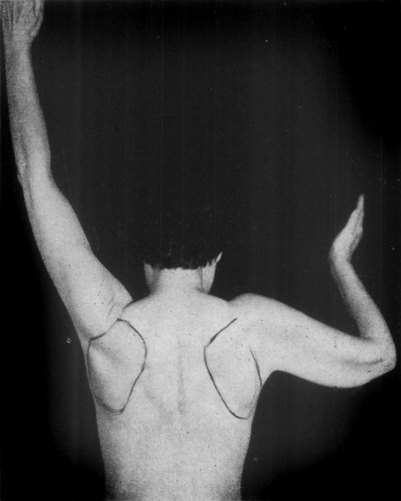
Fig. 1.
This is a typical patient with a frozen right shoulder. Note the angle that the shaft of the humerus makes with the vertebral border of the scapula. All motion is in the scapulothoracic joint. This patient has completely lost all scapulohumeral motion. The scapula and the humerus move as one unit when abduction is attempted. This patient shows the maximum range of abduction that she is able to carry out with a frozen shoulder.
At all times during the course of the disease pain is the most significant clinical manifestation. Although the range of motion of the affected extremity is severely impaired, the pain factor outshadows the loss of motion. The clinical course of frozen shoulders is unpredictable; it may terminate spontaneously at any stage of the disease or it may remain static for many months before regression begins. As a rule, after a variable period of time the pain becomes less intense and function is progressively restored. It is erroneous to believe that in all instances complete restoration of function is attained. In many instances some residual loss of motion, particularly abduction and external rotation, and some weakness of the extremity are demonstrable. Moreover, in the literature the thought is repeatedly conveyed that spontaneous recovery occurs in all instances in six to 36 months. Three individuals in this investigation disclosed frozen shoulders five, six, and eight years respectively after the onset of the disease, with no indication of regression or improvement.
Etiology and Pathogenesis
This study emphasizes that muscular inactivity is the causative agent responsible for frozen shoulders. Any cause which restricts scapulohumeral movements favors muscular inactivity; bicipital tenosynovitis was noted to be the most common factor. Other causes producing restriction or loss of scapulohumeral movements were calcareous tendinitis, sprains, contusions, and fractures in the region of the shoulder joint. Also, lesions at a distance from the shoulder joint, such as fractures of the forearm and wrist which are treated with the arm in the sling position, may produce muscular inactivity about the shoulder.
In the face of muscular inactivity, regardless of the cause, muscle atrophy ensues. This is followed by disturbance in the metabolic processes of all the soft tissues about the shoulder joint, and there follows a slowing down of the circulation and venous and lymphatic stasis. All tissues comprising the musculotendinous cuff and those adjacent to the cuff are implicated in this process. The tissues become saturated with serofibrinous exudates which provide fibrin responsible for the formation of capsular, synovial, fascial, intermuscular and intramuscular adhesions. It must be emphasized that the low grade inflammatory process involves all the normal tissues simultaneously. Microscopic study of sections made from different areas in the musculotendinous cuff, synovial membrane, subacromial bursa and biceps tendon revealed, in all instances of frozen shoulders, findings consistent with a chronic inflammatory process (Fig. 2 A, B, C, D, E). It is common knowledge that functional activity reverses the above process and restores normal circulation, joint function, and normality of the affected tissues.
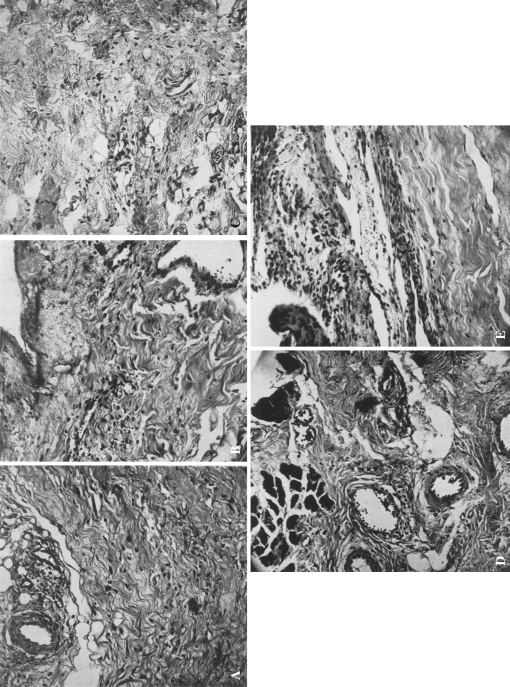
Fig. 2A–E.
The degenerative and inflammatory changes of all the soft tissue components of the scapulohumeral joint of an individual with a frozen shoulder are shown. Note that all these specimens reveal marked edema of the tissues, degeneration of the collagenous fibers, pronounced round cell infiltration of the tissues, increased vascularity, marked thickening of the synovial membrane, and evidence of increased fibrosis. These findings are consistent with a low grade chronic inflammatory process, reparative in nature. (A) A microscopic section (×140) through the coracohumeral ligament. (Again note the marked hypertrophy and increased vascularity of the synovial membrane.) (B) A microscopic section (×140) through the supra sinatus tendon. (C) A microscopic section through the bursal wall. (D) A microscopic section (×140) through the musculotendinous area of the subscapularis muscle. (E) A microscopic section (×140) (through the biceps tendon. (Note the marked thickening of the synovial membrane and increased cellular elements in this tissue).
The anatomy of the shoulder joint lends itself to changes which produce stiffness. Its fibrous capsule is loose and redundant, and with the arm at the side the capsule forms nictitating folds on the inferior and anterior aspects of the scapulohumeral joint. In addition, the short rotator muscles provide large surface areas on which serofibrinous fluid may accumulate. Large surface areas also exist between the outer and inner muscular sleeves (the outer muscular sleeve consists of the deltoid muscle, the inner sleeve of the spinatii and subscapularis muscles). Moreover, the subscapularis recesses (often referred to as subcapularis bursae) and the subacromial bursae are suitable sites for accumulation of serous exudates.
It was interesting to observe that frozen shoulders developed only when muscular inactivity initiated the aforementioned phenomenon in shoulder joints of individuals past 40 years of age or in that period of life when degenerative alterations in the musculotendinous cuff, synovalis, and biceps tendons were demonstrable both macroscopically and microscopically. In a previously recorded study [4] these lesions were first manifested in the fourth decennium and increased in gradient in each successive decade; they are alterations resulting from wear and tear and senescence (Fig. 3). In the presence of these abnormalities the inflammatory process, in most instances, progressed until firm fixation of the scapulohumeral joint by intra- and extra-capsular adhesions ensued. (The term “intracapsular adhesions” is used to designate adhesions in the nictitating folds of the fibrous capsule and synovialis in the anterior and inferior aspects of the scapulohumeral joint binding the synovial side of the articular capsule to the inferior surface of the head of the humerus). All tissues implicated in this process lose their elasticity and become shortened, particularly those on the anterior aspect of the joint.
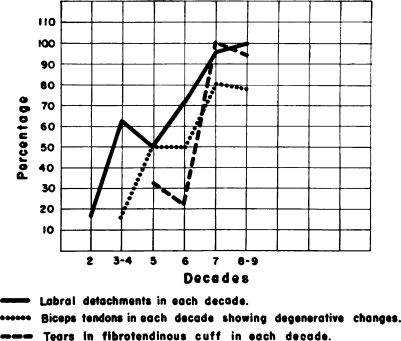
Fig. 3.
This illustrates the degenerative changes noted in a previously recorded study made on 96 shoulder joints in various age groups. Note the progressive rise in gradient in each successive decade of the degenerative lesions implicating the labrum glenoidale, biceps tendon, and musculotendinous (fibrotendinous) cuff. Also note that after the fourth decade there is a sharp rise in the severity of the degenerative alterations.
The coracohumeral ligament in these cases revealed significant alterations. It is converted into a tough inelastic band of fibrous tissue spanning the interval between the coracoid process and the tuberosities of the humerus (Fig. 4). It, together with the shortened subscapularis muscle, acts as powerful check reins to external rotation while the infraspinatus and teres minor muscles restrict the range of internal rotation.
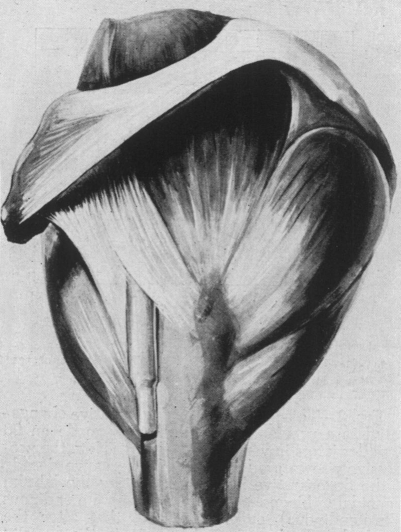
Fig. 4.
Note the topographic anatomy of the coracohumeral ligament as it extends from the coracoid process to the humerus spanning the intertubercular sulcus. This structure is thick, firm, and in a frozen shoulder becomes contracted or freezes in a shortened position and functions as a powerful check-rein to external rotation.
In this series all cases explored disclosed implication of the tendon-tendon-sheath gliding mechanism of the biceps tendon; its involvement may be primary or secondary. The intensity of the inflammatory process involving the biceps tendon and its sheath varied. In some instances the tendon was imbedded in a bed of filmy hemorrhagic adhesions, while in some it was firmly anchored to the undersurface of the capsule in the region of the intertubercular sulcus. (Hitchcock and Bechtol [7] reported similar observations). In three cases the tendon was adherent to the floor of the groove and could only be freed by sharp dissection. In two instances advanced degeneration of the extracapsular portion of the tendon occurred. The tendons were thinned, frayed, and firmly anchored to the floor of the bicipital groove. It appeared as if nature was trying to obliterate the tendon-tendon-sheath mechanism by anchoring the extracapsular portion of the biceps tendon to the shaft of the humerus.
It became apparent that once bicipital tenosynovitis was established, it played a significant role in the symptomatology and course pursued by frozen shoulders. In the light of the observations noted it is reasonable to assume that in a large measure it was responsible for the pain factor in this symptom-complex, particularly when the extremity was put through specific arcs of motion which required a wide range of excursion of the head of the humerus on the tendon. Pain, together with the mechanical obstacles to free motion caused by adhesions between the tendon and tendon-sheath, favors muscular inactivity, a situation which enhances circulatory impairment of the soft tissues and formation of more adhesions. That bicipital tenosynovitis is the responsible agent for pain in both the early and late phases of frozen shoulder was borne out by the facts that in all instances pressure over the intertubercular sulcus and rolling the biceps tendon under the examiner’s thumb elicited excruciating tenderness, also upon obliteration of the tendon-tendon-sheath mechanism by surgical intervention, dramatic and instantaneous relief of pain ensued.
It is common knowledge that in many cases of frozen shoulders, after varying periods of pain and dysfunction of the shoulder, pain disappears and restoration of function follows. In these instances, it is reasonable to assume that the inflammatory process described above subsides, resolution of adhesions occurs, and muscular activity is restored which permits return of normal circulation to the affected tissues. In other instances the process continues until there is complete obliteration of the tendon-tendon-sheath mechanism. Nature achieves this by anchoring the extracapsular portion of the biceps tendon to the shaft of the humerus, usually in the region of the lesser tuberosity. By this method the pain factor is eliminated and restoration of function can begin. The clinical course of the case R. V., a male, of 73 years, illustrates this last clinical course. This individual was examined prior to death. No clinical dysfunction of either shoulder was demonstrable and the patient was unaware of any disability in either extremity. However, he gave a history that 12 to 15 years prior to this examination he had severe pain and limitation of motion, first in the right and then in the left shoulder joint. After an indefinite period of time (two to three years) pain subsided gradually and function returned in both shoulders. From the history and degree of restriction of motion described, it was reasonable to conclude that a frozen shoulder had existed in either extremity. At postmortem, both scapulohumeral joints with the intact musculotendinous cuffs were removed and studied. The intracapsular portions of the biceps tendons were absent, while the extracapsular portions had attained a bony insertion below the lesser tuberosities.
In some instances, nature fails to obliterate completely the biceps tendon gliding mechanism; hence, spontaneous recovery does not occur and pain and stiffness persist.
In general, this investigation disclosed that the clinical course pursued following muscular inactivity of the shoulder, regardless of the cause, is directly dependent upon the degree of degenerative alterations existing in the structures comprising the scapulohumeral joint. For example, frozen shoulders rarely follow muscular inactivity in individuals under 40 years of age in whom only minimal degenerative changes in the musculotendinous cuff are discernible. In older individuals who are in excellent general health, frozen shoulders may develop, but spontaneous recovery occurs in most of them. On the other hand, in individuals past the fourth decade of life and who are afflicted by some debilitating disease which tends to accentuate the severity of degenerative lesions, particularly those in the tissues of the scapulohumeral joint, development of a frozen shoulder following muscular inactivity is the rule.
Treatment
In the light of the aforementioned observations, it becomes obvious that the method of treatment chosen is not the same for all cases of frozen shoulders. If possible, the causative factor producing the syndrome should be determined and eliminated. In many instances elimination of this causative agent will suffice to promote relief of pain and muscle spasm and permit restoration of muscular activity and function. This point is best illustrated in cases of calcareous tendinitis of long standing which have developed frozen shoulders. In such instances, simple excision of the calcareous material will initiate the sequence of events leading to recovery. Likewise, if bicipital tenosynovitis is the precipitating factor of the malady, treatment directed toward alleviation of this lesion may eliminate pain and initiate restoration of function in the scapulohumeral joint.
Early Stages of the Disease
Loss of motion in the scapulohumeral joint, in the early phases of the disease, is the result of pain and muscle spasm. It has been demonstrated many times on the operating table when the patient is under anesthesia that with muscular relaxation considerable freedom of motion is possible, indicating that pain and muscle spasm are chiefly responsible for the loss of motion. The treatment of choice in these individuals is complete bed rest, sedatives to relieve pain, continuous hot fomentations to the shoulder region and active progressive exercises in the supine position. All exercises are performed within the painless arcs of motion. Another valuable adjunct to relieve pain and muscle spasm is blocking the cervical sympathetic ganglia with 10 to 15 cc. of 1 per cent solution of Novocain. Such a regimen is continued for seven to ten days. The arm is then rested in the sling position but active pendulum exercises are encouraged. Exercises are done every hour; each exercise being done ten to 12 times and always within the painless arcs of motion. Within a few days the sling is discarded and crawling-up-the-wall and pulley exercises are added. The success of this method of treatment depends upon the full co-operation of the patient and the insistence and encouragement of the treating physician. A regimen such as described will result in a surprisingly large number of cures within eight to ten weeks. Some patients will refuse a period of bed rest. In these, repeated blocking of the cervical sympathetic ganglia may prove a valuable aid to the therapy described above. (The procedure is performed every four or five days for three to four times.) In spite of a well regulated program of treatment many cases fail to respond to these conservative measures and progress to the later stages of the disease.
Later Stage of the Disease
In addition to pain and muscle spasm, formation of intracapsular and extracapsular adhesions and shortening of the rotator and adductor muscles of the shoulder joint limit the range of motion. Moreover, as previously recorded, in all instances of frozen shoulders, particularly in the later stages of the disease, the biceps tendon discloses varying degrees of involvement. In this series it has been repeatedly demonstrated that if the pain factor is eliminated by obliteration of the tendon-tendon-sheath mechanism of the biceps tendon, restoration of function ensues. This is achieved by severing the biceps tendon at the supraglenoid tubercle and transplanting it to the coracoid process. Hitchcock and Bechtol [7] were the first observers to advocate excision of the intracapsular portion of the tendon. They anchored the end of the tendon to the shaft of the humerus in the floor of the bicipital groove.
Operative Technic
The “S” shaped skin incision described by Hitchcock and Bechtol [7] is used to expose the anterior aspect of the deltoid muscle (Fig. 5A). Next, the anterior fibers of the deltoid are split and retracted, thereby exposing the subacromial bursa, musculotendinous cuff and intertubercular sulcus. The extracapsular portion of the biceps tendon is exposed by dividing longitudinally the transverse humeral ligament and the capsule spanning the intertubercular sulcus. This incision is extended proximally through the fibers of the coracohumeral ligament for a distance of two inches. This exposure provides adequate visualization of the intracapsular portion of the biceps tendon. The biceps tendon is next severed with a long curved scissors close to the supraglenoid tubercle and withdrawn from the joint cavity.
Fig. 5A–C.
(A) This depicts the subacromial region after the deltoid muscle has been split longitudinally. (B) This portrays the long head of the biceps tendon severed at the supraglenoid brim and retracted from the joint cavity; also a trough has been made in the coracoid process. (C) This shows the tendon anchored in its new position, the edges of the musculotendinous cuff approximated by interrupted sutures and the coraco-acromial ligament divided close to the acromion process.
A one-half inch vertical incision is made over the coracoid process through the conjoined tendon fibers of the coracobrachialis, pectoralis major and short head of the biceps muscle. The coracoid process is exposed subperiosteally by sharp dissection and with a small gauge a bony trough is made in its substance (Fig. 5B). The biceps tendon is anchored in the bony trough by interrupted silk sutures and covered by the periosteal-tendinous flaps formed by the conjoined tendon. Two inches of the proximal end of the biceps tendon is sutured with interrupted silk sutures to the combined tendon of the coracobrachialis and short head of the biceps tendon. Fine interrupted silk sutures are employed to approximate the edges of the fibrous capsule and transverse humeral ligament.
At this point it must be emphasized that at no time before, during, or after the procedure must the arm be forced through its normal arcs of movements at the scapulo humeral joint.
It has been found advantageous to divide the coraco-acromial and coracohumeral ligaments in all instances of frozen shoulder in which the biceps tendon was transplanted to the coracoid process. In frozen shoulders the coraco-acromial ligament forms a definite mechanical barrier to the head of the humerus when the extremity is abducted. Division of this ligament permits the head to pass beneath the coraco-acromial arch with greater freedom. If the ligament is divided the site of severance should be close to its insertion into the acromion. As previously recorded the coracohumeral ligament in frozen shoulders is converted into a strong, thickened, shortened, fibrotic band extending from the horizontal limb of the coracoid process to the fibrous capsule as far as both tuberosities. It now acts as a powerful check rein to external rotation and abduction. Division of this structure permits early restoration of scapulohumeral motion.
After the operation, the arm is placed in a sling. Active pendulum exercises are encouraged on the second or third day. These are performed a prescribed number times (ten or 12) every hour on the hour. After five days the sling is discarded. Although the range of motion is progressively increased, it is at all times kept within the tolerance of the patient. Later, crawling up-the-wall and pulley exercises are added. Optimum restoration of function is attained in three to four months. In all cases in this series in which the above operative procedure was performed, pain was immediately relieved and restoration of muscular activity was promptly initiated.
Manipulation for Frozen Shoulders
Manipulation of frozen shoulders is a dangerous and futile procedure. That this is true was confirmed by the observations noted in 11 cases which were manipulated under direct vision. The affected shoulders of these 11 patients were exposed through an anterior deltoid splitting incision. In all instances when the arm was externally rotated, regardless of how gently the maneuver was performed, tears of varying degrees were noted in the subscapularis tendon and in its lower muscle fibers inserting into the shaft of the humerus. In two individuals the tendinous insertion of the subscapularis muscle was completely avulsed from the lesser tuberosity and the tear extended proximally into the musculotendinous cuff for one and one-and-one-half inches respectively. Abduction of the arm in all cases produced transverse tears in the fibrous capsule along the inferior aspect of the neck of the humerus. In three instances the articular surface of the head came into view through tears in the capsule. In three instances rupture of the biceps tendon at the level of the intertubercular sulcus was sustained and in one, fracture of the surgical neck resulted.
Such observations point out the hopelessness of such a procedure. One is forced to conclude that the cases that are benefited by manipulation may be those in which the tendon of the long head of the biceps brachii muscles was ruptured. By so doing, the tendon-tendon-sheath gliding mechanism is obliterated and pain may be eliminated. As previously noted, nature often resorts to this method by anchoring the tendon to the shaft of the humerus.
Analysis of Cases
Thirty-eight cases of frozen shoulder were studied. In this series there were 12 males (31.7 per cent) and 26 females (68.3 per cent); the left shoulder was implicated in 28 cases (73.9 per cent) and the right in 10 (26.1 per cent). The duration of symptoms varied from three to 96 months; in three cases symptoms had existed for 45, 72, and 96 months respectively. In two instances both shoulders were involved. The age ranged from 37 to 61 years; only one individual was under 40 years of age.
Muscular inactivity was the common precipitating factor in all instances. In 16 cases (42.1 per cent) a bicipital tenosynovitis was responsible for the muscular inactivity. Six of the 16 cases were followed from the onset of the malady which began as a bicipital tenosynovitis to the late stage of the disease, when a classic frozen shoulder was demonstrable. In the remaining ten of the 16 cases, a careful history of the onset of the disease forced one to conclude that the bicipital tenosynovitis was the initiating agent. In 22 (57.9 per cent) cases, muscular inactivity of the shoulder was produced by varied causes, such as calcareous tendinitis five cases), falls on the shoulder, immobilization of the arm in the sling position following fractures of the humerus or bones of the forearm, and in two females following a radical mastectomy. It must be emphasized that in all individuals after the fourth decade, varying degrees of degenerative changes exist in the musculotendinous cuff. This was conclusively shown in a previously reported study. Only in the presence of these alterations will muscular inactivity produce a frozen shoulder.
The cardinal clinical features were: (1) loss of the normal scapulohumeral rhythm, (2) fixation of the extremity at the side in a position of internal rotation, (3) loss of scapulohumeral motion except for a few degrees of motion in the anteroposterior plane, (4) exquisite tenderness over the intertubercular groove and (5) rolling the biceps tendon (rolling sign) under the examiner’s thumb elicited tenderness.
Six cases responded to conservative measures, while 32 were treated surgically. In all instances explored, in addition to the macroscopic and microscopic evidence of a low grade chronic inflammatory process implicating the entire musculotendinous cuff and fibrous capsule, involvement of the tendon-tendon-sheath gliding mechanism of the biceps tendon in varying intensities was discernible. In some cases the biceps tendon lay in a mesh of fine, filmy, hemorrhagic adhesions, while in others the tendon was firmly bound by tough adhesions to the groove and to the undersurface of the fibrous capsule. In three instances sharp dissection was necessary in order to free the tendon from the groove. Changes in the subacromial bursa varied from the formation of a few fine adhesions within the bursal sac to complete obliteration of the bursa by fibrous tissue. The coracohumeral ligament in all instances was found to be thickened and frozen in a shortened position.
In the 32 cases which were treated by surgical intervention, pain was eliminated immediately following the operation. The time required to obtain maximum restoration of painless function in the scapulohumeral joint varied from eight to 22 weeks.
Summary and Conclusions
Frozen shoulder is a clinical entity which is produced by muscular inactivity of the shoulder in individuals past 40 years of age.
Bicipital tenosynovitis is the most common etiologic factor producing muscular inactivity. (It initiated the syndrome in 42.1 per cent of the cases in this series).
Pain is the outstanding clinical feature of frozen shoulder; once pain is eliminated restoration of function can begin.
Conservative measures in early cases may abort the syndrome.
In late cases transplantation of the tendon of the long head of the biceps brachii muscle to the coracoid process will eliminate the pain factor and permit painless motion in the shoulder.
In this series optimum restoration of function was obtained in eight to 22 weeks.
Footnotes
(The Classic Article is ©1952 by Lippincott Williams & Wilkins and is reprinted with permission from DePalma AF. Loss of scapulohumeral motion (frozen shoulder). Ann Surg. 1952;135:193–204.)
Submitted for publication February, 1951.
Bibliography
- 1.Abbott, L. C., and J. B. deC. M. Saunders: Acute Traumatic Dislocations of the Tendon of the Long Head of the Biceps Brachii. A Report of Six Cases with Operative Findings. Surgery, 6: 817, 1939.
- 2.Baer, W. S.: Operative Treatment of Subdeltoid Bursitis. Bull. Johns Hopkins Hosp., 18: 282, 1907.
- 3.Codman, E. A.: The Shoulder. The Author, 1934.
- 4.DePalma, A. F., G. Callery, G. Bennett: Variational Anatomy and Degenerative Lesions of the Shoulder Joint. Instructional Course Lectures, Am. Acad. Orthoped. Surg., 1949.
- 5.Duplay, S.: De la periarthrite scapulo-humerale. Rev. frat. d. trav. de med., 53: 226, 1896; translated, M. Week, 4: 253, 1896, On Scapulo-Humeral Periarthritis. M. Press, 59: 571, 1900.
- 6.Gilcreest, E. L.: Rupture of Muscles and Tendons, J. A. M. A., 84: 1819, 1925; Spontaneous Rupture of Long Head of Biceps Flexor Cubiti, S. Clin. North America, 6: 547, 1926; Dislocation and Elongation of Long Head of Biceps Brachii. Ann. Surg., 104: 118, 1936. [DOI] [PMC free article] [PubMed]
- 7.Hitchcock, H. H., and C. O. Bechtol: Painful Shoulder. J. Bone & Joint Surg., 30-A: 263, 1948. [PubMed]
- 8.King, J. M., Jr., and G. W. Holmes: Diagnosis and Treatment of Four Hundred and Fifty Painful Shoulders. J. A. M. A., 89: 1956, 1927.
- 9.Lippmann, R. K.: Bicipital Tenosynovitis. Arch. Surg., 47: 283, 1943.
- 10.Meyer, A. W.: Chronic Functional Lesions of the Shoulder. Arch. Surg., 35: 646, 1937.
- 11.Meyer, A. W.: (a) Unrecognized Occupational Destruction of the Tendon of the Long Head of the Biceps Brachii, Arch. Surg., 2: 130, 1921; (b) Spontaneous Dislocation of the Long Head of Biceps Brachii, ibid. 13: 109, 1926; (c) Spontaneous Dislocation and Destruction of the Tendon of Long Head of Biceps Brachii, ibid, 17: 493, 1928.
- 12.Mumford, E. B., and F. S. Martin: Calcified Deposits in the Subdeltoid Bursitis. J. A. M. A., 97: 690, 1931.
- 13.Pasteur, F.: La teno-bursite bicipitale. J. deradial, et d’electrol., 16: 419, 1932.
- 14.Pasteur, F.: La teno-bursite de la longue portion du biceps. Gas. d. hop., 107: 477, 1934.
- 15.Pasteur, F.: Sur une forone nourcele de periarthralgie et d’ankylose de l’épaule. J. deradial, et d’electrol, 18: 327, 1934.
- 16.Pasteur, F.: Tenobursite bicipitale. Presse med., 41: 142, 1933.