Abstract
After placement of a reverse shoulder endoprosthesis, range of motion is usually still compromised. To what extent this occurs from limitation in motion of the reverse endoprosthesis is, however, unclear. We measured the motion pattern of 16 patients (18 shoulders) during three active and passive range of motion tasks using a six degree-of-freedom electromagnetic tracking device. Despite rotator cuff deficiencies, glenohumeral elevation contributed roughly two-thirds of the total thoracohumeral elevation, which is comparable to healthy subjects. However, patients could not actively use the full range of motion provided by the prosthesis. Although we found considerable interindividual differences in shoulder kinematics, the limitation in glenohumeral range of motion appears related to a lack of generated muscle force and not the design of the prosthesis.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
In situations in which shoulder arthroplasty is necessary and the rotator cuff is degenerated, the reverse shoulder endoprosthesis offers a potentially beneficial surgical option [1]. In standard designs in which stability problems occur, the reverse shoulder endoprosthesis theoretically reduces this problem by reversing the relationship between the scapular and humeral components. It uses a modified mechanical joint design to compensate, especially for defects in the rotator cuff, by moving the ball of the joint to the glenoid and the socket to the humeral head. The resulting design combines a medialized center of rotation with the mechanical advantage of a substantially higher translational stiffness (small displacements of the humeral socket along the surface of the joint lead to relatively large displacements away from the joint center [15]) resulting in a clinically more stable joint. This allows effective deltoid muscle activation without the otherwise necessary rotator cuff contribution to create sufficient translational stiffness. Based on this mechanical advantage, the reverse shoulder endoprosthesis has become an alternative for the treatment of complex proximal humeral fractures, whereas tuberosity nonunion is a frequent complication in standard prosthetic fracture treatment and it is assumed more stable than a shoulder prosthesis with an anatomic design [1, 4].
Two short-term studies (average followup after 33 and 38 months) suggest that the reverse endoprosthesis provides the patient with pain relief [8, 19]. However, restoration of shoulder function is less clear. Postoperative shoulder function differs from “moderate” (forward elevation less than 90°) to “good” (forward elevation greater than 120°) [6, 16]. Given this elevation range, the question remains to what extent does the reverse endoprosthesis actually facilitate the elevation of the arm, because this range might have been the result of scapulothoracic rotation combined with no, or only limited, glenohumeral rotation as has been reported for some patients with regular shoulder endoprostheses [17]. Moreover, the reverse endoprosthesis does not seem to restore normal active external rotation [1]. Whether this limited shoulder function is caused by a lack of muscle force or by mechanical effects resulting from the design of the prosthesis that hinders a larger range of motion is unclear. If the arm can passively be moved further than the result of active elevation, this would be a strong indicator for the existence of a muscle moment deficit most likely resulting from either changes in moment arms or changes in optimal muscle length.
We hypothesized patients with a reverse endoprosthesis would have larger ranges of motion during passive movements than during active movements. In addition, we wondered to what extent movement in the reverse endoprosthesis contributes to movement of the arm.
Materials and Methods
Sixteen patients, eight men and eight women, with recently placed reverse prostheses (Tornier®; Tornier BV, Schiedam, The Netherlands) voluntarily participated in this study. Nine patients had been operated on the right side, five on the left, and two on both sides (18 shoulders). The average time between measurements and surgery was 90 weeks (range, 8–274 weeks). Mean age of the patients was 71 years (range, 58–85 years). The protocol was approved by the medical ethics committee. All patients gave written informed consent before the experiment.
We used an electromagnetic tracking device (Motion Monitor Biomech I; Innovative Sports Training, Chicago, IL) consisting of a transmitter and a number of sensors to measure the three-dimensional position of the upper extremity. A computer calculated the position and orientation of the sensors in the electromagnetic field. We used five sensors attached to a pointer, the sternum, humerus, forearm, and a scapula locator [10] to measure position and orientation of the upper extremity. The sensors on the sternum and acromion were fixed using double-sided adhesive tape and covered with a Fixomull stretch self-adhesive bandage (Beiersdorf AG, Hamburg, Germany). Arm and forearm sensors were fitted using a brace [12]. This kind of method and system has been used in prior studies regarding shoulder kinematics [11, 13, 17].
We used a pointer to digitize 13 bony landmarks relative to their sensors to relate the sensors to local anatomic coordinate systems (Table 1). Segments and joint rotations were calculated using the combination of local coordinate systems constructed from the bony landmarks and the sensor motions. For the humerus, the proximal landmark was chosen in the glenohumeral rotation center estimated by regression analysis using five bony landmarks of the scapula. Local coordinate systems, segment and joint rotations were all defined following the International Society of Biomechanics standardization proposal for the upper extremity [20].
Table 1.
Bony landmarks used to construct local coordinate systems
| Bone | Bony landmarks |
|---|---|
| Thorax | Incisura jugularis |
| Processus spinosus seventh | |
| cervical vertebrae | |
| Processus spinosus eighth | |
| thoracal vertebrae | |
| Processus xiphoideus | |
| Scapula | Angulus inferior |
| Trigonum spinae | |
| Angulus acromialis | |
| Acromioclavicular joint | |
| Processus coracoideus | |
| Humerus | Epicondylus medialis |
| Epicondylus lateralis |
We measured three range of motion (ROM) tasks, which were performed actively and passively. The ROM tasks consisted of elevation in the sagittal (forward flexion) and in the scapular planes at a 45° angle of sagittal and frontal planes (abduction [14]) and axial rotation consisting of internal and external rotation of the arm during 90° of abduction. Subjects were instructed to reach a maximal joint angle in each active ROM task. During the elevation, subjects were instructed to elevate the arm as high as possible. Scapular abduction was maintained by using a semicircular board that subjects could follow as a reference. This board was fixed in a 45° angle relative to the frontal plane. During the passive ROM tasks, the arm was moved by the experimenter until considerable resistance was met.
Because dynamic tracking of the scapula is difficult, we performed measurements in a quasistatic mode using a scapula locator similar to that used in other studies [11, 17]. We asked subjects to perform each task three times. If all three attempts were subsequently available for analysis, only the second attempt was selected for further processing.
We accounted for the motions of the scapula relative to the thorax and the humerus relative to both thorax (thoracohumeral motion) and scapula (glenohumeral motion). Joint angles were defined based on the International Society of Biomechanics standardization proposal of the International Shoulder Group [20]. To ensure consistent angle definitions, data from left shoulders were mirrored to the right before further data processing. The elevation angles of the humerus were expressed as either thoracohumeral angles (the angle of the humerus coordinate system relative to the thorax) or glenohumeral angles (the angle of the humerus coordinate system relative to the scapula coordinate system) [18]. The difference between the thoracohumeral and glenohumeral angles reflects the contribution of scapular motion to the movement of the arm. For both the thoracohumeral and glenohumeral motions, the decomposition order was chosen following the globographic convention, which is plane of elevation, elevation, axial rotation [7]. Negative axial rotation is external rotation and internal rotation is positive axial rotation.
The thoracohumeral and glenohumeral elevation angles and axial rotation values were selected for further analysis. For the elevation tasks, we determined the peak thoracohumeral elevation value for each subject. For the axial rotation tasks, the peak external and internal thoracohumeral rotation values were determined for each subject. We calculated the minimum, maximum, mean, and standard deviation for all subjects for each of the previously mentioned angles.
To assess the contribution of scapular motion, we determined the ratio between thoracohumeral and glenohumeral elevation by dividing the peak (change in) thoracohumeral elevation angle by peak (change in) glenohumeral elevation angle [11].
For each patient, the second trial was selected for further processing. The Shapiro-Wilk test for normality showed the overall data were normally distributed (p = 0.31). We used a paired t-test to determine differences between active and passive tasks. The significance level was set at 0.05.
Results
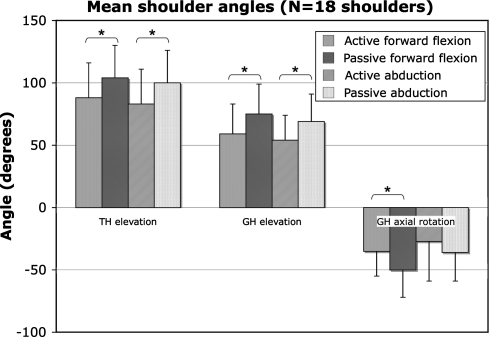
We observed large differences in ROM between patients (Table 1). Despite the large interindividual differences, the passive tasks showed higher elevation angles than the active tasks (Fig. 1). For elevation in the sagittal plane (forward flexion) and for elevation in the scapular plane (abduction), a greater (p < 0.001) thoracohumeral elevation angle was found in the passive condition compared with the active condition (Table 2). The passive glenohumeral elevation angle was also greater (p < 0.001) than the active angle in both the forward flexion and the abduction task, whereas only the forward flexion showed higher (p < 0.01) glenohumeral rotation during the passive condition compared with the active condition. Passive thoracohumeral and glenohumeral external rotation was greater (p ≤ 0.001) than active external rotation during the axial rotation task (Table 3). A similar effect was found for internal rotation during this condition. Passive thoracohumeral and glenohumeral internal rotation was greater (p < 0.001) than active internal rotation (Fig. 1).
Fig. 1.
Mean and standard deviation of the joint angles for 18 shoulders. External rotation is negative axial rotation and internal rotation is positive axial rotation. Asterisks indicate differences between active and passive tasks.
Table 2.
Maximal joint angles (degrees) for 18 shoulders during the elevation tasks*
| Range of motion | Thoracohumeral elevation | Glenohumeral elevation | Glenohumeral axial rotation | |||
|---|---|---|---|---|---|---|
| Forward flexion† | Abduction† | Forward flexion† | Abduction† | Forward†flexion | Abduction | |
| Active task | 88 ± 28 (27–121) | 83 ± 28 (26–119) | 59 ± 24 (6–95) | 54 ± 20 (9–78) | −35 ± 20 (−75–9) | −27 ± 32 (−114–19) |
| Passive task | 104 ± 26 (36–136) | 100 ± 26 (37–127) | 75 ± 24 (15–101) | 69 ± 22 (17–100) | −50 ± 22 (−102– −13) | −36 ± 23 (−89–2) |
Mean ± standard deviation (range); * external rotation is negative axial rotation and internal rotation is positive axial rotation; †significant differences (p < 0.05) between active and passive task.
Table 3.
Maximal joint angles (degrees) for 18 shoulders during the rotation task*
| Range of motion | Thoracohumeral axial rotation | Glenohumeral axial rotation | Glenohumeral elevation | |||
|---|---|---|---|---|---|---|
| External rotation† | Internal rotation† | External rotation† | Internal rotation† | External rotation | Internal rotation† | |
| Active axial rotation | −17 ± 24 | 28 ± 27 | −15 ± 26 | 29 ± 28 | 53 ± 21 | 36 ± 13 |
| Passive axial rotation | −38 ± 25 | 48 ± 21 | −36 ± 26 | 50 ± 18 | 58 ± 16 | 49 ± 14 |
Mean ± standard deviation; *external rotation is negative axial rotation and internal rotation is positive axial rotation; †significant differences (p < 0.05) between active and passive task.
We found a higher (p < 0.05) average ratio between thoracohumeral and glenohumeral elevation and abduction for the active ROM tasks than for the passive ROM tasks showing the glenohumeral joint contributes more to movement of the arm in passive tasks compared with active tasks. Active forward flexion showed the highest mean ratio (1.7 ± 0.8:1), whereas the lowest ratio was found for passive abduction (1.4 ± 0.4:1). Active abduction had a ratio of 1.6 ± 0.4 to one, whereas passive forward flexion had a ratio of 1.5 ± 0.2 to one.
Discussion
Although results in terms of pain reduction are good, the extent in which a reverse endoprosthesis contributes to restoration of arm function is not clear. In particular, the extent in which glenohumeral motion postoperatively contributes to arm elevation is as yet unknown. The aim of this study was to determine to what extent movement in the reverse endoprosthesis contributes to movement of the arm as a whole and determine whether sufficient force could be generated to use the full ROM of the prosthesis. We hypothesized patients with a reverse endoprosthesis would show larger ROM during passive movements than during active movements and that the increase of ROM was mainly the result of a higher contribution of the glenohumeral joint.
The results of this study might have been influenced by some of our methods. We measured motions in a quasistatic mode, because the scapula needed to be palpated after each change of arm position. de Groot et al. [3] showed differences between active and quasistatic movements in healthy subjects were negligible. Because our subjects were patients, it can, however, not be excluded that they might display a higher amount of variation between quasistatic and dynamic measurements.
Considerably higher ROM and ratios (between thoracohumeral and glenohumeral elevation) were found during the passive movements. On average, approximately two-thirds of the entire active elevation is the result of the reverse prosthesis. The mean contribution of glenohumeral movement on the entire ROM increases even further for the active axial rotation task. Nearly all the movement is the effect of axial rotation of the mechanical joint. The reverse endoprosthesis seems to provide on average a major contribution to the movement of the arm as a whole.
The glenohumeral elevation angles in our patients were similar to those for patients with the usual shoulder arthroplasty [17]. Given a comparable setup and testing conditions, no marked difference in glenohumeral elevation was found between a “normal” shoulder arthroplasty (53° ± 11°) and a reverse endoprosthesis (59° ± 26°). The contribution of glenohumeral elevation during thoracohumeral elevation is decreased in patients with a standard arthroplasty compared with healthy subjects. The glenohumeral elevation of that patient group contributes approximately one-third of the total thoracohumeral elevation. The reverse endoprosthesis seems to enable scapulohumeral rhythm during arm elevation similar to that found in healthy subjects [3] in contrast to the “normal” shoulder arthroplasty.
The contribution of glenohumeral movement on the entire ROM differed greatly among subjects, which explains the differences in outcome for postoperative shoulder function found in the literature [6, 16]. However, in general, patients appear unable to generate enough force to use the full ROM of the mechanical joint. Because medialization of the center of rotation causes a decrease in the muscle force needed to reach maximum ROM [4], a medialized rotation center and an increased deltoid lever arm, present in the reverse prosthesis, should result in more powerful abduction of the shoulder despite loss of rotator cuff function [5]. Nonetheless, the shortening range of the deltoid muscle increases as a result of lowering the humerus. The muscle might not be able to fully adapt to these large increases of shortening range causing a decrease in potential muscle force. Future research in which force generation capability is also recorded could elucidate this, especially when combined with musculoskeletal modeling in which muscle forces also be estimated [4].
In addition, a rehabilitation program that specifically focuses on increasing the muscle force under stretching conditions might lead to higher utilization of the ROM of the mechanical joint. The training should mainly focus on the deltoid muscle because this muscle compensates for the deficient rotator cuff muscles [1].
During the active forward flexion task, a lower glenohumeral external peak rotation was found (−35° ± 20°) for patients with a reverse shoulder endoprosthesis compared with patients with a standard arthroplasty (−54° ± 31°) and healthy subjects (−73° ± 13°) [11, 17]. An even lower value was found for external rotation during the active axial rotation task, showing the patients with a reverse shoulder endoprosthesis have difficulty with glenohumeral axial rotation. This might be caused by insufficiency of the teres minor, which is often involved in cuff tear arthropathy [2], but also as a result of function loss in infraspinatus. Further research should evaluate how this lack of external rotation affects the performance of activities of daily living.
We observed substantial differences in patients’ ROM despite a normal glenohumeral contribution during thoracohumeral elevation. Furthermore, the observed limitation in glenohumeral ROM appears the result of a lack of generated muscle force and not the design of the prosthesis. The design of the prosthesis appears to have a beneficial effect on arm motion and most likely also arm function. To what extent increased function might contribute to early loosening of the scapular component [9] is an element that should also be evaluated in the near future.
Acknowledgments
We thank E. Zwitser for her valuable support in the experimental phase of the project.
Footnotes
One of the authors (WJW) has received funding from Tornier SA, France.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
References
- 1.Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(Suppl S):147S–161S. [DOI] [PubMed]
- 2.Boulahia A, Edwards TB, Walch G, Baratta RV. Early results of a reverse design prosthesis in the treatment of arthritis of the shoulder in elderly patients with a large rotator cuff tear. Orthopedics. 2002;25:129–133. [DOI] [PubMed]
- 3.de Groot JH, Valstar ER, Arwert HJ. Velocity effects on the scapulo-humeral rhythm. Clin Biomech (Bristol, Avon). 1998;13:593–602. [DOI] [PubMed]
- 4.de Leest O, Rozing PM, Rozendaal LA, van der Helm FC. Influence of glenohumeral prosthesis geometry and placement on shoulder muscle forces. Clin Orthop Relat Res. 1996;330:222–233. [DOI] [PubMed]
- 5.De Wilde LF, Audenaert EA, Berghs BM. Shoulder prostheses treating cuff tear arthropathy: a comparative biomechanical study. J Orthop Res. 2004;22:1222–1230. [DOI] [PubMed]
- 6.Delloye C, Joris D, Colette A, Eudier A, Dubuc JE. [Mechanical complications of total shoulder inverted prosthesis.] Rev Chir Orthop Reparatrice Appar Mot. 2002;88:410–414. [PubMed]
- 7.Doorenbosch CA, Harlaar J, Veeger DH. The globe system: an unambiguous description of shoulder positions in daily life movements. J Rehabil Res Dev. 2003;40:147–155. [PubMed]
- 8.Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87:1697–1705. [DOI] [PubMed]
- 9.Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88:1742–1747. [DOI] [PubMed]
- 10.Johnson GR, Stuart PR, Mitchell S. A method for the measurement of three-dimensional scapular movement. Clin Biomech. 1993;8:269–273. [DOI] [PubMed]
- 11.Magermans DJ, Chadwick EK, Veeger HE, van der Helm FC. Requirements for upper extremity motions during activities of daily living. Clin Biomech (Bristol, Avon). 2005;20:591–599. [DOI] [PubMed]
- 12.Meskers CG, Fraterman H, van der Helm FC, Vermeulen HM, Rozing PM. Calibration of the ‘Flock of Birds’ electromagnetic tracking device and its application in shoulder motion studies. J Biomech. 1999;32:629–633. [DOI] [PubMed]
- 13.Meskers CG, Vermeulen HM, de Groot JH, van Der Helm FC, Rozing PM. 3D shoulder position measurements using a six-degree-of-freedom electromagnetic tracking device. Clin Biomech (Bristol, Avon). 1998;13:280–292. [DOI] [PubMed]
- 14.Michiels I, Grevenstein J. Kinematics of shoulder abduction in the scapular plane: on the influence of abduction velocity and external load. Clin Biomech. 1995;10:137–143. [DOI] [PubMed]
- 15.Oosterom R, Herder JL, van der Helm FCT, Swieszkowski W, Bersee HEN. Translational stiffness of the replaced shoulder joint. J Biomech. 2003;36:1897–1907. [DOI] [PubMed]
- 16.Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86:388–395. [DOI] [PubMed]
- 17.Veeger HEJ, Magermans DJ, Nagels J, Chadwick EKJ, van der Helm FCT. A kinematical analysis of the shoulder after arthroplasty during a hair combing task. Clin Biomech. 2006;21(Suppl 1):S39–S44. [DOI] [PubMed]
- 18.Veeger HEJ, van der Helm FCT, Rozendal RH. Orientation of the scapula in a simulated wheelchair push. Clin Biomech. 1993;8:81–90. [DOI] [PubMed]
- 19.Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87:1476–1486. [DOI] [PubMed]
- 20.Wu G, van der Helm FC, Veeger HE, Makhsous M, Van Roy P, Anglin C, Nagels J, Karduna AR, McQuade K, Wang X, Werner FW, Buchholz B. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion—Part II: shoulder, elbow, wrist and hand. J Biomech. 2005;38:981–992. [DOI] [PubMed]