Abstract
Tendinopathy is a common clinical problem with athletes and in many occupational settings. Tendinopathy can occur in any tendon, often near its insertion or enthesis where there is an area of stress concentration, and is directly related to the volume of repetitive load to which the tendon is exposed. Recent studies indicate tendinopathy is more likely to occur in situations that increase the “dose” of load to the tendon enthesis – including increased activity, weight, advancing age, and genetic factors. The cells in tendinopathic tendon are rounder, more numerous, and show evidence of oxidative damage and more apoptosis. These cells also produce a matrix that is thicker and weaker with more water, more immature and cartilage-like matrix proteins, and less organization. There is now evidence of a population of regenerating stem cells within tendon. These studies suggest prevention of tendinopathy should be directed at reducing the volume of repetitive loads to below that which induces oxidative-induced apoptosis and cartilage-like genes. The management strategies might involve agents or cells that induce tendon stem cell proliferation, repair and restoration of matrix integrity.
Introduction
Tendons are specialized tissues that connect muscle to bone and transmit the forces generated by muscle to bone, resulting in joint movement. Tendon injuries are common and affect a substantial portion of recreational and professional athletes and those in many occupations involving repetitive work [16, 37, 60, 79, 102]. Tendinopathy (often called tendinitis or tendinosis) is the most common tendon disorder [86, 99]. It is characterized by activity-related pain, focal tendon tenderness, and decreased strength and movement in the affected area. The histological features of tendinopathy are further described in the current study. Tendinopathy can occur in almost any tendon. Common examples include plantar fasciitis, Achilles tendinitis, patellar tendinitis, tennis elbow, golfer’s elbow, and supraspinatus tendinitis. Tendinopathy is poorly understood and has many described remedies with very little evidence to support their efficacy. One of the reasons there are very few, if any, good treatments for tendinopathy is lack of knowledge regarding its pathogenesis.
We summarize recent cellular and molecular findings in tendinopathy to identify potential preventative and treatment strategies and specific areas needing further investigation.
Search Strategies and Criteria
We performed a systematic review of peer-reviewed, original English language papers published on the etiology, histopathology and molecular biology/pathology of tendinopathy using Ovid MEDLINE and PubMed database from 1950 to November 2007. Keywords used in the search were: tendinopathy; pathogenesis; tendon cells; extracellular matrix; proteoglycan; metalloproteinases. Subheadings used in the search were: etiology; pathology; chemistry; metabolism. This analysis revealed 441 papers (432 papers from 1997–2007), of which 86 met our selection criteria. From this analysis we came to the following conclusions:
Common Intrinsic and Extrinsic Associations
Tendinopathy has an increased incidence with age and the male gender [11, 89] and with obesity [36, 47]. Excessive long-distance running, intensity, and hill work are risk factors for acute Achilles tendinopathy [60, 70, 95]; distance and excessive time spent swimming are associated with supraspinatus tendinopathy [98]. There is also an association between tendinopathy and hormone replacement therapy and oral contraceptives in women [47].
Genetic Factors
The siblings of patients with full-thickness rotator cuff tears have twice the risk of developing full-thickness tears, and nearly five times the risk of experiencing symptomatic full-thickness tears [44]. Earlier studies have reported an association between blood type O and the incidence of tendon injuries [51, 52, 59]. Recent genomic studies suggest people who have variants of the tenascin-C gene with 12 and 14 guanine-thymine repeats, or people who have COL5A1 BstUI restriction fragment length polymorphisms (RFLPs) are more likely to develop chronic Achilles tendinopathy than those who do not have those polymorphisms [73, 74]. These findings suggest there is a genetic predisposition to the development of tendinopathy. However, no specific causative gene has been linked to tendinopathy, suggesting that tendinopathy may be polygenic and may involve complex interaction between multiple genes.
Histopathological Changes
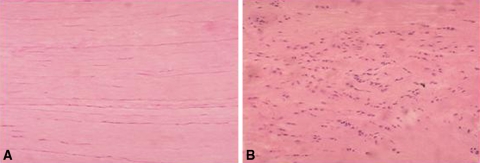
The histopathologic changes in tendinopathy are well-established (Table 1) (Fig. 1) [101]. Normal tendon is brilliant white in color and has a firm fibroelastic texture. In contrast, tendinopathic tendon is grey or brown, and is soft, thin, and fragile [57]. Microscopically, the collagen bundles are disorganized, there is increased ground substance, and the nuclei are darkly stained and round and found in increased numbers. This contrasts to the well organized parallel collagen bundles found in normal tendon with spindle-shaped tenocyte nuclei arranged in parallel alignment [46, 54, 68, 89]. At the electron-microscopic level the collagen fibers in tendinopathic tendon are angulated, vary in diameter and orientation, and have bubbles. There are changes consistent with hypoxia, including lipid vacuoles, enlarged lysosomes, and degranulated endoplasmic retinaculum [54]. Rarely have inflammatory cells been identified in tendinopathic tendon [12, 46, 54, 89]. Tendinopathic tendon often has infiltrations of vascular and small blood vessels [4, 46, 68, 85], an upregulation of vascular endothelial growth factor (VEGF) [80, 84], and ingrowths of small nerves [4, 64, 93]. These pathologic changes are consistent with “degeneration” and attempts of “regeneration”.
Table 1.
Comparison of normal and tendinopathic tendon by macroscopic, light and electron microscopic and ultrasound findings [3, 4, 17, 28, 46, 54, 64, 68, 85, 89]
| Findings | Macroscopic | Grey-scale or color and power doppler ultrasound | Light microscopic | Light microscopic |
|---|---|---|---|---|
| Normal tendon | • Brilliant white• Fibroelastic firm texture | • Parallel hyperechoic or bright white line• Regular uniform fiber structure | • Organized parallel collagen bundles• Spindle shape tenocyte nuclei• Nuclei parallel alignment | • Densely packed collagen fibers• Uniform in diameter and orientation of collagen fibers |
| Tendinopathy tendon | • Grey or brown • Tissue is thin, fragile and disorganized• Loose texture | • Localized widening of the tendon• Local hypoechoic areas• Irregular fiber structure• Neovascularization correlated with tendon changes | • Disorganized collagen bundle• Increased ground substance consisting of proteoglycans and glycosaminoglycans (GAG)• Large mucoid patches and vacuoles between fibers• Round with darker-staining tenocyte nuclei• Markedly increase number of nuclei with loss of parallel alignment• Increase of vascular and nerve ingrowths | • Angulation, bubble formation of collagen fibers• Variations in the diameters and orientation of collagen fibers• Hypoxic changes in tenocytes (lipid vacuoles, enlarged lysosomes and degranulated endoplasmic retinaculum) |
Fig. 1A–B.
The normal tendon and tendinopathy tendon is shown. (A) Normal tendon has parallel, longitudinal architecture with scattered elongated tenocytes. (B) Tendinopathy tendon shows disorganized collagen architecture, high cellularity of rounded tenocytes. (This figure was published in Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, Carpenter JE. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84, © Elsevier 2000, with permission.)
Immunohistochemical studies demonstrate the number of substance P (SP)-positive nerve fibers are higher in painful tendinopathic samples compared to normal tendon samples [64, 93]. Microdialysis demonstrate higher neurotransmitter glutamate levels in chronic painful Achilles and patellar tendinosis compared with pain-free normal control tendons [2]. Microarray studies also report an increased mRNA of glutamate in healing rat Achilles tendon [76] and tendinopathic supraspinatus in rats [75]. The presence of neovascularization and innervation, and the increase of neurotransmitter in tendinopathy may be part of the reason tendinopathy patients often have chronic pain [1, 3, 82]. Danielson et al. [32, 33] recently reported the tenocytes in tendinosis patellar tendon exhibited more immunoreactions for adrenergic receptors and catecholamine. These findings are of relevance as studies have demonstrated stimulation of adrenergic receptors can lead to cell proliferation and/or cell degeneration and apoptosis [6, 23].
Apoptosis
We found an increased amount of apoptosis or programmed cell death in degenerative tendon: there are twice as many apoptotic cells in ruptured supraspinatus tendon as in normal subscapularis tendon [117]. Lian et al. [65] reported an increased apoptosis in patellar tendinopathy in athletes and also showed apoptosis could be induced by high-strain mechanical loading in a rat tibialis anterior tendon model [97]. Activation of c-Jun N-terminal kinase (JNK) [111] and increase of cytochrome c-related activation of caspase-3 [118] might be two potential pathways for the induction of apoptosis in tendinopathy. These two pathways are associated with oxidative stress. Oxidative stress can be induced during high dose cyclic strain in human and animal tendon cells in vitro and in ex vivo [10, 97, 100]. Arnoczky et al. [10] reported cyclical strain in cultured canine flexor tendon cells induces stress activated protein kinase, which in turn can induce apoptosis [100]. In a running rat supraspinatus tendon model, we also have found overuse induces upregulation of stress-related genes such as flice inhibitory protein (FLIP), heat shock protein 27 (HSP27) and testis heat shock-related protein 70 (HST70) [75].
Tendon Cells
Fibroblast-like cells are the major cell type in tendons, and have been histologically classified as elongated tenocytes or ovoid tenoblasts [25, 53]. These cells are important for maintenance of healthy tendon as they can proliferate, produce collagen and maintain the appropriate extracellular matrix [19, 53]. Ovoid tenoblasts – often described as an immature or activated form of tenocytes – have a higher proliferation index and apoptosis index than those of elongated tenocytes [25]. There is evidence that changes occur in the cells (tenocytes appear rounder, proliferate, become necrotic or apoptotic, and have an increased expression of local insulin-like growth factor-1 (IGF-1)) before the overt development of tendinopathy [8, 28, 96]. Other cell types, such as synovial-like cells, smooth muscle cell and endothelial cells, can be found in the endotenon and epitenon of tendon.
Transformation of tendon cells towards a fibrochondrogenic phenotype has been observed in torn rotator cuff tendons [46], and Archambault et al. [7] reported tendinopathic rat supraspinatus tendon had increased expression of cartilage genes such as col2a1, aggrecan, and sox9. Tenocytes from the site of tendinopathy also produce abnormal amounts of collagen III, commonly associated with wound healing, even when the repetitive motion is no longer present [69].
Using a combination of cell markers and flow cytometry analysis, Bi and colleagues [18] identified a unique cell population termed tendon stem/progenitor cells (TSPCs) in human and mouse tendons with several universal stem cell characteristics including clonogenicity (the ability to form clones), multipotency (multidifferentiation potential towards osteogenesis, adipogenesis, and chondrogenesis) and self-renewal capacity (higher doubling capacity than bone marrow stromal cells [BMSCs] from same sources). Moreover, the isolated human or mouse TSPCs could regenerate tendon-like tissues with either HA/TCP or Matrigel in vitro, whereas mouse dermal fibroblasts transplanted with Matrigel did not form any tissue. When transplanted with HA/TCP onto the surface of mouse calvaria, human TSPCs formed condensed collagen fibers inserted into the bone which were similar to Sharpey’s fibers [18]. Terminal differentiation of single cells selected from a group of equivalent precursors may be random, or may be regulated by external environment/signals. Some specific microenvironments/additional signals are essential for tenocyte differentiation and proliferation [94, 114]. A recent report suggests an extracellular matrix (ECM)-rich niche, organized in part by biglycan (Bgn) and fibromodulin (Fmod), controls the self-renewal and differentiation of TSPCs [18]. Depletion of Bgn and Fmod in mice leads to decreased expression of the tendon marker scleraxis (Scx) and of Type I collagen, and increased sensitivity to BMP2 in TSPCs when compared to cells from wild-type mice [18]. BMP12 acts as signaling molecules during embryonic tendon/ligament formation in animal experiments [67, 112, 114] and can stimulate patellar tendon fibroblasts proliferation in humans [40]. Expression of Scx, a member of the basic helix-loop-helix (bHLH) superfamily of transcription factors in the progenitors and cells of all tendon tissues [94], is crucial for differentiation of all force-transmitting and intermuscular tendons in Scx−/− mice [78] and for activation of the COL1a1 gene in mouse tendon fibroblasts in an in vitro study [63]. These studies indicate the genes that coordinate with the matrix regeneration are also important for regenerating TSPC’s.
Extracellular Matrix (ECM)
The extracellular matrix (ECM) is a complex structural entity surrounding and supporting cells. The ECM is composed of three major classes of biomolecules: structural proteins (collagen and elastin), specialized proteins (eg. fibrillin and fibronectin), and proteoglycans [21]. The interaction between tendon cells and EMC is bidirectional: alteration of EMC may be initiated by tendon cells [8, 28], and changes of EMC microenvironment may also lead to cell proliferation, migration, apoptosis, and morphogenesis [108]. The maintenance of the tendon matrix has important consequences for the ability of the tendon to resist mechanical forces and to repair response to injury [58]. Some authors suggest an imbalance in the synthesis and degradation of ECM leads to structural deterioration and degeneration of the tendon [9, 20, 48].
Collagen is the predominant constituent of tendon. There are 27 different collagen molecules identified to date [87]. Although Type I collagen accounts for approximately 65% to 80% of the dry mass of the tendon and represents almost 95% of the total collagen in a normal tendon [53], other collagens including collagen types II, III, IV, V, VI, IX, X, XII, and XIV have also been found in small quantities within tendon [86, 107, 110]. Changes in the collagen content and composition have consistently been found in tendinopathy (Table 2). These changes include: (1) a reduction in the total collagen content, an increase of proportion of types I, III, and V collagen, and an increase of ratio of Type III to Type I collagen; (2) a higher percentage of denatured collagen; and (3) a lower ratio of pentosidine and higher ratios of hydroxylated lysine residues in collagen crosslinks.
Table 2.
Changes of extracellular matrix in tendinopathy
| ECM | Tendinopathy | Species | Tendon | Reference | |
|---|---|---|---|---|---|
| Degenerate tendon | Ruptured tendon | ||||
| Collagen | |||||
| Total | Decrease (protein) | Human | Supraspinatus, subscapularis | Bank et al. [15], Riley et al. [92] | |
| Type I | Increase (mRNA) | Human | Achilles | de Mos et al. [34], Ireland et al. [48] | |
| Decrease (protein) | Human | Posterior tibial | Goncalves-Neto et al. [42] | ||
| Type III | Increase (mRNA) | Human | Achilles | de Mos et al. [34], Ireland et al. [48] | |
| Increase (protein) | Human | Supraspinatus, subscapularis, posterior tibial | Goncalves-Neto et al. [42], Riley et al. [92] | ||
| Increase (protein) | Equine | Superficial digital flexor | Birch et al. [20] | ||
| Increase (protein) | Human | Achilles | Eriksen et al. [35] | ||
| Type III/Type I | Increase (mRNA) | Human | Achilles | Ireland et al. [48] | |
| Increase (protein) | Equine | Superficial digital flexor | Birch et al. [20] | ||
| Type V | Increase (protein) | Human | Posterior tibial | Goncalves-Neto et al. [42] | |
| Denatured collagen* | Increase (protein) | Increase (protein) | Human | Achilles, Supraspinatus | de Mos et al. [34], Riley et al. [88] |
| Crosslinks/CTH | |||||
| Pentosidine | Decrease (protein) | Human | Achilles | de Mos et al. [34] | |
| Hydroxylysine | Increase (protein) | Human | Achilles | de Mos et al. [34] | |
| Increase (protein) | Human | Supraspinatus | Bank et al. [15] | ||
| HP | No change (mRNA) | Human | Achilles | de Mos et al. [34] | |
| Increase (protein) | Human | Supraspinatus | Bank et al. [15] | ||
| LP | No change | Human | Achilles | de Mos et al. [34] | |
| Increase (protein) | Human | Supraspinatus | Bank et al. [15] | ||
| Proteoglycans | |||||
| Versican | Decrease (mRNA) | Decrease (mRNA) | Human | Achilles | Corps et al. [30] |
| No change (mRNA) | No change (mRNA) | Human | Achilles | Corps et al. [29] | |
| V0 | No change (mRNA) | No change (mRNA) | Human | Achilles | Corps et al. [30] |
| V1 | Decrease (mRNA) | Decrease (mRNA) | Human | Achilles | Corps et al. [30] |
| V2 | Decrease (mRNA) | Decrease (mRNA) | Human | Achilles | Corps et al. [30] |
| V3 | Decrease (mRNA) | Decrease (mRNA) | Human | Achilles | Corps et al. [30] |
| Aggrecan | Increase (mRNA) | No change (mRNA) | Human | Achilles | Corps et al. [29] |
| Increase (mRNA) | Rat | Supraspinatus | Archambault et al. [7] | ||
| Biglycan | Increase (mRNA) | No change (mRNA) | Human | Achilles | Corps et al. [29] |
| Decorin | No change (mRNA) | Decrease (mRNA) | Human | Achilles | Corps et al. [29] |
| GAG (staining) | Increase (protein) | Human | Achilles | Maffulli et al. [68] | |
| Sulphated GAG | Increase (protein) | Human | Patellar | Fu et al. [39] | |
| Noncollagen glycoproteins | |||||
| Fibronectin | Increase (protein) | Human | Supraspinatus; Achilles | Lehto et al. [62], Tillander et al. [105] | |
| Tenascin (300-kd isoform) | Increase (protein) | Increase (protein) | Human | Supraspinatus | Riley et al. [90] |
* Using selective proteolysis of denatured collagen by alpha-chymotrypsin as described by Bank et al. [14].
CTH = collagen triple helix; HP = hydroxylysylpyridinoline; LP = lysylpyridinoline; GAG = glycosaminoglycan; V0-3 = Versican splice variants 0–3.
Proteoglycans are protein/polysaccharide complexes in the ECM that trap water and affect the viscoelastic properties of the tissue, helping the tissue resist compressive forces [115]. Proteoglycans consist of a protein core with attached glycosaminoglycans (GAGs). Tendon contains a wide variety of proteoglycans, including the large aggregating proteoglycans and a variety of small leucine-rich proteoglycans (SLRPs) [116]. Proteoglycans and their constituent GAGs can influence many important physiological processes in tendon, including ion transport, water retention, the diffusion of nutrients, mediating cell-matrix interactions, resistance of compression and sequestration of growth factors and enzymes in the matrix [43, 116]. Animal studies demonstrate biglycan may serve both a structural [109] and a signaling role [77] in developing tendon and biglycan and collagen VI are coexpressed in tendon development [61]. It is possible the presence of tendon fibrocartilage proteoglycan/glycosaminoglycan in normal supraspinatus is part of a normal functional adaptation to mechanical forces in tendon [91].
Studies from gene-knockout mice demonstrate targeted deletion of decorin, fibromodulin, or lumican or deletion of both lumican and fibromodulin cause abnormal collagen fibril and fibril bundle morphology [24, 31, 72], indicating these proteoglycans play an important role in regulation of collagen fibril formation and maturation. Although increases of aggrecan and biglycan in tendons have been implicated in early tendon healing process [104], the changes of other type of proteoglycans in tendinopathy are different to those in normal and healing tendons (Table 2). Very little is known about the changes in noncollagen glycoproteins in tendinopathy. Fibronectin and tenascin-C are key factors in the tendon repairing process by promoting fibroblast migration, and adhesion of fibroblasts to fibrin [41, 106]. In addition to the genomic study [73] showing variants of the tenascin-C gene are associated with Achilles tendon injury, a persistent increase in expression of fibronectin and tenascin-C has been reported in tendinopathy (Table 2) and may contribute to the pathogenic matrix remodeling in tendinopathy [90, 105].
Metalloproteinases and their Inhibitors
Metalloproteases (MMPs) are a large family of enzymes that degrade all tendon matrix components, and these enzymes and their inhibitors play a major role in the degradation of matrix during development, adaptation, and repair [87, 108]. In addition to their important role in normal physiological events in tendon homeostasis and repair [108], these enzymes may be key effectors of the pathological processes in tendon disease [26].
Arnoczky et al. demonstrated MMP inhibitors can prevent the activation of MMP-13 and inhibit pericellular matrix degeneration and the loss of material properties associated with stress deprivation in an in vitro study [9]. They reported increases in the activity and mRNA expression of MMPs-1, -9, -11, and -13 in tendinopathy (Table 3). Elevation of MMP-3 is associated with tendon remodeling and repair in normal and injured tendons [19, 48, 88]. Decreases of MMP-3 activity and mRNA expression were found in supraspinatus and Achilles tendinopathy (Table 3).
Table 3.
Changes of matrix metalloproteases and their inhibitors in tendinopathy
| Name | Tendinopathy | Species | Tendon | Reference | |
|---|---|---|---|---|---|
| Degenerate tendon | Ruptured tendon | ||||
| MMP-1 | Increase (activity) | Increase (activity & mRNA) | Human | Supraspinatus, Achilles, P\patellar | Fu et al. [38], Jones et al. [49], Riley et al. [88] |
| MMP-2 | Increase (activity & mRNA) | Reduce (activity) | Human | Supraspinatus, Achilles | de Mos et al. [34], Riley et al. [88] |
| MMP-3 | Decrease (mRNA) | Decrease (activity & mRNA) | Human | Supraspinatus, rotator cuff, Achilles | Jones et al. [49], Lo et al. [66], Riley et al. [88] |
| Increase (activity) | Decrease (mRNA) | Human | Achilles | de Mos et al. [34] | |
| MMP-7 | Decrease (mRNA) | Human | Achilles | Jones et al. [49] | |
| MMP-9 | Increase (activity & mRNA) | Increase (mRNA) | Human | Achilles | de Mos et al. [34], Jones et al. [49] |
| MMP-10 | Decrease (mRNA) | Human | Achilles | Jones et al. [49] | |
| MMP-11 | Increase (mRNA) | Increase (mRNA) | Human | Achilles | Jones et al. [49] |
| MMP-12 | Decrease (mRNA) | Human | Achilles | Jones et al. [49] | |
| MMP-13 | Increase (activity & mRNA) | Increase (activity & mRNA) | Human | Achilles, rotator cuff | de Mos et al. [34], Lo et al. [66] |
| MMP-14 | Increase (mRNA) | Human | Achilles | Jones et al. [49] | |
| MMP-16 | Increase (mRNA) | Human | Achilles | Jones et al. [49] | |
| MMP-17 | Increase (mRNA) | Human | Achilles | Jones et al. [49] | |
| MMP-19 | Increase (mRNA) | Human | Achilles | Jones et al. [49] | |
| MMP-23 | Increase (mRNA) | Human | Achilles | Jones et al. [49] | |
| MMP-24 | Decrease (mRNA) | Human | Achilles | Jones et al. [49] | |
| MMP-25 | Increase (mRNA) | Human | Achilles | Jones et al. [49] | |
| MMP-27 | Decrease (mRNA) | Human | Achilles | Jones et al. [49] | |
| MMP-28 | Decrease (mRNA) | Human | Achilles | Jones et al. [49] | |
| ADAM-8 | Increase (mRNA) | Human | Achilles | Jones et al. [49] | |
| ADAM-12 | Increase (mRNA) | Increase (mRNA) | Human | Achilles | Jones et al. [49] |
| ADAMTS-2 | Increase (mRNA) | Human | Achilles | Jones et al. [49] | |
| ADAMTS-3 | Increase (mRNA) | Human | Achilles | Jones et al. [49] | |
| ADAMTS-4 | Increase (mRNA) | Human | Achilles | Jones et al. [49] | |
| ADAMTS-5 | Decrease (mRNA) | Human | Achilles | Jones et al. [49] | |
| ADAMTS-7 | Decrease (mRNA) | Human | Achilles | Jones et al. [49] | |
| ADAMTS-13 | Decrease (mRNA) | Human | Achilles | Jones et al. [49] | |
| TIMP-1 | Decrease (protein) | Increase (mRNA) | Human | Achilles, patellar | Fu et al. [38], Jones et al. [49] |
| TIMP-2 | Decrease (mRNA) | Human | Rotator cuff, Achilles | Jones et al. [49], Lo et al. [66] | |
| TIMP-3 | Decrease (mRNA) | Decrease (mRNA) | Human | Rotator cuff, Achilles | Jones et al. [49], Lo et al. [66] |
| TIMP-4 | Decrease (mRNA) | Human | Rotator cuff, Achilles | Jones et al. [49], Lo et al. [66] | |
MMP = matrix metalloproteinase; ADAM = a disintegrin and metalloproteinase; ADAMTS = a disintegrin and metalloprotease with thrombospondin-like repeat; TIMP = tissue inhibitors of metalloproteinase.
A disintegrin and metalloproteinase (ADAM) is a transmembrane protein that contains a disintegrin and metalloprotease domain and, therefore, it potentially has both cell adhesion and protease activities [83]. A novel family of extracellular proteases, a disintegrin-like and metalloprotease with thrombospondin motifs (ADAMTS), apparently plays an important role in proteoglycan turnover in tendon [50, 103]. Nineteen ADAMTSs have been identified so far, but many of them remain to be fully characterized [50]. ADAMTS-2, -3, and -14 function as key regulators of collagen fibril assembly [27]. ADAMTS-1 and -4 are capable of cleaving certain matrix proteoglycans such as versican, brevican, and aggrecan [81, 113]. ADAMTS-4 also cleaves nonproteoglycan ECM components such as fibromodulin and decorin [55]. However, there is very little knowledge about the roles of ADAMs and ADAMTS played in tendinopathy (Table 3).
The activities of MMPs are normally tightly controlled in vivo, with regulation at the levels of transcription, translation, activation, and inhibition [87]. The activities of MMPs are inhibited by a family of tissue inhibitors, the tissue inhibitors of metalloproteinases (TIMPs). This family contains four human gene products, namely TIMP1, TIMP2, TIMP3, and TIMP4 [71]. All TIMP members inhibit MMP members to varying degrees [13], however, only TIMP-3 is a potent inhibitor of members of ADAM and ADAMTS [5, 45, 56]. The activity of MMPs is inhibited reversibly by TIMPs in a noncovalent fashion in a 1:1 stoichiometry [22]. Decreases in TIMP-2, -3, and -4 are consistently found in tendinopathy (Table 3). MMP inhibitors prevent the activation of MMP-13 and inhibit pericellular matrix degeneration and the loss of material properties in stress-deprived tendons in vitro [9].
The data suggest the balance between metalloproteinases and their inhibitors is likely essential in the maintenance of tendon ECM homeostasis, and an imbalance may result in uncontrolled tendon damage.
Discussion
The aim of this review was to identify recent advances in the understanding of tendinopathy, particularly from a cell and molecular biology perspective. There has been much new information and there are many gaps in our understanding of the pathogenesis of tendinopathy. These gaps are most glaring from a timeline perspective, or the sequence of events. The research uses small windows to look into a complex event that changes in location and time.
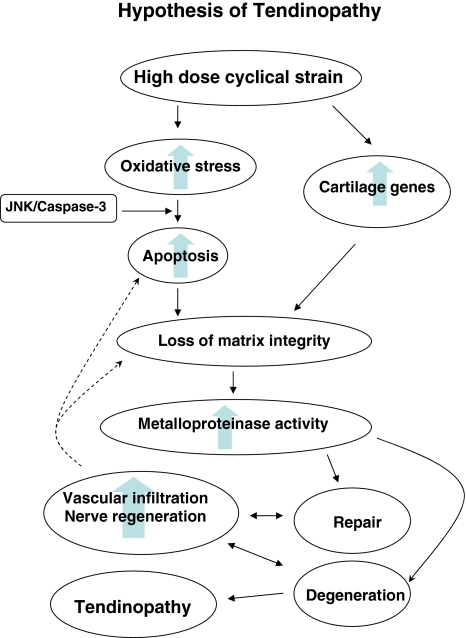
Our current hypothesis is that tendinopathy is induced when tendon cells experience a large volume of repetitive load (Fig. 2). Tendons of certain anatomical locations are more susceptible as are individuals who are older, heavier and male in those genetically predisposed to tendinopathy. There is debate as to whether a loss of integrity of the matrix, or the cells in the tendon matrix, initiate the changes in tendinopathy. Given the high incidence about (70%) of tendinopathy in the shoulders of “elite” asymptomatic swimmers [98] we favor the later. High doses of cyclical strain induce genes for two major pathways (Fig. 2): (1) oxidative stress – apoptosis; and (2) cartilage-like genes.
Fig. 2.
A conceptual diagram shows our current hypothesis on the pathogenesis of tendinopathy.
The interaction between these two pathways is undetermined. Once tendinopathy is initiated the tendon cells become rounded and apoptotic and produce a matrix that contains less Type I collagen and is more cartilaginous and “immature” in nature. Once the normal cell – matrix complex is disrupted, “relative” stress deprivation is induced [8, 9] and metalloproteinase matrix destruction is initiated. The enthesis becomes more painful, more vascular and mechanically inferior to normal tendon. The histologic data suggest attempts at repair with vascular and neuronal infiltration occur and that if the matrix is not adequately repaired the adrenergic responses associated with this process may lead to persistent pain and/or complex regional pain syndrome. The new information on tendon stem cells is important as it implies these cells, if induced and directed to the correct location, can reverse the degenerative process.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Alfredson H. The chronic painful Achilles and patellar tendon: research on basic biology and treatment. Scand J Med Sci Sports. 2005;15:252–259. [DOI] [PubMed]
- 2.Alfredson H, Lorentzon R. Chronic tendon pain: no signs of chemical inflammation but high concentrations of the neurotransmitter glutamate. Implications for treatment? Curr Drug Targets. 2002;3:43–54. [DOI] [PubMed]
- 3.Alfredson H, Ohberg L. Neovascularisation in chronic painful patellar tendinosis–promising results after sclerosing neovessels outside the tendon challenge the need for surgery. Knee Surg Sports Traumatol Arthrosc. 2005;13:74–80. [DOI] [PubMed]
- 4.Alfredson H, Ohberg L, Forsgren S. Is vasculo-neural ingrowth the cause of pain in chronic Achilles tendinosis? An investigation using ultrasonography and colour Doppler, immunohistochemistry, and diagnostic injections. Knee Surg Sports Traumatol Arthrosc. 2003;11:334–338. [DOI] [PubMed]
- 5.Amour A, Knight CG, Webster A, Slocombe PM, Stephens PE, Knauper V, Docherty AJ, Murphy G. The in vitro activity of ADAM-10 is inhibited by TIMP-1 and TIMP-3. FEBS Lett. 2000;473:275–279. [DOI] [PubMed]
- 6.Anesini C, Borda E. Modulatory effect of the adrenergic system upon fibroblast proliferation: participation of beta 3-adrenoceptors. Auton Autacoid Pharmacol. 2002;22:177–186. [DOI] [PubMed]
- 7.Archambault JM, Jelinsky SA, Lake SP, Hill AA, Glaser DL, Soslowsky LJ. Rat supraspinatus tendon expresses cartilage markers with overuse. J Orthop Res. 2007;25:617–624. [DOI] [PubMed]
- 8.Arnoczky SP, Lavagnino M, Egerbacher M. The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? Int J Exp Pathol. 2007;88:217–226. [DOI] [PMC free article] [PubMed]
- 9.Arnoczky SP, Lavagnino M, Egerbacher M, Caballero O, Gardner K. Matrix metalloproteinase inhibitors prevent a decrease in the mechanical properties of stress-deprived tendons: an in vitro experimental study. Am J Sports Med. 2007;35:763–769. [DOI] [PubMed]
- 10.Arnoczky SP, Tiam T, Lavagnino M, Gardner K, Schuler P, Morse P. Activation of stress-activated protein kinases (SAPK) in tendon cells following cyclic strain: the effects of strain frequency, strain magnitude, and cytosolic calcium. J Orthop Res. 2002;20:947–956. [DOI] [PubMed]
- 11.Astrom M. Partial rupture in chronic achilles tendinopathy. A retrospective analysis of 342 cases. Acta Orthop Scand. 1998;69:404–407. [DOI] [PubMed]
- 12.Astrom M, Rausing A. Chronic Achilles tendinopathy. A survey of surgical and histopathologic findings. Clinl Orthop Relat Res. 1995;316:151–164. [PubMed]
- 13.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–3727. [DOI] [PubMed]
- 14.Bank RA, Krikken M, Beekman B, Stoop R, Maroudas A, Lafeber FP, te Koppele JM. A simplified measurement of degraded collagen in tissues: application in healthy, fibrillated and osteoarthritic cartilage. Matrix Biology. 1997;16:233–243. [DOI] [PubMed]
- 15.Bank RA, TeKoppele JM, Oostingh G, Hazleman BL, Riley GP. Lysylhydroxylation and non-reducible crosslinking of human supraspinatus tendon collagen: changes with age and in chronic rotator cuff tendinitis. Ann Rheum Dis. 1999;58:35–41. [DOI] [PMC free article] [PubMed]
- 16.Baquie P, Brukner P. Injuries presenting to an Australian sports medicine centre: a 12-month study. Clin J Sport Med. 1997;7:28–31. [DOI] [PubMed]
- 17.Bertolotto M, Perrone R, Martinoli C, Rollandi GA, Patetta R, Derchi LE. High resolution ultrasound anatomy of normal Achilles tendon. Br J Radiol. 1995;68:986–991. [DOI] [PubMed]
- 18.Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. [DOI] [PubMed]
- 19.Birch HL. Tendon matrix composition and turnover in relation to functional requirements. Int J Exp Pathol. 2007;88:241–248. [DOI] [PMC free article] [PubMed]
- 20.Birch HL, Bailey AJ, Goodship AE. Macroscopic ‘degeneration’ of equine superficial digital flexor tendon is accompanied by a change in extracellular matrix composition. Equine Vet J. 1998;30:534–539. [DOI] [PubMed]
- 21.Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol. 2003;200:423–428. [DOI] [PubMed]
- 22.Bramono DS, Richmond JC, Weitzel PP, Kaplan DL, Altman GH. Matrix metalloproteinases and their clinical applications in orthopaedics. Clin Orthop Relat Res. 2004;428:272–285. [DOI] [PubMed]
- 23.Burniston JG, Tan LB, Goldspink DF. Beta2-Adrenergic receptor stimulation in vivo induces apoptosis in the rat heart and soleus muscle. J Appl Physiol. 2005;98:1379–1386. [DOI] [PubMed]
- 24.Chakravarti S. Functions of lumican and fibromodulin: lessons from knockout mice. Glycoconj J. 2002;19:287–293. [DOI] [PubMed]
- 25.Chuen FS, Chuk CY, Ping WY, Nar WW, Kim HL, Ming CK. Immunohistochemical characterization of cells in adult human patellar tendons. J Histochem Cytochem. 2004;52:1151–1157. [DOI] [PubMed]
- 26.Clegg PD, Strassburg S, Smith RK. Cell phenotypic variation in normal and damaged tendons. Int J Exp Pathol. 2007;88:227–235. [DOI] [PMC free article] [PubMed]
- 27.Colige A, Vandenberghe I, Thiry M, Lambert CA, Van Beeumen J, Li SW, Prockop DJ, Lapiere CM, Nusgens BV. Cloning and characterization of ADAMTS-14, a novel ADAMTS displaying high homology with ADAMTS-2 and ADAMTS-3. J Biol Chem. 2002;277:5756–5766. [DOI] [PubMed]
- 28.Cook JL, Feller JA, Bonar SF, Khan KM. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes’ patellar tendons. J Orthop Res. 2004;22:334–338. [DOI] [PubMed]
- 29.Corps AN, Robinson AH, Movin T, Costa ML, Hazleman BL, Riley GP. Increased expression of aggrecan and biglycan mRNA in Achilles tendinopathy. Rheumatology (Oxford). 2006;45:291–294. [DOI] [PubMed]
- 30.Corps AN, Robinson AH, Movin T, Costa ML, Ireland DC, Hazleman BL, Riley GP. Versican splice variant messenger RNA expression in normal human Achilles tendon and tendinopathies. Rheumatology (Oxford). 2004;43:969–972. [DOI] [PubMed]
- 31.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. [DOI] [PMC free article] [PubMed]
- 32.Danielson P, Alfredson H, Forsgren S. In situ hybridization studies confirming recent findings of the existence of a local nonneuronal catecholamine production in human patellar tendinosis. Microsc Res Tech. 2007;70:908–911. [DOI] [PubMed]
- 33.Danielson P, Alfredson H, Forsgren S. Studies on the importance of sympathetic innervation, adrenergic receptors, and a possible local catecholamine production in the development of patellar tendinopathy (tendinosis) in man. Microsc Res Tech. 2007;70:310–324. [DOI] [PubMed]
- 34.de Mos M, van El B, DeGroot J, Jahr H, van Schie HT, van Arkel ER, Tol H, Heijboer R, van Osch GJ, Verhaar JA. Achilles tendinosis: changes in biochemical composition and collagen turnover rate. Am J Sports Med. 2007;35:1549–1556. [DOI] [PubMed]
- 35.Eriksen HA, Pajala A, Leppilahti J, Risteli J. Increased content of type III collagen at the rupture site of human Achilles tendon. J Orthop Res. 2002;20:1352–1357. [DOI] [PubMed]
- 36.Frey C, Zamora J. The effects of obesity on orthopaedic foot and ankle pathology. Foot Ankle Int. 2007;28:996–999. [DOI] [PubMed]
- 37.Frost P, Bonde JPE, Mikkelsen S, Andersen JH, Fallentin N, Kaergaard A, Thomsen JF. Risk of shoulder tendinitis in relation to shoulder loads in monotonous repetitive work. Am J Indust Med. 2002;41:11–18. [DOI] [PubMed]
- 38.Fu SC, Chan BP, Wang W, Pau HM, Chan KM, Rolf CG. Increased expression of matrix metalloproteinase 1 (MMP1) in 11 patients with patellar tendinosis. Acta Orthop Scand. 2002;73:658–662. [DOI] [PubMed]
- 39.Fu SC, Chan KM, Rolf CG. Increased deposition of sulfated glycosaminoglycans in human patellar tendinopathy. Clin J Sport Med. 2007;17:129–134. [DOI] [PubMed]
- 40.Fu SC, Wong YP, Chan BP, Pau HM, Cheuk YC, Lee KM, Chan KM. The roles of bone morphogenetic protein (BMP) 12 in stimulating the proliferation and matrix production of human patellar tendon fibroblasts. Life Sci. 2003;72:2965–2974. [DOI] [PubMed]
- 41.Gelberman RH, Steinberg D, Amiel D, Akeson W. Fibroblast chemotaxis after tendon repair. J Hand Surg (Am). 1991;16:686–693. [DOI] [PubMed]
- 42.Goncalves-Neto J, Witzel SS, Teodoro WR, Carvalho-Junior AE, Fernandes TD, Yoshinari HH. Changes in collagen matrix composition in human posterior tibial tendon dysfunction. Joint Bone Spine. 2002;69:189–194. [DOI] [PubMed]
- 43.Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. Faseb J. 1992;6:861–870. [PubMed]
- 44.Harvie P, Ostlere SJ, Teh J, McNally EG, Clipsham K, Burston BJ, Pollard TC, Carr AJ. Genetic influences in the aetiology of tears of the rotator cuff. Sibling risk of a full-thickness tear. J Bone Joint Surg Br. 2004;86:696–700. [DOI] [PubMed]
- 45.Hashimoto G, Aoki T, Nakamura H, Tanzawa K, Okada Y. Inhibition of ADAMTS4 (aggrecanase-1) by tissue inhibitors of metalloproteinases (TIMP-1, 2, 3 and 4). FEBS Lett. 2001;494:192–195. [DOI] [PubMed]
- 46.Hashimoto T, Nobuhara K, Hamada T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop Relat Res. 2003;415:111–120. [DOI] [PubMed]
- 47.Holmes GB, Lin J. Etiologic factors associated with symptomatic achilles tendinopathy. Foot Ankle Int. 2006;27:952–959. [DOI] [PubMed]
- 48.Ireland D, Harrall R, Curry V, Holloway G, Hackney R, Hazleman B, Riley G. Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol. 2001;20:159–169. [DOI] [PubMed]
- 49.Jones GC, Corps AN, Pennington CJ, Clark IM, Edwards DR, Bradley MM, Hazleman BL, Riley GP. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human achilles tendon. Arthritis Rheum. 2006;54:832–842. [DOI] [PubMed]
- 50.Jones GC, Riley GP. ADAMTS proteinases: a multi-domain, multi-functional family with roles in extracellular matrix turnover and arthritis. Arthritis Res Ther. 2005;7:160–169. [DOI] [PMC free article] [PubMed]
- 51.Jozsa L, Balint JB, Kannus P, Reffy A, Barzo M. Distribution of blood groups in patients with tendon rupture. An analysis of 832 cases. J Bone Joint Surg Br. 1989;71:272–274. [DOI] [PubMed]
- 52.Jozsa L, Barzo M, Balint JB. Correlations between the ABO blood group system and tendon rupture. Magy Traumatol Orthop Helyreallito Seb. 1990;33:101–104. [PubMed]
- 53.Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10:312–320. [DOI] [PubMed]
- 54.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed]
- 55.Kashiwagi M, Enghild JJ, Gendron C, Hughes C, Caterson B, Itoh Y, Nagase H. Altered proteolytic activities of ADAMTS-4 expressed by C-terminal processing. J Biol Chem. 2004;279:10109–10119. [DOI] [PubMed]
- 56.Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5). J Biol Chem. 2001;276:12501–12504. [DOI] [PubMed]
- 57.Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393–408. [DOI] [PubMed]
- 58.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. [DOI] [PubMed]
- 59.Kujala UM, Jarvinen M, Natri A, Lehto M, Nelimarkka O, Hurme M, Virta L, Finne J. ABO blood groups and musculoskeletal injuries. Injury. 1992;23:131–133. [DOI] [PubMed]
- 60.Kujala UM, Sarna S, Kaprio J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15:133–135. [DOI] [PubMed]
- 61.Lechner BE, Lim JH, Mercado ML, Fallon JR. Developmental regulation of biglycan expression in muscle and tendon. Muscle Nerve. 2006;34:347–355. [DOI] [PubMed]
- 62.Lehto M, Jozsa L, Kvist M, Jarvinen M, Balint BJ, Reffy A. Fibronectin in the ruptured human Achilles tendon and its paratenon. An immunoperoxidase study. Ann Chir Gynaecol. 1990;79:72–77. [PubMed]
- 63.Lejard V, Brideau G, Blais F, Salingcarnboriboon R, Wagner G, Roehrl MH, Noda M, Duprez D, Houillier P, Rossert J. Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. J Biol Chem. 2007;282:17665–17675. [DOI] [PubMed]
- 64.Lian O, Dahl J, Ackermann PW, Frihagen F, Engebretsen L, Bahr R. Pronociceptive and antinociceptive neuromediators in patellar tendinopathy. Am J Sports Med. 2006;34:1801–1808. [DOI] [PubMed]
- 65.Lian O, Scott A, Engebretsen L, Bahr R, Duronio V, Khan K. Excessive apoptosis in patellar tendinopathy in athletes. Am J Sports Med. 2007;35:605–611. [DOI] [PubMed]
- 66.Lo IK, Marchuk LL, Hollinshead R, Hart DA, Frank CB. Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase mRNA levels are specifically altered in torn rotator cuff tendons. Am J Sports Med. 2004;32:1223–1229. [DOI] [PubMed]
- 67.Lou J, Tu Y, Burns M, Silva MJ, Manske P. BMP-12 gene transfer augmentation of lacerated tendon repair. J Orthop Res. 2001;19:1199–1202. [DOI] [PubMed]
- 68.Maffulli N, Barrass V, Ewen SW. Light microscopic histology of achilles tendon ruptures. A comparison with unruptured tendons. Am J Sports Med. 2000;28:857–863. [DOI] [PubMed]
- 69.Maffulli N, Ewen SW, Waterston SW, Reaper J, Barrass V. Tenocytes from ruptured and tendinopathic achilles tendons produce greater quantities of type III collagen than tenocytes from normal achilles tendons. An in vitro model of human tendon healing. Am J Sports Med. 2000;28:499–505. [DOI] [PubMed]
- 70.Maffulli N, Sharma P, Luscombe KL. Achilles tendinopathy: aetiology and management. Journal of the Royal Society of Medicine. 2004;97(10):472–476. [DOI] [PMC free article] [PubMed]
- 71.Magra M, Maffulli N. Matrix metalloproteases: a role in overuse tendinopathies. Br J Sports Med. 2005;39:789–791. [DOI] [PMC free article] [PubMed]
- 72.Matheson S, Larjava H, Hakkinen L. Distinctive localization and function for lumican, fibromodulin and decorin to regulate collagen fibril organization in periodontal tissues. J Periodontal Res. 2005;40:312–324. [DOI] [PubMed]
- 73.Mokone GG, Gajjar M, September AV, Schwellnus MP, Greenberg J, Noakes TD, Collins M. The guanine-thymine dinucleotide repeat polymorphism within the tenascin-C gene is associated with achilles tendon injuries. Am J Sports Med. 2005;33:1016–1021. [DOI] [PubMed]
- 74.Mokone GG, Schwellnus MP, Noakes TD, Collins M. The COL5A1 gene and Achilles tendon pathology. Scand J Med Sci Sports. 2006;16:19–26. [DOI] [PubMed]
- 75.Molloy TJ, Kemp MW, Wang Y, Murrell GAC. Microarray analysis of the tendinopathic rat supraspinatus tendon: glutamate signaling and its potential role in tendon degeneration. J Appl Physiol. 2006;101:1702–1709. [DOI] [PubMed]
- 76.Molloy TJ, Wang Y, Horner A, Skerry TM, Murrell GAC. Microarray analysis of healing rat Achilles tendon: evidence for glutamate signalling mechanisms and embryonic gene expression in healing tendon tissue. J Orthop Res. 2006;24:842–855. [DOI] [PubMed]
- 77.Moreno M, Munoz R, Aroca F, Labarca M, Brandan E, Larrain J. Biglycan is a new extracellular component of the Chordin-BMP4 signaling pathway. Embo J. 2005;24:1397–1405. [DOI] [PMC free article] [PubMed]
- 78.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134:2697–2708. [DOI] [PubMed]
- 79.Murray IR, Murray SA, MacKenzie K, Coleman S. How evidence based is the management of two common sports injuries in a sports injury clinic? Br J Sports Med. 2005;39:912–916; discussion 916. [DOI] [PMC free article] [PubMed]
- 80.Nakama LH, King KB, Abrahamsson S, Rempel DM. VEGF, VEGFR-1, and CTGF cell densities in tendon are increased with cyclical loading: An in vivo tendinopathy model. J Orthop Res. 2006;24:393–400. [DOI] [PubMed]
- 81.Nakamura H, Fujii Y, Inoki I, Sugimoto K, Tanzawa K, Matsuki H, Miura R, Yamaguchi Y, Okada Y. Brevican is degraded by matrix metalloproteinases and aggrecanase-1 (ADAMTS4) at different sites. J Biol Chem. 2000;275:38885–38890. [DOI] [PubMed]
- 82.Ohberg L, Alfredson H. Ultrasound guided sclerosis of neovessels in painful chronic Achilles tendinosis: pilot study of a new treatment. Br J Sports Med. 2002;36:173–175; discussion 176–177. [DOI] [PMC free article] [PubMed]
- 83.Primakoff P, Myles DG. The ADAM gene family: surface proteins with adhesion and protease activity. Trends Genet. 2000;16:83–87. [DOI] [PubMed]
- 84.Pufe T, Petersen W, Tillmann B, Mentlein R. The angiogenic peptide vascular endothelial growth factor is expressed in foetal and ruptured tendons. Virchows Archiv. 2001;439:579–585. [DOI] [PubMed]
- 85.Richards PJ, Win T, Jones PW. The distribution of microvascular response in Achilles tendonopathy assessed by colour and power Doppler. Skeletal Radiol. 2005;34:336–342. [DOI] [PubMed]
- 86.Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford). 2004;43:131–142. [DOI] [PubMed]
- 87.Riley GP. Gene expression and matrix turnover in overused and damaged tendons. Scand J Med Sci Sports. 2005;15:241–251. [DOI] [PubMed]
- 88.Riley GP, Curry V, DeGroot J, van El B, Verzijl N, Hazleman BL, Bank RA. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002;21:185–195. [DOI] [PubMed]
- 89.Riley GP, Goddard MJ, Hazleman BL. Histopathological assessment and pathological significance of matrix degeneration in supraspinatus tendons. Rheumatology (Oxford). 2001;40:229–230. [DOI] [PubMed]
- 90.Riley GP, Harrall RL, Cawston TE, Hazleman BL, Mackie EJ. Tenascin-C and human tendon degeneration. Am J Pathol. 1996;149:933–943. [PMC free article] [PubMed]
- 91.Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Glycosaminoglycans of human rotator cuff tendons: changes with age and in chronic rotator cuff tendinitis. Ann Rheum Dis. 1994;53:367–376. [DOI] [PMC free article] [PubMed]
- 92.Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis. 1994;53:359–366. [DOI] [PMC free article] [PubMed]
- 93.Schubert TE, Weidler C, Lerch K, Hofstadter F, Straub RH. Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis. 2005;64:1083–1086. [DOI] [PMC free article] [PubMed]
- 94.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. [DOI] [PubMed]
- 95.Scott A, Ashe MC. Common tendinopathies in the upper and lower extremities. Curr Sports Med Rep. 2006;5:233–241. [DOI] [PubMed]
- 96.Scott A, Cook JL, Hart DA, Walker DC, Duronio V, Khan KM. Tenocyte responses to mechanical loading in vivo: a role for local insulin-like growth factor 1 signaling in early tendinosis in rats. Arthritis Rheum. 2007;56:871–881. [DOI] [PubMed]
- 97.Scott A, Khan KM, Heer J, Cook JL, Lian O, Duronio V. High strain mechanical loading rapidly induces tendon apoptosis: an ex vivo rat tibialis anterior model. Br J Sports Med. 2005;39:e25. [DOI] [PMC free article] [PubMed]
- 98.Sein ML. Shoulder Pain in Elite Swimmers. Sydney, Australia: Medicine, University of New South Wales; 2006.
- 99.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202. [DOI] [PubMed]
- 100.Skutek M, van Griensven M, Zeichen J, Brauer N, Bosch U. Cyclic mechanical stretching of human patellar tendon fibroblasts: activation of JNK and modulation of apoptosis. Knee Surg Sports Traumatol Arthrosc. 2003;11:122–129. [DOI] [PubMed]
- 101.Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, Carpenter JE. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. [DOI] [PubMed]
- 102.Tanaka S, Petersen M, Cameron L. Prevalence and risk factors of tendinitis and related disorders of the distal upper extremity among U.S. workers: comparison to carpal tunnel syndrome. Am J Indust Med. 2001;39:328–335. [DOI] [PubMed]
- 103.Tang BL. ADAMTS: a novel family of extracellular matrix proteases. Int J Biochem Cell Biol. 2001;33:33–44. [DOI] [PubMed]
- 104.Thomopoulos S, Hattersley G, Rosen V, Mertens M, Galatz L, Williams GR, Soslowsky LJ. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J Orthop Res. 2002;20:454–463. [DOI] [PubMed]
- 105.Tillander B, Franzen L, Norlin R. Fibronectin, MMP-1 and histologic changes in rotator cuff disease. J Orthop Res. 2002;20:1358–1364. [DOI] [PubMed]
- 106.Trebaul A, Chan EK, Midwood KS. Regulation of fibroblast migration by tenascin-C. Biochem Soc Trans. 2007;35(Pt 4):695–697. [DOI] [PubMed]
- 107.Tsuzaki M, Yamauchi M, Banes AJ. Tendon collagens: extracellular matrix composition in shear stress and tensile components of flexor tendons. Connect Tissue Res. 1993;29:141–152. [DOI] [PubMed]
- 108.Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev. 2000;14:2123–2133. [DOI] [PubMed]
- 109.Wadhwa S, Embree MC, Bi Y, Young MF. Regulation, regulatory activities, and function of biglycan. Crit Rev Eukaryot Gene Expr. 2004;14:301–315. [DOI] [PubMed]
- 110.Waggett AD, Ralphs JR, Kwan AP, Woodnutt D, Benjamin M. Characterization of collagens and proteoglycans at the insertion of the human Achilles tendon. Matrix Biol. 1998;16:457–470. [DOI] [PubMed]
- 111.Wang F, Murrell GA, Wang MX. Oxidative stress-induced c-Jun N-terminal kinase (JNK) activation in tendon cells upregulates MMP1 mRNA and protein expression. J Orthop Res. 2007;25:378–389. [DOI] [PubMed]
- 112.Wang QW, Chen ZL, Piao YJ. Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J Biosci Bioeng. 2005;100:418–422. [DOI] [PubMed]
- 113.Westling J, Gottschall PE, Thompson VP, Cockburn A, Perides G, Zimmermann DR, Sandy JD. ADAMTS4 (aggrecanase-1) cleaves human brain versican V2 at Glu405-Gln406 to generate glial hyaluronate binding protein. Biochem J. 2004;377(Pt 3):787–795. [DOI] [PMC free article] [PubMed]
- 114.Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N, Dube JL, DiBlasio-Smith E, Nove J, Song JJ, Wozney JM, Rosen V. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321–330. [DOI] [PMC free article] [PubMed]
- 115.Yanagishita M. Function of proteoglycans in the extracellular matrix. Acta Pathol Jpn. 1993;43:283–293. [DOI] [PubMed]
- 116.Yoon JH, Halper J. Tendon proteoglycans: biochemistry and function. J Musculoskelet Neuronal Interact. 2005;5:22–34. [PubMed]
- 117.Yuan J, Murrell GA, Wei AQ, Wang MX. Apoptosis in rotator cuff tendonopathy. J Orthop Res. 2002;20:1372–1379. [DOI] [PubMed]
- 118.Yuan J, Murrell GAC, Trickett A, Wang MX. Involvement of cytochrome c release and caspase-3 activation in the oxidative stress-induced apoptosis in human tendon fibroblasts. Biochimica et Biophysica Acta. 2003;1641:35–41. [DOI] [PubMed]