Abstract
Mechanical overloading is a major causative factor of tendinopathy; however, its underlying mechanisms are unclear. We hypothesized mechanical overloading would damage tendons and alter genes associated with tendinopathy in a load-dependent manner. To test this hypothesis, we fatigue loaded rat patellar tendons in vivo and measured expression of the matrix-degrading enzyme MMP-13 and the inflammatory cytokine IL-1β. We also examined these responses in cultured tenocytes exposed to intermittent hydrostatic pressure in vitro. Additionally, we hypothesized load-induced changes in tenocyte MMP-13 expression would be dependent on expression of IL-1β. In vivo fatigue loading at 1.7% strain caused overt microstructural damage and upregulated expression of MMP-13 and IL-1β, while 0.6% strain produced only minor changes in matrix microstructure and downregulated expression of both MMP-13 and IL-1β. Loading of cultured tenocytes at 2.5 and 7.5 MPa produced comparable changes in expression to those of in vivo tendon loading. Blocking IL-1β expression with siRNA suppressed load-induced both MMP-13 mRNA expression and activity. The data suggest fatigue loading alters expression of MMP-13 and IL-1β in tendons in vivo and tenocytes in vitro in a load-dependent manner. The data also suggest MMP-13 is regulated by both IL-1β-dependent and IL-1β-independent pathways.
Introduction
Degenerative tendon injury, or tendinopathy, is one of the most common disorders of the musculoskeletal system [31]. Tendinopathy affects millions of people, especially those who perform repetitive tasks in their jobs, sports, or daily activities. Mechanical loading is considered a major causative factor for tendinopathy; however, its etiology is complex [20, 35, 44]. Overuse, repeated movements, sudden injuries, and aging can all contribute to degenerative changes in tendon, which may occur in the absence (tendinosis) or presence (tendinitis) of an inflammatory response [22, 28].
Defining the mechanisms that underlie tendinopathy requires an understanding of the interrelationships among mechanical loading, tissue damage and subsequent cellular and tissue level responses. These tissue responses include changes in the expression of matrix-degrading enzymes, like collagenases, and inflammatory cytokines, such as interleukins. Clinical histopathologic findings demonstrate altered expression of several collagenases in tendinopathy [16, 27, 32, 33], while animal exercise and overuse have produced both inflammatory and degenerative changes in tendons [5, 9, 29, 34, 38]. Similarly, mechanical overloading of tendon explants [13, 15] and tendon cells [2, 4, 8, 26, 36, 41, 45, 46] in vitro led to increases in both collagenase and inflammatory mediators, in conjunction with a loss of mechanical integrity. MMP-13 (collagenase-3), which has been implicated in osteoarthritis, rheumatoid arthritis [43], and periodontal diseases [17] is upregulated in patients with complete tears of rotator cuff tendons [27], while the inflammatory mediator IL-1β induces MMP-13 in numerous cells and tissues [10, 18, 39, 42].
Because repetitive loading has been proposed as a cause of tendinopathy, we hypothesized the application of repetitive loads to rat patellar tendons in vivo and to cultured rat tenocytes in vitro would alter genes associated with tendinopathy in a load-dependent manner. Furthermore, because IL-1β induces MMP-13 [10, 18, 39, 42], we hypothesized changes in tenocyte MMP-13 expression would be dependent on expression of IL-1β.
Materials and Methods
We utilized an in vivo animal model and an in vitro tenocyte model to evaluate whether repetitive loading would alter genes associated with tendinopathy in a load-dependent manner. Adult female Sprague-Dawley rats (N = 48; 390 ± 30 g) (Charles River Laboratories, Wilmington, MA, USA) were used in the in vivo model. Under isoflurane anesthesia, left patellae were surgically exposed and clamped to a 50 lb (222 N) load cell and actuator of a servohydraulic testing system (Instron 8841, Canton, MA, USA). All procedures were approved by the Institutional Animal Care and Use Committee. Tendons were fatigue loaded based on a damage accumulation loading protocol adapted from cortical bone fatigue damage studies [19]. After preloading and initial length measured, tendons were cyclically loaded between 1 and 35 N (∼40% maximum monotonic strength) at 1 Hz until reaching either 0.6% or 1.7% increases in elongation (relative to initial length) at peak cyclic load beyond baseline measurement. These loading endpoints were chosen because they produced different and repeatable levels of damage in our previous ex vivo tendon fatigue studies. Sham-operated animals, treated identically except for loading, and naïve control animals, were included in the study. Animals were allowed to resume normal cage activity for 1 or 3 days (n = 6/load group/time point) after loading. Animals were then sacrificed and hindlimbs dissected and frozen in liquid N2 prior to RNA and protein analysis. Animal numbers were chosen based on preliminary studies of gene expression using this loading protocol. For microstructural damage analysis, additional animals (n = 2/load group/time point) were used and sacrificed immediately after loading, and the hindlimbs fixed in 10% neutral buffered formalin and embedded in methacrylate [23].
We utilized an in vitro tenocyte model to determine whether MMP-13 expression would be dependent on expression of IL-1β. A clonal cell line derived from rat patellar tendon was cultured in DMEM with 10% FBS, then starved in DMEM containing 1% FBS for 18 hours prior to intermittent hydrostatic pressure loading at 0, 2.5, 5.0, or 7.5 MPa at 1 Hz for 1 hour using a custom-made loading device. After loading, cells were lysed for isolation of total RNA and proteins. In some experiments, cells were either transfected for 24 hours prior to serum starvation with si-IL-1β RNA or a scrambled RNA control (Upstate Biologics, Lake Placid, NY, USA).
Total RNA isolated from tendons or cultured tenocytes (RNeasy Kit, Eppendorf, Westbury, NY, USA) was reverse transcribed with MMLV reverse transcriptase and an Oligo(dT)12–18 primer and expression of MMP-13 was quantitated by real time PCR (ABI Prism 7900HT Real-Time PCR System, Applied Biosystems, Framingham, MA, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for normalization.
Extracts from tendon tissue or cultured tenocytes containing approximately 10 μg of total protein were separated by electrophoresis on 10% SDS PAGE, transferred electrophoretically onto nitrocellulose membranes, blocked with a commercial blocking reagent (Amersham Biosciences, Piscataway, NJ, USA) and incubated overnight with antibody against MMP-13 (rabbit polyclonal IgG, Santa Cruz Biotechnology, Santa Cruz, CA, USA) or IL-1β (goat polyclonal IgG, Santa Cruz Biotechnology) followed by peroxidase-conjugated secondary antibodies. Levels of MMP-13 or IL-1β proteins were detected using ECL Western blotting analysis system (Amersham Biosciences). To measure MMP-13 activity, extracts of tendon tissue or concentrates of tenocyte media were assayed using an MMP-13 assay kit (Sensolyte, Anaspec, Inc., San Jose, CA, USA) following the manufacturer’s instructions.
For microstructural analysis, 200 μm thick, sagittal sections were cut using a diamond wafering saw and mounted unstained. Tendon morphology was examined using a laser multiphoton microscope (LSM 510; Carl Zeiss, Jena, Germany) tuned to 840 nm. Backward second harmonic signals (generated by the collagen fibers) [47, 50] were collected using an external detector through a narrow bandpass (450/40 nm) filter. Area fraction of damage was quantitated in (400 × 400 μm) fields from at least 10 multiphoton micrographs per tendon.
We compared expression of MMP-13 and IL-1β at the mRNA and protein level for 0.6% and 1.7% cyclic strain in vivo and intermittent hydrostatic pressure loading at 0, 2.5, 5.0, or 7.5 MPa in vitro using ANOVA with Bonferroni post-hoc testing for multiple comparisons. We used Minitab v13 (Minitab, Inc., State Collage, PA, USA) for all analyses.
Results
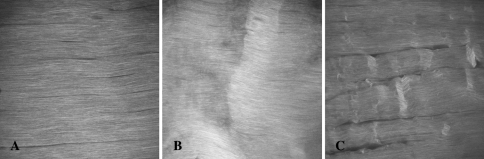
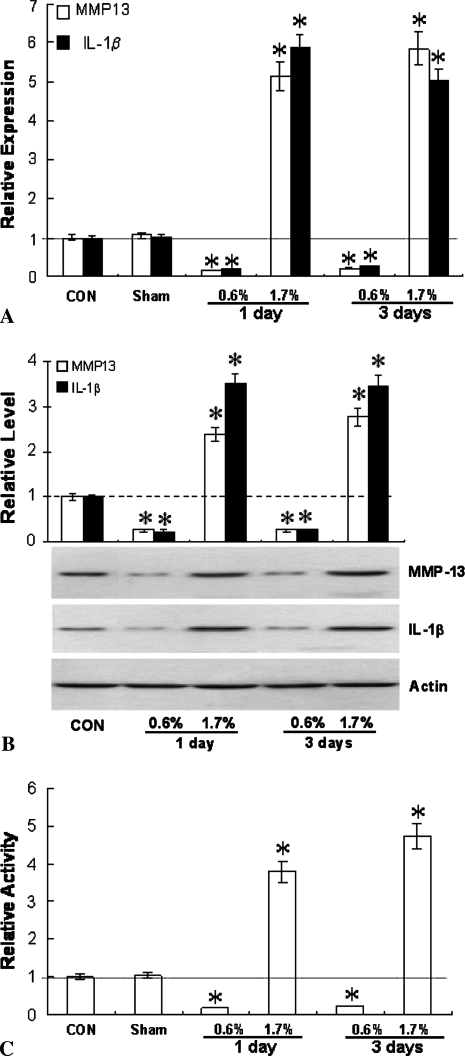
Loading rat patellar tendons in vivo to 0.6% strain and 1.7% strain produced distinctly different microstructural damage patterns (Fig. 1). At 0.6%, second harmonic imaging revealed kinked collagen fiber deformation in isolated areas with a mean damage area fraction of 4.1%. At 1.7% strain, lateral fiber separation (tearing) was clearly evident, in addition to the previously observed kinked fiber deformation. The mean damage area fraction also increased to 10.3%. Fatigue loading altered the expression of MMP-13 and IL-1β. The effects were similar at both 1 and 3 days after fatigue, but highly dependent on the level of load. At 1.7% strain, MMP-13 and IL-1β mRNA levels increased (p < 0.001) five- to six-fold over control and sham-operated tissues (Fig. 2A). In contrast, loading to 0.6% strain suppressed (p < 0.001) expression of both MMP-13 and IL-1β by nearly 70%. In all instances, changes in MMP-13 mRNA levels were accompanied by comparable changes (p < 0.001) in MMP-13 protein (Fig. 2B) and enzyme activity (Fig. 2C).
Fig. 1A–C.
Mechanical loading causes microstructural damage in rat patellar tendon in a loading intensity dependent manner. (A) Second harmonic generation microscopic images show a control tendon characterized by intact, aligned, densely packed fibers with no patterns of disruption. (B) In 0.6% strain loaded tendons, localized transverse patterns of kinked fiber deformation were evident with no fiber rupture. (C) Tendons loaded to 1.7% exhibited, in addition to kinked fiber deformation, longitudinal separation among the fibers. Field of view = 400 μm.
Fig. 2A–C.
Fatigue loading of patellar tendons in vivo alters expression of MMP-13 and IL-1β. (A) IL-1β and MMP-13 mRNA were suppressed by low-level strain (0.6%) loading, but upregulated by higher strain (1.7%) loading. Changes in (B) MMP-13 and IL-1β protein and (C) MMP-13 activity due to loading were similar to those seen in (A) for mRNA. * denotes significant difference from the appropriate sham-operated group (p ≤ 0.001 based on ANOVA and Bonferroni post-hoc test).
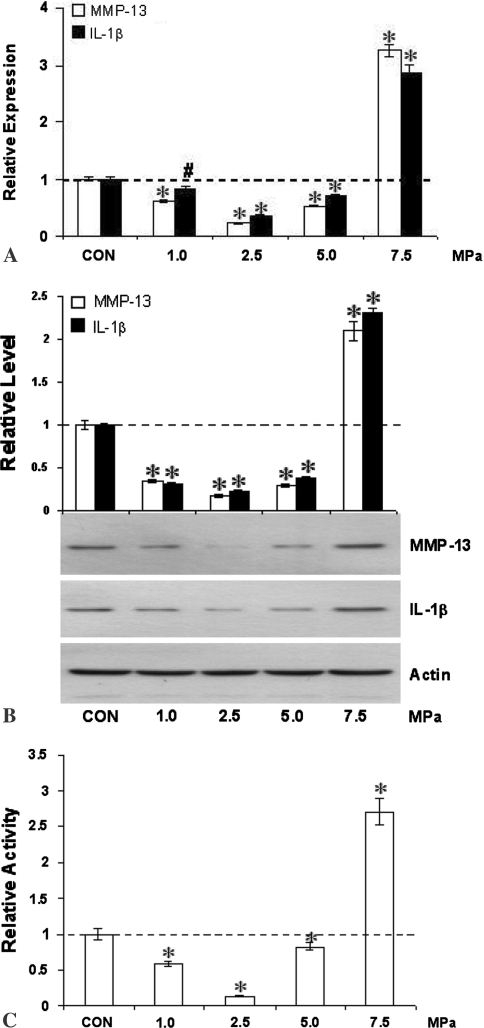
In vitro responses of cultured tenocytes to intermittent hydrostatic pressure closely mimicked the effects observed following fatigue loading in vivo. MMP-13 mRNA (Fig. 3A), protein (Fig. 3B) and enzyme activity in conditioned medium (Fig. 3C) showed similar biphasic responses to intermittent hydrostatic pressure. At 1.0 to 5.0 MPa, expression was reduced (p ≤ 0.003) to less than 50% of control levels, while at 7.5 MPa expression was stimulated (p < 0.001) roughly two- to three-fold. As observed in vivo, responses of MMP-13 closely mirrored those of IL-1β (Fig. 3A).
Fig. 3A–C.
Intermittent hydrostatic pressure alters expression and activity of MMP-13, and expression of IL-1β by tenocytes in vitro. Expression of MMP-13 and IL-1β (A) mRNA, (B) protein, and (C) MMP-13 enzyme activity were downregulated at 1.0, 2.5, and 5.0 MPa while they were upregulated at 7.5 MPa in response to intermittent hydrostatic pressure loading. Differences from control are indicated by * (p ≤ 0.001) and # (p = 0.003).
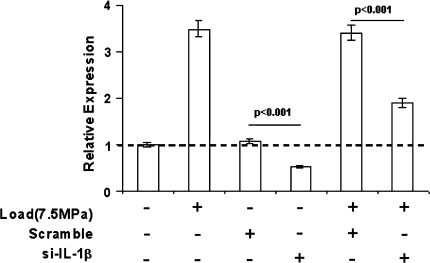
Cells transfected with siRNA directed against IL-1β exhibited reductions (p < 0.001) in both basal and load-induced MMP-13 expression relative to cells transfected with a scrambled siRNA sequence (Fig. 4) As in previous experiments, changes in MMP-13 mRNA were accompanied by similar changes in enzyme activity.
Fig. 4.
MMP-13 expression in cultured tenocytes is partially dependent on IL-1β. Tenocytes were either transfected with si-IL-1β RNA or a control scrambled siRNA, or left untransfected prior to culture in the presence or absence of intermittent hydrostatic pressure (7.5 MPa), then assayed for MMP-13 mRNA expression. Significance levels are presented for differences between the treatment groups indicated by horizontal bars.
Discussion
Mechanical overloading is a major causative factor of tendinopathy; however, its underlying mechanisms are unclear. Presuming this is the case one would presume loading would alter genes associated with tendinopathy in a load-dependent manner. We therefore hypothesized the application of repetitive loads to rat patellar tendons in vivo and to cultured rat tenocytes in vitro would alter genes associated with tendinopathy, IL-1β and MMP-13, in a load-dependent manner. Furthermore, because IL-1β induces MMP-13 we hypothesized changes in tenocyte MMP-13 expression would be dependent on expression of IL-1β.
A limitation of these models, however, is that fatigue loading is applied acutely and so may not fully reflect the loading or response patterns associated with chronic damage accumulated over long periods of time in vivo. Similarly, intermittent hydrostatic pressure clearly does not directly model tendon stretching; however, the effects of intermittent hydrostatic pressure on cultured tenocytes in vitro were similar to those seen in tendons subjected to repetitive stretching in vivo. This suggests the possibility that diverse mechanical stimuli may activate a common set of cellular responses.
Our data demonstrate that in vivo fatigue loading of rat patellar tendons to two strain endpoints produced distinct tissue damage patterns and opposite effects on expression of both MMP-13 and IL-1β. High strain, which produced matrix disruption that included severe fiber angulation and longitudinal fiber separation and increased MMP-13 level, were consistent with those of late-stage clinical tendinopathy [16, 20, 21, 27, 37]. By contrast, low strain, which produced matrix deformations (kinks) without overt tearing, suppressed both matrix remodeling (MMP-13) and inflammatory (IL-1β) responses. Whether low-level strain caused increases in these markers that were early and transient, or slow to develop (ie, after 3 days post-load) is not yet clear. Interestingly, however, physiological levels of mechanical loading have been reported to protect tissues like cartilage against degeneration resulting from arthritis or similar pathologic conditions [1, 40, 48]. We have observed similar suppressive effects on MMP expression in chondrocytes [49], and comparable mechanisms may apply in tendon. Whether the microstructural changes produced by 0.6% strain are permanent or will ultimately be “repaired” remains to be determined.
We focused on MMP-13 (collagenase-3) in this study for two reasons. First, MMP-13 increases in human tendinopathy [27]. Second, MMP-13 in rats may play a predominant role in matrix degradation, comparable to that ascribed to MMP-1 in humans. MMP-1 is the principal interstitial collagenase in human tendons; however, a gene strictly homologous to human MMP-1 has not been identified in the rat. Previous studies demonstrate stress deprivation of rat tail tendons in vitro results in an immediate increase in MMP-13 mRNA expression and protein synthesis, and a correspondingly large decrease in the material properties of these stress-deprived tendons [24, 25].
IL-1β is a major inflammatory mediator in both human and rat, and induces MMP-1, -3 and -13 in human tendon cells in vitro and ex vivo [3, 4, 42]. These findings, coupled with observations that IL-1β mRNA can be induced in tendon cells both by exogenous IL-1β and by mechanical loading, have led to the suggestion that IL-1β may participate in a positive feedback loop that triggers MMP-dependent matrix destruction [41]. Our data showing MMP-13 and IL-1β are coregulated in vivo and that IL-1β is in part responsible for the upregulation of tenocyte MMP-13 expression in response to mechanical loading indicate similar pathways may operate in rat tendon.
Finally, our in vitro result demonstrating expression of MMP-13 is only partly dependent on IL-1β raises the possibility inflammatory suppression may not fully inhibit MMP-based tendon degradation, while therapies directly aimed at MMPs may be more effective [6]. Indeed, injection of an MMP inhibitor, aprotinin, seems beneficial in treatment of Achilles and patellar tendinopathies [7, 11, 12]. On the other hand, another broad spectrum MMP inhibitor, marimastat, itself produced a painful tendinopathy in patients [14, 30]. The basis for these paradoxical findings remains unknown, but it is possible effective treatment or prevention of tendinopathy may require MMP inhibition strategies that are not broadly based, but rather selective for individual MMP family members. The close association we demonstrated between MMP-13 expression and tendinopathic changes induced by fatigue loading suggests this is a likely candidate for further investigation as a therapeutic target.
Acknowledgments
We thank Vincent Wang, PhD, Lakshmi Rajan, PhD, Philip Nasser, MS, Kenneth Accousti, MD, Jonathan Braman, MD, and Raymond Klug, MD.
Footnotes
One or more of the authors (HBS, MBS, ELF) has received funding from the National Institutes of Health. This study was supported by grants from the Aircast Foundation and NIH Grants AR41210, AR52743, and AR50968.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Agarwal S, Long P, Gassner R, Piesco NP, Buckley MJ. Cyclic tensile strain suppresses catabolic effects of interleukin-1beta in fibrochondrocytes from the temporomandibular joint. Arthritis Rheum. 2001;44:608–617. [DOI] [PMC free article] [PubMed]
- 2.Almekinders LC, Banes AJ, Ballenger CA. Effects of repetitive motion on human fibroblasts. Med Sci Sports Exerc. 1993;25:603–607. [PubMed]
- 3.Archambault J, Tsuzaki M, Herzog W, Banes AJ. Stretch and interleukin-1beta induce matrix metalloproteinases in rabbit tendon cells in vitro. J Orthop Res. 2002;20:36–39. [DOI] [PubMed]
- 4.Archambault JM, Elfervig-Wall MK, Tsuzaki M, Herzog W, Banes AJ. Rabbit tendon cells produce MMP-3 in response to fluid flow without significant calcium transients. J Biomech. 2002;35:303–309. [DOI] [PubMed]
- 5.Archambault JM, Hart DA, Herzog W. Response of rabbit Achilles tendon to chronic repetitive loading. Connect Tissue Res. 2001;42:13–23. [DOI] [PubMed]
- 6.Arnoczky SP, Lavagnino M, Egerbacher M, Caballero O, Gardner K. Matrix metalloproteinase inhibitors prevent a decrease in the mechanical properties of stress-deprived tendons: an in vitro experimental study. Am J Sports Med. 2007;35:763–769. [DOI] [PubMed]
- 7.Aubin F, Javaudin L, Rochcongar P. Case reports of aprotinin in Achilles tendinopathies with athletes. J Pharmacie Clinique. 1997;16:270–273.
- 8.Banes AJ, Horesovsky G, Larson C, Tsuzaki M, Judex S, Archambault J, Zernicke R, Herzog W, Kelley S, Miller L. Mechanical load stimulates expression of novel genes in vivo and in vitro in avian flexor tendon cells. Osteoarthritis Cartilage. 1999;7:141–153. [DOI] [PubMed]
- 9.Barbe MF, Barr AE, Gorzelany I, Amin M, Gaughan JP, Safadi FF. Chronic repetitive reaching and grasping results in decreased motor performance and widespread tissue responses in a rat model of MSD. J Orthop Res. 2003;21:167–176. [DOI] [PMC free article] [PubMed]
- 10.Borden P, Solymar D, Sucharczuk A, Lindman B, Cannon P, Heller RA. Cytokine control of interstitial collagenase and collagenase-3 gene expression in human chondrocytes. J Biol Chem. 1996;271:23577–23581. [DOI] [PubMed]
- 11.Capasso G, Maffulli N, Testa V, Sgambato A. Preliminary results with peritendinous protease inhibitor injections in the management of Achilles tendonitis. Eur J Sports Traumatol Relat Res. 1993;15:37–43.
- 12.Capasso G, Testa V, Maffulli N, Bifulco G. Aprotinin, corticosteroids and normosaline in the management of patellar tendinopathy in athletes: a prospective ramdomized study. Sports Exerc Inj. 1997;3:111–115.
- 13.Devkota AC, Tsuzaki M, Almekinders LC, Banes AJ, Weinhold PS. Distributing a fixed amount of cyclic loading to tendon explants over longer periods induces greater cellular and mechanical responses. J Orthop Res. 2007;25:1078–1086. [DOI] [PubMed]
- 14.Drummond AH, Beckett P, Brown PD, Bone EA, Davidson AH, Galloway WA, Gearing AJ, Huxley P, Laber D, McCourt M, Whittaker M, Wood LM, Wright A. Preclinical and clinical studies of MMP inhibitors in cancer. Ann NY Acad Sci. 1999;878:228–235. [DOI] [PubMed]
- 15.Flick J, Devkota A, Tsuzaki M, Almekinders L, Weinhold P. Cyclic loading alters biomechanical properties and secretion of PGE2 and NO from tendon explants. Clin Biomech (Bristol, Avon). 2006;21:99–106. [DOI] [PubMed]
- 16.Fu SC, Chan BP, Wang W, Pau HM, Chan KM, Rolf CG. Increased expression of matrix metalloproteinase 1 (MMP1) in 11 patients with patellar tendinosis. Acta Orthop Scand. 2002;73:658–662. [DOI] [PubMed]
- 17.Hernandez M, Valenzuela MA, Lopez-Otin C, Alvarez J, Lopez JM, Vernal R, Gamonal J. Matrix metalloproteinase-13 is highly expressed in destructive periodontal disease activity. J Periodontol. 2006;77:1863–1870. [DOI] [PubMed]
- 18.Ilgenli T, Vardar-Sengul S, Gurkan A, Sorsa T, Stackelberg S, Kose T, Atilla G. Gingival crevicular fluid matrix metalloproteinase-13 levels and molecular forms in various types of periodontal diseases. Oral Dis. 2006;12:573–579. [DOI] [PubMed]
- 19.Jepsen KJ, Davy DT. Comparison of damage accumulation measures in human cortical bone. J Biomech. 1997;30:891–894. [DOI] [PubMed]
- 20.Jozsa L, Kannus P. Histopathological findings in spontaneous tendon ruptures. Scand J Med Sci Sports. 1997;7:113–118. [DOI] [PubMed]
- 21.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed]
- 22.Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393–408. [DOI] [PubMed]
- 23.Laudier D, Schaffler MB, Flatow EL, Wang VM. Novel procedure for high-fidelity tendon histology. J Orthop Res. 2007;25:390–395. [DOI] [PubMed]
- 24.Lavagnino M, Arnoczky SP, Egerbacher M, Gardner KL, Burns ME. Isolated fibrillar damage in tendons stimulates local collagenase mRNA expression and protein synthesis. J Biomech. 2006;39:2355–2362. [DOI] [PubMed]
- 25.Lavagnino M, Arnoczky SP, Tian T, Vaupel Z. Effect of amplitude and frequency of cyclic tensile strain on the inhibition of MMP-1 mRNA expression in tendon cells: an in vitro study. Connect Tissue Res. 2003;44:181–187. [DOI] [PubMed]
- 26.Li Z, Yang G, Khan M, Stone D, Woo SL, Wang JH. Inflammatory response of human tendon fibroblasts to cyclic mechanical stretching. Am J Sports Med. 2004;32:435–440. [DOI] [PubMed]
- 27.Lo IK, Marchuk LL, Hollinshead R, Hart DA, Frank CB. Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase mRNA levels are specifically altered in torn rotator cuff tendons. Am J Sports Med. 2004;32:1223–1229. [DOI] [PubMed]
- 28.Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22:675–692. [DOI] [PubMed]
- 29.Nakama LH, King KB, Abrahamsson S, Rempel DM. VEGF, VEGFR-1, and CTGF cell densities in tendon are increased with cyclical loading: An in vivo tendinopathy model. J Orthop Res. 2006;24:393–400. [DOI] [PubMed]
- 30.Nemunaitis J, Poole C, Primrose J, Rosemurgy A, Malfetano J, Brown P, Berrington A, Cornish A, Lynch K, Rasmussen H, Kerr D, Cox D, Millar A. Combined analysis of studies of the effects of the matrix metalloproteinase inhibitor marimastat on serum tumor markers in advanced cancer: selection of a biologically active and tolerable dose for longer-term studies. Clin Cancer Res. 1998;4:1101–1109. [PubMed]
- 31.Renstrom P, Woo S. Tendinopathy: a major medical problem in sport. In: Woo S, Renstrom P, Arnoczky S, eds. Tendinopathy in Athletes. Oxford, UK: Blackwell; 2007:1–9.
- 32.Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford). 2004;43:131–142. [DOI] [PubMed]
- 33.Riley GP, Curry V, DeGroot J, van El B, Verzijl N, Hazleman BL, Bank RA. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002;21:185–195. [DOI] [PubMed]
- 34.Scott A, Cook JL, Hart DA, Walker DC, Duronio V, Khan KM. Tenocyte responses to mechanical loading in vivo: a role for local insulin-like growth factor 1 signaling in early tendinosis in rats. Arthritis Rheum. 2007;56:871–881. [DOI] [PubMed]
- 35.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202. [DOI] [PubMed]
- 36.Skutek M, van Griensven M, Zeichen J, Brauer N, Bosch U. Cyclic mechanical stretching enhances secretion of interleukin 6 in human tendon fibroblasts. Knee Surg Sports Traumatol Arthrosc. 2001;9:322–326. [DOI] [PubMed]
- 37.Sonnabend DH, Yu Y, Howlett CR, Harper GD, Walsh WR. Laminated tears of the human rotator cuff: a histologic and immunochemical study. J Shoulder Elbow Surg. 2001;10:109–115. [DOI] [PubMed]
- 38.Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, Carpenter JE. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. [DOI] [PubMed]
- 39.Sun HB, Yokota H. Reduction of cytokine-induced expression and activity of MMP-1 and MMP-13 by mechanical strain in MH7A rheumatoid synovial cells. Matrix Biol. 2002;21:263–270. [DOI] [PubMed]
- 40.Trindade MC, Shida J, Ikenoue T, Lee MS, Lin EY, Yaszay B, Yerby S, Goodman SB, Schurman DJ, Smith RL. Intermittent hydrostatic pressure inhibits matrix metalloproteinase and pro-inflammatory mediator release from human osteoarthritic chondrocytes in vitro. Osteoarthritis Cartilage. 2004;12:729–735. [DOI] [PubMed]
- 41.Tsuzaki M, Bynum D, Almekinders L, Yang X, Faber J, Banes AJ. ATP modulates load-inducible IL-1beta, COX 2, and MMP-3 gene expression in human tendon cells. J Cell Biochem. 2003;89:556–562. [DOI] [PubMed]
- 42.Tsuzaki M, Guyton G, Garrett W, Archambault JM, Herzog W, Almekinders L, Bynum D, Yang X, Banes AJ. IL-1 beta induces COX2, MMP-1, -3 and -13, ADAMTS-4, IL-1 beta and IL-6 in human tendon cells. J Orthop Res. 2003;21:256–264. [DOI] [PubMed]
- 43.Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157–164. [DOI] [PMC free article] [PubMed]
- 44.Wang JH, Iosifidis MI, Fu FH. Biomechanical basis for tendinopathy. Clin Orthop Relat Res. 2006;443:320–332. [DOI] [PubMed]
- 45.Wang JH, Jia F, Yang G, Yang S, Campbell BH, Stone D, Woo SL. Cyclic mechanical stretching of human tendon fibroblasts increases the production of prostaglandin E2 and levels of cyclooxygenase expression: a novel in vitro model study. Connect Tissue Res. 2003;44:128–133. [DOI] [PubMed]
- 46.Wang JH, Li Z, Yang G, Khan M. Repetitively stretched tendon fibroblasts produce inflammatory mediators. Clin Orthop Relat Res. 2004;422:243–250. [DOI] [PubMed]
- 47.Williams RM, Zipfel WR, Webb WW. Interpreting second-harmonic generation images of collagen I fibrils. Biophys J. 2005;88:1377–1386. [DOI] [PMC free article] [PubMed]
- 48.Xu Z, Buckley MJ, Evans CH, Agarwal S. Cyclic tensile strain acts as an antagonist of IL-1 beta actions in chondrocytes. J Immunol. 2000;165:453–460. [DOI] [PMC free article] [PubMed]
- 49.Yokota H, Goldring MB, Sun HB. CITED2-mediated regulation of MMP-1 and MMP-13 in human chondrocytes under flow shear. J Biol Chem. 2003;278:47275–47280. [DOI] [PubMed]
- 50.Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci USA. 2003;100:7075–7080. [DOI] [PMC free article] [PubMed]