Abstract
A histologically normal insertion site does not regenerate following rotator cuff tendon-to-bone repair, which is likely due to abnormal or insufficient gene expression and/or cell differentiation at the repair site. Techniques to manipulate the biologic events following tendon repair may improve healing. We used a sheep infraspinatus repair model to evaluate the effect of osteoinductive growth factors and BMP-12 on tendon-to-bone healing. Magnetic resonance imaging and histology showed increased formation of new bone and fibrocartilage at the healing tendon attachment site in the treated animals, and biomechanical testing showed improved load-to-failure. Other techniques with potential to augment repair site biology include use of platelets isolated from autologous blood to deliver growth factors to a tendon repair site. Modalities that improve local vascularity, such as pulsed ultrasound, have the potential to augment rotator cuff healing. Important information about the biology of tendon healing can also be gained from studies of substances that inhibit healing, such as nicotine and antiinflammatory medications. Future approaches may include the use of stem cells and transcription factors to induce formation of the native tendon-bone insertion site after rotator cuff repair surgery.
Introduction
Rotator cuff repair usually requires tendon-to-bone healing, and prior studies have reported a relatively high rate of failure of tendon healing following repair [8, 26, 28, 30, 34]. Because of the relatively high failure rate of surgical repair, there is strong clinical relevance to methods to improve rotator cuff tendon healing. Numerous animal studies examining healing between tendon and bone demonstrate a normal tendon-bone insertion site is not regenerated following tendon-to-bone repair [11, 31, 55]. Rotator cuff healing occurs by reactive scar formation rather than regeneration of a histologically normal insertion site (Fig. 1A–B). The overall structure, composition, and organization of a normal insertion site do not regenerate. Specifically, the zone of calcified cartilage does not reform [31]. The poor healing response likely relates to insufficient expression of genes that direct formation of the complex structure and composition of the insertion site.
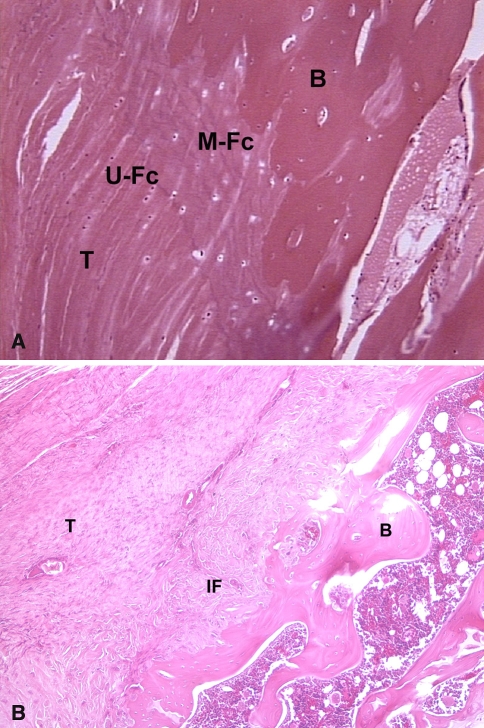
Fig. 1A–B.
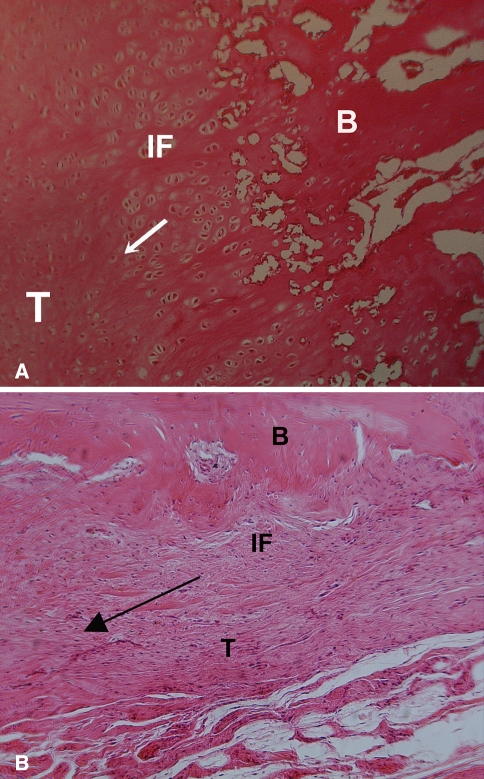
(A) Histologic section of a normal supraspinatus tendon insertion site demonstrates the four zones of a direct insertion: tendon (T), unmineralized fibrocartilage (U-Fc), mineralized fibrocartilage (M-Fc), and bone (B). (B) This histologic section of the tendon-bone attachment site 4 weeks after supraspinatus tendon repair in a rat model shows the site is characterized by a fibrovascular scar tissue interface (IF) without formation of an intermediate zone of fibrocartilage between tendon (T) and bone (B).
It is conceivable the structure of the rotator cuff insertion could be reformed following tendon repair if it were possible to recapitulate the events that occur during embryonic development. Several factors may explain why this does not occur during healing of tissues in the postnatal organism. These include abnormal and/or insufficient expression of genes that direct insertion site formation, insufficient numbers of undifferentiated cells at the healing interface, and excessive loads on the healing tendon (tendon-bone interface motion). The insufficiency of appropriate molecular signals (gene expression) and cell differentiation may occur because of the presence of inflammation in the postnatal organism. This review will explore novel concepts to improve rotator cuff tendon healing that are based on these factors.
Inflammation plays a critical role in wound healing. Wounds in the embryo and early fetus are known to heal by tissue regeneration rather than scar formation, in a process that has been termed “scarless healing.” The absence of a substantial inflammatory response in fetal wounds is likely an important factor in scarless healing. While macrophages accumulate as part of the inflammatory response to wounds in the postnatal organism, they do not accumulate in wounds made in the mouse fetus. The transition stage after which macrophages accumulate in a fetal wound is coincident with the stage in mouse fetal development after which wounds heal by scar. This information indicates inflammation leads to healing by scar tissue rather than regeneration of phenotypically normal tissue. The rapid influx of inflammatory cells following rotator cuff tendon repair may result in cellular and molecular signals that ultimately lead to fibrosis rather than tissue regeneration.
Given these abnormalities in the cellular and molecular signals in the healing environment, attention has turned to methods to augment the biologic response following rotator cuff repair. Because cytokines play important roles in cell chemotaxis, proliferation, matrix synthesis, and cell differentiation, these molecules have the potential to improve rotator cuff tendon healing. For example, a recent study reported expression of basic fibroblast growth factor (bFGF), insulin-like growth factor-1 (IGF-1), platelet-derived growth factor (PDGF), and transforming growth factor-β (TGF-β) in a rabbit supraspinatus tendon defect model [39]. There was sequential expression of these factors in fibroblasts, blood cells, and vascular endothelial cells. These data demonstrate cytokines play a role in tissue formation and support the potential for growth factors to improve tendon healing.
In this review we discuss the results of two separate studies evaluating rotator cuff repair in sheep using a mixture of osteoinductive factors (BMP-2–7 and fibroblast growth factor) and BMP-12 [55]. This is followed by a detailed review of the current knowledge regarding biologic augmentation of rotator cuff repair healing. We also review material on tendon-to-tendon healing because some longitudinal tears require side-to-side tendon repair. We conclude by discussing speculative approaches to improvement of rotator cuff biology and repair and we will identify areas for further study. We have chosen studies for review that presented significant results and that had detailed descriptions of study methodology.
Bone Morphogenetic Proteins for Augmentation of Rotator Cuff Tendon-to-Bone Repair
Because tendon-to-bone healing appears to depend upon bone ingrowth into the fibrovascular interface tissue and outer tendon, we hypothesized osteoinductive growth factors would improve rotator cuff tendon healing. We tested an osteoinductive bone protein extract derived from bovine cortical bone [55]. The extract contains bone morphogenetic proteins 2–7 (BMP-2–7), transforming growth factor-β-1–3, and fibroblast growth factor (FGF).
Seventy-two skeletally mature female sheep underwent detachment followed by immediate repair of the infraspinatus tendon to the greater tuberosity using sutures through bone tunnels. The experimental animals (n = 24) received the growth factors at the tendon-bone interface using a type I collagen sponge carrier (Fig. 2). The two control groups (n = 24 each) consisted of repairs treated with the collagen sponge carrier alone or no implant. Animals were euthanized at 6 and 12 weeks and the repaired rotator cuff was evaluated using MRI, plain radiographs, histology, and mechanical testing. We measured the gap between tendon and bone at the repair site and the volume of newly formed bone and soft tissue on the MRI. Mechanical testing was performed on an Instron machine to measure load-to-failure of the tendon-bone construct (structural property). We normalized the data by dividing the load data by the MRI measurements of tissue volume in order to derive an estimate of the material properties of the healed tendon attachment site.
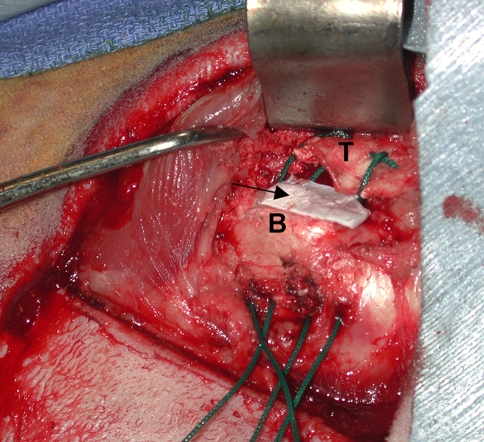
Fig. 2.
A collagen sponge (arrow) is used as a carrier vehicle to deliver growth factors to the tendon-bone attachment site in a sheep infraspinatus repair model (T = tendon, B = bone).
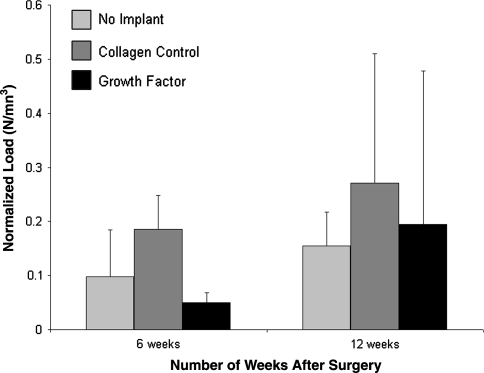
Similar to other studies using the sheep model, we found a gap consistently formed between the end of the repaired tendon and bone, with reparative scar tissue and new bone spanning the gap. Thus, in effect this model tests the effects of growth factors on scar tissue formation in a gap between tendon and bone. MRI showed the volume of newly formed bone (p < 0.05) and soft tissue (p < 0.05) in the tendon-bone gap were greater in the growth-factor-treated animals compared to the collagen sponge control group at both time points (Figs. 3, 4). There was a fibrovascular tissue in the interface between tendon and bone, with a more robust fibrocartilage zone between the bone and tendon in the growth-factor-treated animals (Fig. 5A–B). The repairs treated with the osteoinductive growth factors had greater (p < 0.05) failure loads at 6 weeks and 12 weeks. However, when the data were normalized by tissue volume, there were no differences between groups, suggesting the growth factor treatment results in formation of poor quality scar tissue rather than true tissue regeneration (Fig. 6). This study was the first to demonstrate the possibility of increasing tissue formation in a tendon-bone gap using a biologic agent. These results were consistent with previous studies of the effect of growth factors on tendon and ligament healing that reported formation of new tissue with inferior material properties [24, 62].
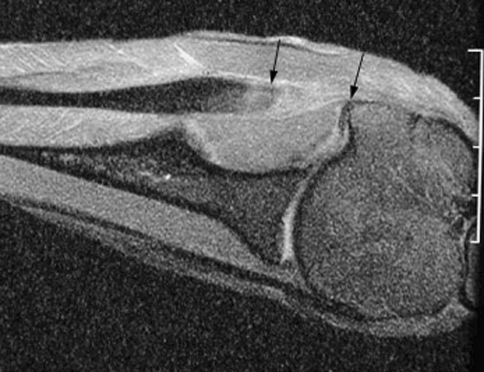
Fig. 3.
MR image of the healed tendon-bone interface 6 weeks after infraspinatus tendon repair to the greater tuberosity in a sheep model reveals the clear gap between the end of the repaired tendon and bone (between arrows). The low signal intensity native tendon is easily distinguished from the fibrovascular scar tissue interface.
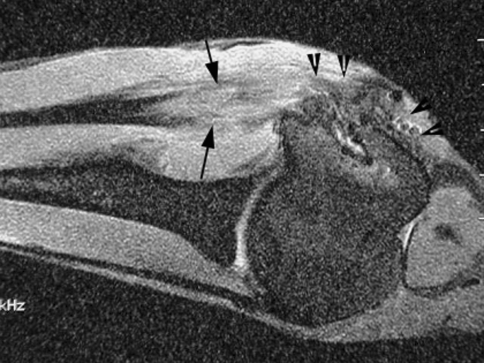
Fig. 4.
MR image shows robust formation of new soft tissue (between arrows) in the tendon-bone interface and new bone formation (arrow heads) at the greater tuberosity 12 weeks after repair with a mixture of osteoinductive growth factors applied at the tendon attachment site on a collagen sponge carrier. Compare to the healed attachment site in an untreated repair in Fig. 3.
Fig. 5A–B.
A histologic section shows fibrovascular tissue in the interface (IF) between tendon and bone at 6 weeks, with a more robust fibrocartilage interface zone between the bone (B) and tendon (T) in the (A) growth-factor treated animals compared to (B) controls that received the collagen sponge alone. The arrow indicates the direction of pull of the tendon.
Fig. 6.
The ultimate load-to-failure was higher in the animals treated with the osteoinductive growth factors; however, when the data were normalized by tissue volume there were no differences between groups. Figure reprinted with permission and © The Journal of Bone and Joint Surgery, Inc. from Rodeo SA, Potter HG, Kawamura S, Turner AS, Kim HJ, Atkinson BL. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am. 2007; 89:2485–2497.
Bone morphogenetic protein-12 (BMP-12), also known as growth and differentiation factor-7 (GDF-7) and cartilage-derived morphogenetic protein-3 (CDMP-3); BMP-13 (GDF-6 and CDMP-2); and BMP-14 (GDF-5 and CDMP-1) are novel molecules that are expressed at tendon insertion sites during embryonic development [75]. These signaling molecules have substantially different biologic activity compared to the osteoinductive BMPs (BMP-2, -4, -7) and induce formation of tendon and fibrocartilage. Prior studies have reported induction of neo-tendon/ligament in rats when implanted subcutaneously or intramuscularly [75] improved healing of tendon lacerations in various animal models with administration of either one of these three growth factors, as evidenced by superior mechanical properties relative to untreated controls [3, 4, 23–25, 54, 71] and improved healing after tendon repair [44, 53].
Based on this preliminary work, we have evaluated the effect of recombinant human BMP-12 (rhBMP-12) in rotator cuff tendon-to-bone healing in the sheep infraspinatus repair model. The rhBMP-12 was tested using various delivery vehicles (hyaluranon paste, hyaluranon sponge, absorbable type I collagen sponge, and type I/III collagen sponge) using eight animals per treatment. The type I/III collagen sponge alone was evaluated in 10 sheep (treated control), and another 14 sheep underwent the tendon repair with no implant (untreated control). The model consisted of complete detachment and subsequent double-row suture reattachment of the infraspinatus tendon to the proximal humerus. Radiographic, mechanical testing, and biochemical evaluations were performed at 8 weeks, and ultrasound imaging was performed at 4 and 8 weeks. The rhBMP-12-treated repairs had increased load-to-failure and stiffness compared to the sponge carrier alone or untreated repairs. Repairs treated with rhBMP-12/type I collagen sponges were three times stronger (p < 0.0001) than untreated repairs after 8 weeks, while the rhBMP-12/hyaluronan sponge was 2.1 times stronger (p = 0.01) than untreated repairs. Repairs treated with rhBMP-12/type I/III collagen sponge were 2.4 times stronger (p = 0.0004) than repairs treated with this collagen sponge alone. Repairs treated with rhBMP-12 in a hyaluronan paste were similar to untreated repairs, likely due to shorter BMP-12 retention times. The stiffness for repairs treated with rhBMP-12/type I or type I/III collagen sponges were 2.1 times greater (p < 0.0003) than untreated repairs, while the stiffness for repairs treated with rhBMP-12/hyaluronan sponge were 1.7 times greater (p = 0.025) than untreated repairs. Ultrasound evaluation demonstrated a gap (10–18 mm) between the tendon and the bone in all repair groups at both time points, with no differences between groups. Histologic evaluation suggested reestablishment of collagen fiber continuity between the bone and the fibrovascular interface scar tissue, with increased glycosaminoglycan content in the rhBMP-12-treated repairs. There was a positive correlation between the glycosaminoglycan content of the repairs and the maximum load. These results suggest rhBMP-12 may play an important role in efforts to improve rotator cuff repair healing.
Literature Review
The Role of Inflammation on Tendon-Bone Healing
Using a rodent model to examine the temporal and spatial pattern of accumulation of hematopoietic lineage cells in the early phase of tendon-to-bone healing, we found neutrophils, recruited phagocytic macrophages, and resident anabolic macrophages accumulate sequentially in the healing tendon graft, with progressive cell ingrowth from the interface towards the inner tendon [37]. Neutrophils and recruited macrophages were seen in the tendon-bone interface 4 days after surgery, while resident macrophages were not present until 11 days, consistent with a proinflammatory stage in the early healing period transitioning to a proregenerative stage as healing progresses. The infiltrating macrophages likely contribute to the formation of a fibrous scar tissue interface rather than a normal insertion site by producing inflammatory cytokines.
A recent study investigated the role of proinflammatory (IL-6) and antiinflammatory (IL-4) cytokines in tendon healing by creating a full-thickness partial patellar tenotomy in IL-6 and IL-4 knockout mice [42]. The tendons in the IL-6 knockout mice displayed lower material properties when compared to control and IL-4 knockout mice 3 weeks postinjury. Although there was upregulation of the proinflammatory cytokine TNF-α, this compensation was inadequate to overcome the loss of IL-6 as both collagen fiber continuity and mechanical properties were decreased when compared to control and IL-4 knockout mice. Alternatively, at the earlier observation points, the tendons in the IL-4 knockout mice exhibited superior material properties when compared to control and IL-6 knockout mice, with improved collagen fiber organization when compared to control mice at 6 weeks postinjury. This study is meaningful because it suggests some inflammatory response is required to initiate repair of injured tendon, with an intricate interplay between proinflammatory and antiinflammatory cytokines in early tendon healing.
In a rat supraspinatus tendon overuse injury model, mRNA expression levels of inflammatory markers five-lipoxygenase-activating protein (FLAP) and COX-2 were initially increased at 3 days, decreased by 1 week, peaked at 8 weeks, and decreased by 16 weeks of overuse [52]. As for angiogenesis, VEGF (vascular endothelial growth factor) and vWF (von Willebrand factor) were maximally increased at 3 days and expressed for up to 16 weeks, indicating new blood vessel formation occurs very early in the time course of overuse injury. These results suggest the elevation of inflammatory and angiogenic markers after mechanical overload may be responsible for the eventual tendon degeneration and inferior tissue quality seen in rotator cuff tendinopathy. Nonsurgical interventions could be designed to modify this biological response in hopes of decreasing the progression of tendon degeneration.
The Role of Cytokines on Rotator Cuff Tendon-to-Bone Repair
Cytokines are active at various stages of the healing process and work with other signaling molecules. They can influence cell chemotaxis, proliferation, differentiation, and matrix synthesis by way of autocrine or paracrine signaling. In a rat model of rotator cuff repair, delivery of cells expressing PDGF-β with a polyglycolic acid scaffold showed restoration of normal crimp patterning and collagen bundle alignment compared to suture repair only [70]. Other studies support the role of cytokines in tendon repair. In a rat patellar tendon defect model, there was an increased proliferative response when PDGF-BB was supplemented on Day 3 after surgery by way of microsyringe injection, whereas supplementation on Day 7 improved peak load and pyridinoline content after administration of the highest dosage of PDGF [12]. These studies demonstrate improved healing with cytokines is dependent on the dosage, timing, and delivery vehicle used. It is unlikely any one single factor will have an important effect when used in isolation. Rather, it seems more probable numerous factors (such as an autologous bone marrow-derived cascade) will produce the desired clinical effects.
PDGF can also be isolated from autologous blood. Platelets contain several cytokines that play important roles in initiating the early healing response in bone and tendon. These cytokines include bFGF, TGF-β, IGF-1,2, VEGF, and PDGF. Using a rotator cuff defect model in rats, the addition of an exogenous fibrin clot at the defect site exhibited increased cellularity and vascularity, poor collagenous organization, and did not exhibit full defect closure even at 12 weeks [66]. By 3 weeks after injury the fibrin-clot-treated animals had inferior maximum stress and total specimen modulus with respect to the untreated defect animals. It was believed application of the exogenous clot might interfere with natural clot formation and interfere with the healing process.
Currently there are commercially-available systems to create a “platelet-rich plasma” (PRP) or “platelet gel” from autologous blood. Autologous blood is spun through a simple centrifugation process to form a dense, suturable fibrin matrix that can be easily placed directly at the tendon repair site. We are currently conducting a prospective, randomized, patient-blinded study to evaluate the effect of a platelet-rich fibrin matrix placed at the rotator cuff tendon attachment site. Tendon healing will be evaluated directly using ultrasonography to identify recurrent tears or failed healing, as well as the thickness of the tendon at the attachment site.
The Role of Bone Morphogenetic Proteins-12, -13, and -14 for Cuff Tendon-to-Bone Repair
Our findings using BMP-12 discussed above are supported by prior studies that have evaluated bone morphogenetic proteins-12, -13, and -14. Injection of recombinant adenovirus mediated-BMP-12 into a complete flexor digitorum profundus tendon laceration and repair model in chickens resulted in a two-fold increase in tensile strength and stiffness of the repaired tendons 4 weeks after surgery [44]. In a supraspinatus tendon defect model in rats, a recent study reported delivery of rhCDMP-2 (BMP-13) with a collagen sponge improved maximum loads to failure 4 and 6 weeks after surgery when compared to the untreated tendons [49]. At 6 weeks the CDMP-2-treated repairs were approximately 80% as strong as the failure strength for unoperated supraspinatus tendons.
Further support for the role of these signaling molecules comes from studies demonstrating their absence delays tendon healing. Repaired Achilles’ tendons in mice deficient in GDF-5 (BMP-14) took approximately 7 days longer to achieve similar peak cell density, glycosaminoglycan content, collagen content, collagen fibril size, and revascularization compared to phenotypically normal control littermates [13]. These histologic, biochemical, and ultrastructural findings paralleled the inferior structural properties exhibited by GDF-5-deficient Achilles’ tendons when compared to control tissue at 5 weeks after surgery. CDMP-1 (BMP-14) is localized predominantly in the torn edge and in the bursal side in human patients with full-thickness rotator cuff tears [50]. This finding suggests the cells in the torn rotator cuff are capable of producing this signaling molecule responsible for tendon formation, but questions remain regarding what role, if any, CDMP-1 plays in the healing of injured rotator cuff.
Angiogenesis in Rotator Cuff Tendon-Bone Healing
It is well-established the rotator cuff tendon is hypovascular in the “critical zone” adjacent to the distal insertion site. Recent work from our institution using contrast-enhanced power Doppler sonography allowed for evaluation of vascularity following rotator cuff repair [22]. There was increased vascularity immediately following surgery, followed by a decrease in vascularity over time. The most robust vascular flow was observed at the peritendinous region, while the lowest vascularity was at the anchor site or cancellous trough. Augmentation with biologic factors at this anchor site may improve the quality of tendon-bone healing.
With use of orthogonal polarization spectral imaging, it was possible to visualize and quantify in vivo the microcirculation of the rotator cuff during arthroscopic surgery [7]. Eleven patients with a degenerative rotator cuff lesion had less functional capillary density at the edge of the rotator cuff lesion compared to the unaffected tendon insertion zone near the greater tuberosity. These findings were corroborated with immunohistochemical staining with monoclonal antibodies for the endothelial surface marker CD31. This study provides additional evidence for the relative hypovascularity near the critical area of degenerative tendon lesions.
Several cytokines are known to have a potent angiogenic effect, including VEGF and bFGF. No studies have directly evaluated the role of these molecules in rotator cuff repair. However, several modalities that appear effective for rotator cuff repair may act via increased vascularity at the repair site. For example, a recent prospective, randomized, double-blinded, placebo-controlled clinical trial reported topical glyceryl trinitrate improved pain scores, muscle force, and activities of daily living in individuals with rotator cuff tendonitis [51]. Although the mechanism responsible for the positive clinical effect is unknown, nitric oxide (the active metabolite contained in topical glyceryl trinitrate) is a potent local vasodilator known to improve blood flow to tissues.
Physical modalities such as low-intensity pulsed ultrasound (LIPUS) and shock wave treatments affect local vascularity and may improve tendon healing. This modality augments fracture healing and bone formation, and promotes earlier bone formation and trabecular bone expansion at the patellar tendon-patella healing junction after partial patellectomy in rabbits [45, 56]. In an ovine ACL reconstruction model, this modality increased cellular activity at the tendon-bone interface, improved local vascularity and tendon-bone integration, and improved mechanical properties at the healing tendon-bone interface [73]. These studies indicate rotator cuff tendon healing may be improved by methods that increase local repair site vascularity.
The Role of Matrix Metalloproteinases in Rotator Cuff Repair
Matrix metalloproteinases (MMPs) are zinc-dependent proteases capable of degrading extracellular matrix proteins such as collagen and elastin, and MMP activity is not only integral to wound healing but also to tissue development and graft remodeling. Despite their role in connective tissue healing, there are few studies on the role of MMPs in tendon-bone healing. In a study of matrix type-1 MMP (MT1-MMP) gene deficiency, knockout mice developed severe arthropathy and the joints in these animals had “ghost cartilage” present at sites of ligament insertion into cartilage and bone [35]. MT1-MMP expression was also noted at the tendon-bone interface in 8-day-old wild-type mice. In a rotator cuff defect model in rabbits, MMP-2 was localized to the edges of the tendon defect and expressed and activated during healing, while tissue inhibitor of metalloproteinase-1 (TIMP-1) expression was induced in cells at both the tendon edges and the reparative tissue [14]. It seems MMP-2 and TIMP-1 play an important role in the remodeling of a torn supraspinatus tendon during the healing process.
The Role of Corticosteroids on Tendon-Bone Healing
Subacromial corticosteroid injections are commonly used in the initial management of rotator cuff disease. Although these injections may alleviate inflammation and provide pain relief, there are several reports of spontaneous tendon ruptures following the use of local corticosteroid injections [15, 60, 64]. In patients with ruptures of the supraspinatus tendon, the local administration of a corticosteroid relieved pain but had no effect in improving shoulder range of motion [18]. However, it is still not known what dosage and number of injections are safe in the management of rotator cuff disease, and there is much variability in patient response to corticosteroid injection. For example, a randomized, controlled clinical trial of patients with chronic cuff tendinosis or partial cuff tear reported subacromial injection of betamethasone no more effective in improving the quality of life, Neer impingement sign, and range of motion than xylocaine alone at 2, 6, 12, and 24 weeks after injection [1].
A recent in vitro study attempted to characterize the direct effects of corticosteroids on the mechanical properties and collagen crosslink amount of isolated collagen fascicles [33]. Incubating rat tail collagen fascicles in either high or low concentration of methylprednisolone acetate for 3 or 7 days reduced tensile strength by approximately 45% to 66% compared with saline-treated controls. The corticosteroid incubation did not affect the intermolecular crosslinking of the collagen fascicles, suggesting factors other than crosslinking are responsible for the observed decrease in ultimate stress to failure. This study has clinical relevance because corticosteroids may weaken the individual collagen fascicles in the injected tendon and make it more prone to rupture.
Animal models have provided insight into possible causes for poor tendon healing after local corticosteroid administration. Several mechanisms for delayed tendon healing after local steroid injection have been proposed, including inhibition of tendon cell proliferation, decreased collagen synthesis, or increased collagen breakdown [5, 38, 58]. A recent study reported dexamethasone inhibition of rat Achilles’ tendon cell migration paralleled the decreases in α-smooth muscle actin mRNA and protein expression levels, providing yet another mechanism for delayed tendon healing after steroid injection [69]. In a rat infraspinatus tendon defect model, a single dose of methylprednisolone increased the type III-to-type I collagen expression ratio by at least fourfold above the control level in animals with a tendon defect only, a tendon defect plus steroid-treatment, and an uninjured tendon plus steroid-treatment [74]. Additionally, 1 week after injection the steroid-treated animals had softer and duller tendons on gross observation, while the animals with a tendon defect or a tendon defect plus steroid treatment exhibited evidence of granulation tissue and neovascularization at the same time. It was believed a single dose of corticosteroid does not alter the acute phase response of an injured rotator cuff tendon in the rat, but the same steroid dose in uninjured rotator cuff tendon initiates a short-term alteration in collagen composition similar to that of structural injury. After local corticosteroid injections in patients, these negative effects on tenocyte proliferation, migration, and alteration of extracellular matrix protein expression may cause tendon weakening and possibly even spontaneous tendon rupture.
Effect of Anabolic-Androgenic Steroids on Tendon-Bone Healing
Anabolic-androgenic steroids (AASs) may be used by body builders, elite athletes, and recreational sports competitors to improve strength, muscle hypertrophy, increase training load capacity, and improve recovery for optimizing athletic performance. High doses and repetitive use of AASs can have various adverse effects on multiple organs and systems including, but not limited to, the cardiovascular, hepatic, dermatologic, musculoskeletal, reproductive-endocrine, and even psychiatric [46]. For example, there have been several reported cases of upper and lower extremity tendon rupture [17, 19, 43, 61, 63, 72], which may be caused by increased forces being transmitted from the hypertrophied musculature to tendon. Anabolic steroids may alter tendon crimp morphology, which can affect risk of tendon rupture [40]. On the other hand, there is a legitimate role for controlled use of AASs as an adjuvant medical therapy in the treatment of cachexia for patients with chronic disease [6]. In orthopaedics, AASs may be beneficial for fracture healing, soft tissue healing, and postoperative rehabilitation [21]. In a rabbit model AASs reduce immobilization-induced muscle atrophy [65].
Experimental animal models have been used to investigate the effects of anabolic steroids in the presence of exercise on biomechanical properties of tendon [36, 48]. These studies demonstrate anabolic steroid use during exercise produces a stiffer tendon which absorbs less energy and fails with less elongation. A recent in vivo study reported AAS treatment can impair Achilles’ tendon remodeling by down-regulating matrix metallopeptidase activity, and thus increase the potential for injury [47]. In an in vitro rotator cuff tendon model, human supraspinatus tenocytes treated with nandrolone decanoate and subjected to mechanical load had more organized actin cytoskeleton and increased collagen matrix remodeling and mechanical properties [68]. More research is required to determine the effect of AASs on tendon healing, but this was the first known study to document the acute effect of anabolic steroids on human rotator cuff tendon cells. The knowledge gained from these studies may provide insight into the biologic effects of anabolic steroids on tendon.
Pharmacologic Agents that Inhibit Rotator Cuff Tendon Healing
Further insight into the cellular events that initiate and regulate rotator cuff tendon healing and biological methods to augment such healing can also be gained from consideration of agents that have an inhibitory effect on healing. A recent study [27] reported nicotine caused a delay in tendon-to-bone healing in a rat supraspinatus tendon repair model. The mechanical properties of the healing tendon-bone interface in the nicotine group lagged behind those in the saline control group at all observation points. Maximum stress was lower in the nicotine-treated group at 10 days. Inflammation persisted longer in the nicotine group than in the saline control group. Cellular density, cellular proliferation, and type I collagen expression were lower in the nicotine group at the early time points. These biological differences were believed to at least partly explain the inferior mechanical properties in the nicotine group and the delay in scar degradation and remodeling. These findings suggest clinicians should consider asking patients who are undergoing rotator cuff repair to refrain from smoking for at least 16 consecutive weeks—8 weeks prior to and 8 weeks after surgery.
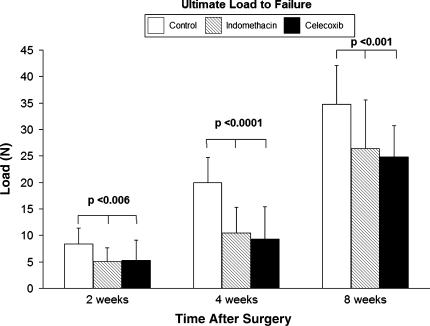
In a controlled laboratory study, nonsteroidal antiinflammatory drugs (NSAIDs) inhibited supraspinatus tendon-to-bone healing in a rat rotator cuff repair model [16]. Both indomethacin, a traditional nonselective NSAID, and celecoxib, a cyclooxygenase-2-specific NSAID, inhibited tendon-bone healing based on gross observations, histologic, and biomechanical criteria. At the later observation points, the tendon and tendon-bone interface tissue in both treatment groups were distinctly less robust and less organized compared to the control cohort, and five NSAID-treated tendons completely failed to heal to bone. When compared to nontreated controls, both NSAID-treated cohorts demonstrated a poorly organized tendon-bone interface tissue and osteoclasts persisted longer in the NSAID-treated animals. There were lower failure loads in both NSAID-treated groups compared with the control group at all observation points (Fig. 7). The inhibition in healing in both NSAID-treated groups suggests cyclooxygenase-2 plays an important role in healing, and administration of these medications may have detrimental effects on tendon-bone healing. We currently recommend patients refrain from using these medications for 6 weeks after rotator cuff repair surgery. Further studies are necessary to provide more evidence about the safe dosage and timing of NSAID use following rotator cuff repair.
Fig. 7.
Ultimate load-to-failure was lower (p < 0.001) in animals treated with NSAIDs.
Effect of Mechanical Load History on Tendon-Bone Healing
Using animal models, a number of previous studies [9, 29, 32] evaluating the effect of increased or decreased loading on healing tendons and ligaments have generally suggested the mechanical properties increase with increased loading, and decrease with decreased loading. A rotator cuff repair model in rats was used to study the effect of loading environment on the healing tendon-bone insertion site [67]. Postoperative activity level was controlled in three groups through cast immobilization, cage activity, or exercise. The investigators reported the immobilization group had increased collagen organization, an increased ratio of type I collagen to type III collagen (indicative of deceased scar), increased expression of collagen XII, collagen II, and aggrecan, and superior viscoelastic properties compared to the exercise group. These results demonstrate the tendon-bone healing tissue quality is improved with decreased loading.
It is also established that mechanical load history plays an important role in tissue organization. The organization of newly forming collagen fibrils at the microstructural level and the overall organization of fiber bundles at the macrostructural level are likely determined in large part by mechanical load history. The complex structure and organization of the tendon-bone insertion site where collagen fibers become anchored into fibrocartilage and then into bone is likely dependent upon appropriate mechanical signals; however, very little is currently known about the exact type and magnitude of load that will lead to optimal restoration of tissue organization at the healing tendon attachment site.
Excessive loading across the healing insertion site may cause microdamage at the interface, ultimately preventing collagen fiber integration with bone. It is possible tendon-bone healing may be improved by an initial period of immobilization followed by later remobilization. There is a need for an animal model that allows precise control of postoperative mechanical loading of the repaired tendon-bone interface.
Future (Speculative) Approaches to Augmentation of Rotator Cuff Tendon Healing
The Promise of Stem Cells for Rotator Cuff Repair
Bone marrow-derived mesenchymal stem cells (MSCs) could improve rotator cuff tendon-to-bone repair because of their capacity for self-renewal and totipotency. There are currently no published animal studies utilizing stem cells to augment tendon-bone healing after rotator cuff repair. However, important information can be gained from understanding the effect of mesenchymal stem cells on healing after anterior cruciate ligament (ACL) reconstruction. In a rabbit model of ACL reconstruction, tendon grafts coated with MSCs in a fibrin glue carrier demonstrated large areas of immature fibrocartilage cells at the tendon-bone junction at 2 weeks [41]. By 8 weeks the MSC-treated grafts resembled the chondral enthesis of normal ACL insertions with the presence of a mature zone of fibrocartilage blending into the adjacent bone and tendon substance rather than disorganized fibrovascular granulation tissue. This improvement in tissue quality was associated with higher failure load and stiffness in the MSC-treated grafts compared to fibrin glue-treated grafts at 8 weeks after surgery.
The Promise of Transcription Factors in Tendon-Bone Healing
Embryonic development studies have identified scleraxis as a potential marker specific for tendons and ligaments [2, 10, 57]. Scleraxis is a basic helix-loop-helix (bHLH) transcription factor that is expressed in tendons from the early progenitor stage to the formation of mature tendons in chick embryos, mouse embryos, and mouse limb tendons, suggesting scleraxis might regulate the tendon cell fate. Using the scleraxis gene as a tendon cell marker, tendon progenitors in the proximomedial limb bud mesenchyme appear induced by ectodermal signals and restricted by bone morphogenetic signaling within the mesenchyme [57]. Recent studies reported tenomodulin (a novel type II transmembrane glycoprotein) is upregulated in tenocytes during chick development by scleraxis, and the loss of tenomodulin expression in gene-targeted mice leads to reduced tenocyte density, decreased tenocyte proliferation, and altered tendon ultrastructure [20, 59]. This suggests tenomodulin promotes tendon cell proliferation and collagen fibril maturation, and may be used as a late marker of tendon formation.
Discussion
There appears strong potential for augmentation of rotator cuff tendon healing using biologic agents and methods. We have reviewed preclinical data demonstrating several different approaches that may prove valuable in improving rotator cuff tendon healing. We have explored novel concepts relating to molecular signals (gene expression) and cell differentiation at the healing tendon-bone interface. Deficiencies in the cellular and molecular aspects of healing may occur because of the presence of inflammation in the postnatal organism.
There are important limitations in knowledge that currently limit the clinical application of various biologic approaches to augment rotator cuff tendon healing. Much more information is required to understand the role of inflammatory cells and mediators in the healing process. It is likely that signals produced by inflammatory cells soon after surgical repair play an important role in the initiation and regulation of the healing process; however, further information is required to identify how these signals control healing.
One of the most important limitations in current knowledge relates to the effect of mechanical load on tendon-to-bone healing. There is currently very little information available about the effect of timing, magnitude, and type of mechanical load on tendon-to-bone healing. Development of an appropriate animal model to test the effects of mechanical load application on tendon-to-bone healing will provide insight into improving postoperative physical therapy prescription.
Cytokines clearly have the potential to improve cell proliferation and differentiation, neovascularization, and matrix synthesis at the healing tendon-bone interface. One of the principal challenges to the use of cytokines and growth factors is the identification of optimal delivery vehicles that will localize the factor to the repair site for a relevant length of time and appropriate concentration. The ideal delivery vehicle may also serve as a scaffold to support the reparative response. Gene therapy techniques hold promise for cytokine delivery, as the gene for the molecule of interest could be transfected into cells that are subsequently delivered to the repair site. Further study is required to identify the optimal dose and timing of cytokine administration. It is likely healing will be optimized by a combination of cytokines, and further study is required to identify the types and timing of cytokines that will improve tendon healing.
Stem cells also have great potential for biologic augmentation of healing. These pluripotential cells can be differentiated into relevant cell types at the repair site by stimuli present (or delivered) in the local environment. However, much basic information is still required to identify the specific signals (chemical and/or physical) that control stem cell differentiation. Further study is also required to identify the optimal method to deliver stem cells to the repair site.
In summary, there appears strong potential for novel approaches to improve the biology of rotator cuff tendon repair. Osteoinductive cytokines can improve bone formation at the healing tendon-bone interface. Bone morphogenetic protein-12 increases fibrocartilage formation at the healing tendon-bone interface, leading to improved tendon load to failure. Future approaches to improve tendon healing may include use of stem cells and transcription factors. Healing may also be improved by manipulation of factors that are known to inhibit healing, such as inflammatory cells, inflammatory mediators, and MMPs. Finally, manipulation of the mechanical load history on the healing tendon-bone interface may improve healing and decrease the rate of incomplete and failed healing. Further studies in these areas will lead to clinical application of techniques and methods to ultimately improve the outcome of repair for our patients.
Footnotes
One or more of the authors (SAR) has received funding from Wyeth Research, Inc.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Alvarez CM, Litchfield R, Jackowski D, Griffin S, Kirkley A. A prospective, double-blind, randomized clinical trial comparing subacromial injection of betamethasone and xylocaine to xylocaine alone in chronic rotator cuff tendinosis. Am J Sports Med. 2005;33:255–262. [DOI] [PubMed]
- 2.Asou Y, Nifuji A, Tsuji K, Shinomiya K, Olson EN, Koopman P, Noda M. Coordinated expression of scleraxis and Sox9 genes during embryonic development of tendons and cartilage. J Orthop Res. 2002;20:827–833. [DOI] [PubMed]
- 3.Aspenberg P, Forslund C. Enhanced tendon healing with GDF 5 and 6. Acta Orthop Scand. 1999;70:51–54. [DOI] [PubMed]
- 4.Aspenberg P, Forslund C. Bone morphogenetic proteins and tendon repair. Scand J Med Sci Sports. 2000;10:372–375. [DOI] [PubMed]
- 5.Balasubramaniam P, Prathap K. The effect of injection of hydrocortisone into rabbit calcaneal tendons. J Bone Joint Surg Br. 1972;54:729–734. [PubMed]
- 6.Basaria S, Wahlstrom JT, Dobs AS. Clinical review 138: Anabolic-androgenic steroid therapy in the treatment of chronic diseases. J Clin Endocrinol Metab. 2001;86:5108–5117. [DOI] [PubMed]
- 7.Biberthaler P, Wiedemann E, Nerlich A, Kettler M, Mussack T, Deckelmann S, Mutschler W. Microcirculation associated with degenerative rotator cuff lesions: in vivo assessment with orthogonal polarization spectral imaging during arthroscopy of the shoulder. J Bone Joint Surg Am. 2003;85:475–480. [PubMed]
- 8.Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229–1240. [DOI] [PubMed]
- 9.Bray RC, Shrive NG, Frank CB, Chimich DD. The early effects of joint immobilization on medial collateral ligament healing in an ACL-deficient knee: a gross anatomic and biomechanical investigation in the adult rabbit model. J Orthop Res. 1992;10:157–166. [DOI] [PubMed]
- 10.Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. [DOI] [PubMed]
- 11.Carpenter JE, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg. 1998;7:599–605. [DOI] [PubMed]
- 12.Chan BP, Fu SC, Qin L, Rolf C, Chan KM. Supplementation-time dependence of growth factors in promoting tendon healing. Clin Orthop Relat Res. 2006;448:240–247. [DOI] [PubMed]
- 13.Chhabra A, Tsou D, Clark RT, Gaschen V, Hunziker EB, Mikic B. GDF-5 deficiency in mice delays Achilles tendon healing. J Orthop Res. 2003;21:826–835. [DOI] [PubMed]
- 14.Choi HR, Kondo S, Hirose K, Ishiguro N, Hasegawa Y, Iwata H. Expression and enzymatic activity of MMP-2 during healing process of the acute supraspinatus tendon tear in rabbits. J Orthop Res. 2002;20:927–933. [DOI] [PubMed]
- 15.Clark SC, Jones MW, Choudhury RR, Smith E. Bilateral patellar tendon rupture secondary to repeated local steroid injections. J Accid Emerg Med. 1995;12:300–301. [DOI] [PMC free article] [PubMed]
- 16.Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34:362–369. [DOI] [PubMed]
- 17.Cope MR, Ali A, Bayliss NC. Biceps rupture in body builders: three case reports of rupture of the long head of the biceps at the tendon-labrum junction. J Shoulder Elbow Surg. 2004;13:580–582. [DOI] [PubMed]
- 18.Darlington LG, Coomes EN. The effects of local steroid injection for supraspinatus tears. Rheumatol Rehabil. 1977;16:172–179. [DOI] [PubMed]
- 19.David HG, Green JT, Grant AJ, Wilson CA. Simultaneous bilateral quadriceps rupture: a complication of anabolic steroid abuse. J Bone Joint Surg Br. 1995;77:159–160. [PubMed]
- 20.Docheva D, Hunziker EB, Fässler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25:699–705. [DOI] [PMC free article] [PubMed]
- 21.Evans NA. Current concepts in anabolic-androgenic steroids. Am J Sports Med. 2004;32:534–542. [DOI] [PubMed]
- 22.Fealy S, Adler RS, Drakos MC, Kelly AM, Allen AA, Cordasco FA, Warren RF, O’Brien SJ. Patterns of vascular and anatomical response after rotator cuff repair. Am J Sports Med. 2006;34:120–127. [DOI] [PubMed]
- 23.Forslund C, Aspenberg P. Tendon healing stimulated by injected CDMP-2. Med Sci Sports Exerc. 2001;33:685–687. [DOI] [PubMed]
- 24.Forslund C, Aspenberg P. Improved healing of transected rabbit Achilles tendon after a single injection of cartilage-derived morphogenetic protein-2. Am J Sports Med. 2003;31:555–559. [DOI] [PubMed]
- 25.Forslund C, Rueger D, Aspenberg P. A comparative dose-response study of cartilage-derived morphogenetic protein (CDMP)-1, -2, and -3 for tendon healing in rats. J Orthop Res. 2003;21:617–621. [DOI] [PubMed]
- 26.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219–224. [DOI] [PubMed]
- 27.Galatz LM, Silva MJ, Rothermich SY, Zaegel MA, Havlioglu N, Thomopoulos S. Nicotine delays tendon-to-bone healing in a rat shoulder model. J Bone Joint Surg Am. 2006;88:2027–2034. [DOI] [PubMed]
- 28.Gazielly DF, Gleyze P, Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Relat Res. 1994;304:43–53. [PubMed]
- 29.Gelberman RH, Woo SL, Lothringer K, Akeson WH, Amiel D. Effects of intermittent passive mobilization on healing canine flexor tendons. J Hand Surg Am. 1982;7:170–175. [DOI] [PubMed]
- 30.Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82:505–515. [DOI] [PubMed]
- 31.Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair. a preliminary study. J Bone Joint Surg Am. 1999;81:1281–1290. [DOI] [PubMed]
- 32.Gomez M, Woo SL, Amiel D, Harwood F, Kitabayashi L, Matyas J. The effects of increased tension on healing medial collateral ligaments. Am J Sports Med. 1991;19:347–354. [DOI] [PubMed]
- 33.Haraldsson BT, Langberg H, Aagaard P, Zuurmond AM, van El B, Degroot J, Kjaer M, Magnusson SP. Corticosteroids reduce the tensile strength of isolated collagen fascicles. Am J Sports Med. 2006;34:1992–1997. [DOI] [PubMed]
- 34.Harryman DT, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA. Repairs of the rotator cuff: correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73:982–989. [PubMed]
- 35.Holmbeck K, Bianco P, Chrysovergis K, Yamada S, Birkedal-Hansen H. MT1-MMP-dependent, apoptotic remodeling of unmineralized cartilage: a critical process in skeletal growth. J Cell Biol. 2003;163:661–671. [DOI] [PMC free article] [PubMed]
- 36.Inhofe PD, Grana WA, Egle D, Min KW, Tomasek J. The effects of anabolic steroids on rat tendon: an ultrastructural, biomechanical, and biochemical analysis. Am J Sports Med. 1995;23:227–232. [DOI] [PubMed]
- 37.Kawamura S, Ying L, Kim HJ, Dynybil C, Rodeo SA. Macrophages accumulate in the early phase of tendon-bone healing. J Orthop Res. 2005;23:1425–1432. [DOI] [PubMed]
- 38.Kennedy JC, Willis RB. The effects of local steroid injections on tendons: a biomechanical and microscopic correlation study. Am J Sports Med. 1976;4:11–21. [DOI] [PubMed]
- 39.Kobayashi M, Itoi E, Minagawa H, Miyakoshi N, Takahashi S, Tuoheti Y, Okada K, Shimada Y. Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J Shoulder Elbow Surg. 2006;15:371–377. [DOI] [PubMed]
- 40.Laseter JT, Russell JA. Anabolic steroid-induced tendon pathology: a review of the literature. Med Sci Sports Exerc. 1991;23:1–3. [PubMed]
- 41.Lim JK, Hui J, Li L, Thambyah A, Goh J, Lee EH. Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy. 2004;20:899–910. [DOI] [PubMed]
- 42.Lin TW, Cardenas L, Glaser DL, Soslowsky LJ. Tendon healing in interleukin-4 and interleukin-6 knockout mice. J Biomech. 2006;39:61–69. [DOI] [PubMed]
- 43.Liow RY, Tavares S. Bilateral rupture of the quadriceps tendon associated with anabolic steroids. Br J Sports Med. 1995;29:77–79. [DOI] [PMC free article] [PubMed]
- 44.Lou J, Tu Y, Burns M, Silva MJ, Manske P. BMP-12 gene transfer augmentation of lacerated tendon repair. J Orthop Res. 2001;19:1199–1202. [DOI] [PubMed]
- 45.Lu H, Qin L, Fok P, Cheung W, Lee K, Guo X, Wong W, Leung K. Low-intensity pulsed ultrasound accelerates bone-tendon junction healing: a partial patellectomy model in rabbits. Am J Sports Med. 2006;34:1287–1296. [DOI] [PubMed]
- 46.Maravelias C, Dona A, Stefanidou M, Spiliopoulou C. Adverse effects of anabolic steroids in athletes: a constant threat. Toxicol Lett. 2005;158:167–175. [DOI] [PubMed]
- 47.Marqueti RC, Parizotto NA, Chriguer RS, Perez SE, Selistre-de-Araujo HS. Androgenic-anabolic steroids associated with mechanical loading inhibit matrix metallopeptidase activity and affect the remodeling of the achilles tendon in rats. Am J Sports Med. 2006;34:1274–1280. [DOI] [PubMed]
- 48.Miles JW, Grana WA, Egle D, Min KW, Chitwood J. The effect of anabolic steroids on the biomechanical and histological properties of rat tendon. J Bone Joint Surg Am. 1992;74:411–422. [PubMed]
- 49.Murray DH, Kubiak EN, Jazrawi LM, Araghi A, Kummer F, Loebenberg MI, Zuckerman JD. The effect of cartilage-derived morphogenetic protein 2 on initial healing of a rotator cuff defect in a rat model. J Shoulder Elbow Surg. 2007;16:251–254. [DOI] [PubMed]
- 50.Nakase T, Sugamoto K, Miyamoto T, Tsumaki N, Luyten FP, Inui H, Myoui A, Tomita T, Yoshikawa H. Activation of cartilage-derived morphogenetic protein-1 in torn rotator cuff. Clin Orthop Relat Res. 2002;399:140–145. [DOI] [PubMed]
- 51.Paoloni JA, Appleyard RC, Nelson J, Murrell GA. Topical glyceryl trinitrate application in the treatment of chronic supraspinatus tendinopathy: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2005;33:806–813. [DOI] [PubMed]
- 52.Perry SM, McIlhenny SE, Hoffman MC, Soslowsky LJ. Inflammatory and angiogenic mRNA levels are altered in a supraspinatus tendon overuse animal model. J Shoulder Elbow Surg. 2005;14:S79–S83. [DOI] [PubMed]
- 53.Rickert M, Jung M, Adiyaman M, Richter W, Simank HG. A growth and differentiation factor-5 (GDF-5) coated suture stimulates tendon healing in an Achilles tendon model in rats. Growth Factors. 2001;19:115–126. [DOI] [PubMed]
- 54.Rickert M, Wang H, Wieloch P, Lorenz H, Steck E, Sabo D, Richter W. Adenovirus-mediated gene transfer of growth and differentiation factor-5 into tenocytes and the healing rat achilles tendon. Connect Tiss Res. 2005;46:175–183. [DOI] [PubMed]
- 55.Rodeo SA, Potter HG, Kawamura S, Turner AS, Kim HJ, Atkinson BL. Biologic augmentation of rotator cuff tendon healing using a mixture of osteoinductive growth factors: an experimental study in sheep. J Bone Joint Surg Am. 2007;89:2485–2497. [DOI] [PubMed]
- 56.Rubin C, Bolander M, Ryaby JP, Hadjiargyrou M. The use of low-intensity ultrasound to accelerate the healing of fractures. J Bone Joint Surg Am. 2001;83:259–270. [DOI] [PubMed]
- 57.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. [DOI] [PubMed]
- 58.Scutt N, Rolf CG, Scutt A. Glucocorticoids inhibit tenocyte proliferation and tendon progenitor cell recruitment. J Orthop Res. 2006;24:173–182. [DOI] [PubMed]
- 59.Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol. 2006;298:234–247. [DOI] [PubMed]
- 60.Smith AG, Kosygan K, Williams H, Newman RJ. Common extensor tendon rupture following corticosteroid injection for lateral tendinosis of the elbow. Br J Sports Med. 1999;33:423–424. [DOI] [PMC free article] [PubMed]
- 61.Sollender JL, Rayan GM, Barden GA. Triceps tendon rupture in weight lifters. J Shoulder Elbow Surg. 1998;7:151–153. [DOI] [PubMed]
- 62.Spindler KP, Dawson JM, Stahlman GC, Davidson JM, Nanney LB. The biomechanical response to doses of TGF-beta 2 in the healing rabbit medial collateral ligament. J Orthop Res. 2003;21:245–249. [DOI] [PubMed]
- 63.Stannard JP, Bucknell AL. Rupture of the triceps tendon associated with steroid injections. Am J Sports Med. 1993;21:482–485. [DOI] [PubMed]
- 64.Sweetnam R. Corticosteroid arthropathy and tendon rupture. J Bone Joint Surg Br. 1969;51:397–398. [PubMed]
- 65.Taylor DC, Brooks DE, Ryan JB. Anabolic-androgenic steroid administration causes hypertrophy of immobilized and nonimmobilized skeletal muscle in a sedentary rabbit model. Am J Sports Med. 1999;27:718–727. [DOI] [PubMed]
- 66.Thomopoulos S, Soslowsky LJ, Flanagan CL, Tun S, Keefer CC, Mastaw J, Carpenter JE. The effect of fibrin clot on healing rat supraspinatus tendon defects. J Shoulder Elbow Surg. 2002;11:239–247. [DOI] [PubMed]
- 67.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125:106–113. [DOI] [PubMed]
- 68.Triantafillopoulos IK, Banes AJ, Bowman KF Jr, Maloney M, Garrett WE Jr, Karas SG. Nandrolone decanoate and load increase remodeling and strength in human supraspinatus bioartificial tendons. Am J Sports Med. 2004;32:934–943. [DOI] [PubMed]
- 69.Tsai WC, Tang FT, Wong MK, Pang JH. Inhibition of tendon cell migration by dexamethasone is correlated with reduced alpha-smooth muscle actin gene expression: a potential mechanism of delayed tendon healing. J Orthop Res. 2003;21:265–271. [DOI] [PubMed]
- 70.Uggen JC, Dines J, Uggen CW, Mason JS, Razzano P, Dines D, Grande DA. Tendon gene therapy modulates the local repair environment in the shoulder. J Am Osteopath Assoc. 2005;105:20–21. [PubMed]
- 71.Virchenko O, Fahlgren A, Skoglund B, Aspenberg P. CDMP-2 injection improves early tendon healing in a rabbit model for surgical repair. Scand J Med Sci Sports. 2005;15:260–264. [DOI] [PubMed]
- 72.Visuri T, Lindholm H. Bilateral distal biceps tendon avulsions with use of anabolic steroids. Med Sci Sports Exerc. 1994;26:941–944. [PubMed]
- 73.Walsh WR, Stephens P, Vizesi F, Bruce W, Huckle J, Yu Y. Effects of low-intensity pulsed ultrasound on tendon-bone healing in an intra-articular sheep knee model. Arthroscopy. 2007;23:197–204. [DOI] [PubMed]
- 74.Wei AS, Callaci JJ, Juknelis D, Marra G, Tonino P, Freedman KB, Wezeman FH. The effect of corticosteroid on collagen expression in injured rotator cuff tendon. J Bone Joint Surg Am. 2006;88:1331–1338. [DOI] [PMC free article] [PubMed]
- 75.Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N, Dube JL, DiBlasio-Smith E, Nove J, Song JJ, Wozney JM, Rosen V. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321–330. [DOI] [PMC free article] [PubMed]