Abstract
Healing of the rat Achilles tendon is sensitive to mechanical loading, and the callus strength is reduced by
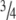 after 14 days, if loading is prevented. Exogenous GDFs stimulate tendon healing. This response is influenced by loading: without loading, cartilage and bone formation is initiated. This implies BMP signaling is crucial during tendon healing and influenced by mechanical loading. We therefore asked if mechanical loading influences the gene expression of the BMP signaling system in intact and healing tendons, and how the BMP signaling system changes during healing. The genes were four BMPs (OP-1/BMP-7, GDF-5/CDMP-1/BMP-14, GDF-6/CDMP-2/BMP-13, and GDF-7/CDMP-3/BMP-12), two receptors (BMPR1b and BMPR2), and the antagonists follistatin and noggin. The Achilles tendon was transected in rats and left to heal. Half of the rats had one Achilles tendon unloaded by injection of Botox in the calf muscles. Ten tendons were analyzed before transection and for each of four time points. All genes except noggin were expressed at all time points, but followed different patterns during healing. Loading strongly decreased the expression of follistatin, which could lead to increased signaling. The BMP system appears involved in tendon maintenance and healing, and may respond to mechanical loading.
after 14 days, if loading is prevented. Exogenous GDFs stimulate tendon healing. This response is influenced by loading: without loading, cartilage and bone formation is initiated. This implies BMP signaling is crucial during tendon healing and influenced by mechanical loading. We therefore asked if mechanical loading influences the gene expression of the BMP signaling system in intact and healing tendons, and how the BMP signaling system changes during healing. The genes were four BMPs (OP-1/BMP-7, GDF-5/CDMP-1/BMP-14, GDF-6/CDMP-2/BMP-13, and GDF-7/CDMP-3/BMP-12), two receptors (BMPR1b and BMPR2), and the antagonists follistatin and noggin. The Achilles tendon was transected in rats and left to heal. Half of the rats had one Achilles tendon unloaded by injection of Botox in the calf muscles. Ten tendons were analyzed before transection and for each of four time points. All genes except noggin were expressed at all time points, but followed different patterns during healing. Loading strongly decreased the expression of follistatin, which could lead to increased signaling. The BMP system appears involved in tendon maintenance and healing, and may respond to mechanical loading.
Introduction
Mechanical loading and biochemical signaling both control tissue healing. It is unknown how they interact, and to what extent mechanics controls biochemistry or vice versa. The bone morphogenetic protein (BMP) signaling system, with its ligands, antagonists, and receptors is important for bone repair and regeneration, but its role in the healing of tendon is largely unknown. Intramuscular injections of growth differentiation factor 5 (GDF-5), -6, and -7 have been reported to induce tendon- or ligament-like tissue in rats [17], and absence of GDF-5 has been reported to affect ultrastructure, composition, and biomechanical integrity of Achilles tendons in mice [11]. GDF-5 might therefore have a role in establishment and maintenance of tendon properties [11]. Knockout mice lacking GDF-5 exhibit an increase of irregularly shaped Type I collagen fibrils and a time-dependent alternation in mechanical behavior [3]. These mice also display a delayed tendon healing [2]. GDF-5 has therefore been identified as important during early tendon healing [2].
Our previous studies have demonstrated that a collagen sponge containing GDF-5 or GDF-6 protein [1] or single injections of GDF-5, -6, or -7 [7] can accelerate or enhance tendon healing. The healing effect of GDF-5 has also been confirmed by a GDF-5-protein-coated suture, which temporarily gives thicker, stiffer, and stronger tendons [15]. GDF-6 also increases the strength in healing rotator cuff tendons in rats [12]. GDF-6 and GDF-7 have been detected in samples from healthy human patellar tendons with immunohistochemical staining [8, 18]. Both were located in active tenoblasts and mesenchymal cells (pericytes in the endotenon) but not in tenocytes. RhGDF-7 could stimulate proliferation of tendon fibroblasts in vitro, and the gene expression of procollagen Type I and Type III was increased after rhGDF-7 stimulation while the gene expression of decorin was decreased [8]. RhGDF-6 also increased the proliferation of tendon fibroblasts in vitro [18]. The gene expression of procollagen Type I increased after treatment with rhGDF-6 and gene expression of procollagen III, decorin, and biglycan remained unchanged [18]. These results suggested GDF-6 and GDF-7 might be involved in matrix remodeling and have a role in tissue regeneration in tendons, although less in early tendon healing [8, 18].
Mechanical stimulation is crucial for tendon healing [16]. Moreover, mechanical stimulation can direct the response to injected GDFs in a tendon healing model, so that its bone inductive capacity is inhibited in favor of formation of a tendon-like tissue [6]. Since we have previously demonstrated administration of exogenous protein improves tendon healing and mechanical influence is crucial for healing the same model [6], we chose to study the role of mechanical loading for BMP signaling in tendons and during tendon healing.
We asked three questions: (1) Does mechanical loading in intact tendons influence the gene expression of the BMP signaling system? (2) Is the gene expression affected by mechanical loading during healing? (3) How is the BMP signaling system changing during different phases of tendon healing?
Materials and Methods
We divided 50 female Sprague-Dawley rats (Scanbur BK, Stockholm, Sweden) with intact tendons into two groups. One of the groups received botulinum toxin A (Botox®, Allergan Inc., Irvine, CA) into the right calf muscles for unloading, and the other group were loaded controls. The rats weighed approximately 220 g and were 67 to 70 days old. After 5 days 5 rats in each group were sacrificed. The remainder underwent tendon transection and equal numbers in each group were sacrificed after 3, 8, 14 and 21 more days (n = 5 for each group at each time). All tendon samples were analyzed for eight different genes (agonists, antagonists and receptors) belonging to the BMP signaling system. The animals were housed two or three per cage at 21°C in a 12-hour light and dark cycle and were given food and water ad libitum. All 50 specimens were analyzed for all genes, and no data were excluded or lost. This study was approved by the regional ethics committee for animal experiments and adhered to the institutional guidelines for the care and treatment of laboratory animals.
The rats to be unloaded were anesthetized with isoflurane gas (Forene®, Abbot Scandinavia, Solna, Sweden) and the right hind leg was shaved. We injected botulinum toxin A into the gastrocnemius lateralis and medianus and the soleus muscles at a dose of 1 U in each muscle (total dose 3 U). The total injected volume was 0.06 mL. The rats were thereafter allowed unrestricted activity for 5 days. After 5 days we harvested five loaded and five unloaded tendons. The remainder underwent tendon transection. We anesthetized the rats with isoflurane gas and administered antibiotics (Engemycin; Intervet, Boxmeer, Holland) and analgesics (Temgesic; Schering-Plough, Brussels, Belgium) preoperatively. The right hind leg was shaved and the surgery was performed under aseptic conditions. We made a transverse incision in the skin lateral to the right Achilles tendon. The surrounding fascia was opened longitudinally and the Achilles tendon complex was exposed. We removed the plantaris tendon and cut the Achilles tendon transversely, proximal to the calcaneal insertion. A 3-mm segment of the Achilles tendon was removed and the wound was closed. The rats were allowed unrestricted activity during healing. Tendon regenerates (calluses) were harvested under anesthesia before sacrifice after 3, 8, 14, and 21 days postoperatively. We anesthetized the rats with isoflurane gas and shaved the skin above the right Achilles tendon and washed it with 70% ethanol. The healing tissue was dissected free from all extraneous soft tissue under sterile conditions. The tissue was harvested, quickly rinsed in saline and snap frozen in liquid nitrogen and stored at −80°C until RNA extraction.
We performed extraction of total RNA using the TRIspin method [14], which is a combination of the Trizol method and RNAeasy Total RNA Kit (Qiagen, MERCK Eurolab, Stockholm, Sweden). We placed the frozen tendons one by one in a liquid-nitrogen-cooled vessel and pulverized using a Retsch Mixer Mill MM 200 (Retsch GmbH, Haan, Germany). One mL of TRIzol Reagent (Life Technologies, Gibco BRL) was added to the powdered tissue (2 mL of TRIzol was added if the tissue weighed more than 100 mg) and was left to thaw for approximately 1 hour at room temperature. The samples were transferred to new tubes and incubated at room temperature for 30 minutes. We stored the samples at −80°C until further extraction. The samples were thawed at room temperature. Chloroform (300 μL) was added followed by vigorous mixing for 15 seconds. We incubated the samples for 3 to 5 minutes and centrifuged them at 12,000 rpm for 15 minutes at 4°C. The top aqueous layer was transferred to a new tube containing 70% ethanol (600 μL) and RNA was further purified using the RNAeasy Total RNA Kit. Potential DNA contamination was eliminated by DNase (RNase-Free DNase Set, Qiagen, MERCK Eurolab, Stockholm, Sweden) and the RNA was stored at −80°C. RNA yield and integrity was analyzed with the RNA 6000 nano kit in a Bioanalyzer 2100 (Agilent Technologies Inc, Waldbronn, Germany). Each sample had 0.1 μg total RNA reverse-transcribed into cDNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems Warrington, UK). We purchased primers for OP-1, GDF-6, GDF-7, follistatin, noggin, BMPR1b, and BMPR2 from Applied Biosystems. Primers for GDF-5 were designed using PrimerExpress 2.0 software (Applied Biosystems).
Forward primer: AGGCAACAGCAGCGTGAAG (final conc. 300 nM)
Reverse primer: GGTCATCTTGCCCTTTGTCAA (final conc. 300 nM)
Probe: CCTGGCCAACACCATCACCAGCTT (final conc. 100 nM)
The primers were designed to bind to separate exons to avoid coamplification of genomic DNA. BLASTn ensured gene specificity of the primers. Amplification was performed in 15-μL reactions using TaqMan Fast PCR MasterMix (Applied Biosystems). Each sample was analyzed in duplicate and samples where CT values differed more than 0.5 were reanalyzed. We conducted real-time PCR reactions using a standard curve methodology to quantitate the specific gene targets of interest. The standard curve was made with embryonic rat RNA (Labinova AB, Upplands Väsby, Sweden) and each sample was normalized to 18S rRNA, which was stably expressed across time. Reactions with no RT and no template were added as negative controls.
Intact tendons and healing tendons were analyzed separately. In both cases, we tested the hypothesis that there would be differences between loaded and unloaded tendons. Intact tendons were analyzed by t-test. The healing tendons were analyzed by two-way ANOVA using loading status and time after tendon transection as independent variables. This ANOVA also tested the hypothesis that gene expression would be time dependent. We corrected for multiple testing using Bonferroni’s method (significance at p < 0.007).
Results
All genes were detected at all time points regardless of mechanical stimulation, with the following exceptions: noggin was never detected (except in the embryonic rat RNA controls), and GDF-6 was not expressed in one of the intact unloaded tendons.
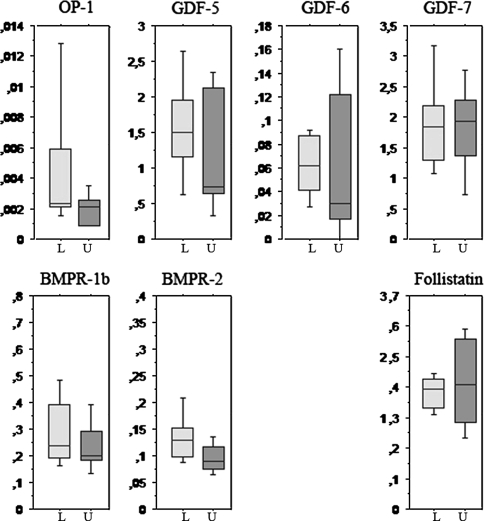
Loading had no detectable effects on any of the agonists, antagonists or receptors in intact tendons (Fig. 1).
Fig. 1.
This box plot shows the expression of the studied genes in intact tendons. The vertical axis shows the ratio to a housekeeping gene (18S rRNA). The horizontal axis shows loading status where loaded tendons (L) are light grey, and unloaded tendons (U) are darker grey. Loading had no major effect on any of the genes. N = 5 for each box.
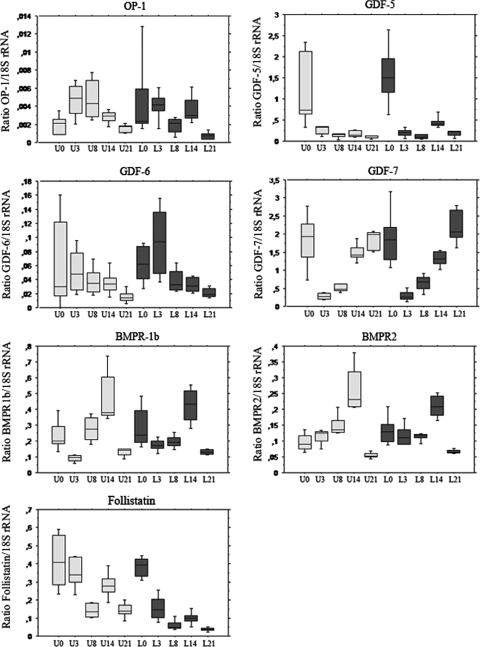
During healing, loading decreased follistatin expression by more than half (p = 0.0001) and tended to slightly increase GDF-5. Follistatin expression decreased continuously during healing, especially in the loaded tendons, where its expression was reduced by over half at 3 days and 90% at 21 days compared to intact tendons. The decrease was less in the unloaded tendons: at 21 days, the expression was four times higher in unloaded tendons compared to loaded.
All genes showed changes (p = 0.0001 for each one) during the time of the healing process, but the time sequences differed (Fig. 2). GDF-5 and GDF-7 were more expressed at all time points compared to OP-1 and GDF-6. The expression of GDF-5 and GDF-7 was lower during healing compared to normal tendons. Their expression almost disappeared during the earliest phase of healing. GDF-7 was back at the level of intact tendons at 21 days, but GDF-5 remained low. OP-1 and GDF-6 showed a reciprocal pattern with high expression during early healing. Both receptors also showed a slight increase during healing, with a peak at 14 days and then a decrease.
Fig. 2.
This box plot shows the expression of the studied genes in all groups. The vertical axis shows the ratio to a housekeeping gene (18S rRNA). The horizontal axis shows time after tendon transection. L0 indicates intact loaded tendons. L3, L8, L14 and L21 indicate loaded healing tendons 3, 8, 14 and 21 days after tendon transection respectively. B0 indicates unloaded intact tendons analyzed 5 days after Botox injection. B3, B8, B14 and B21 indicate unloaded healing tendons 3, 8, 14 and 21 days after tendon transection respectively. Loaded tendons (L) are darker, and unloaded (U) are lighter. N = 5 for each box. During healing, loading decreased follistatin expression by more than half (p = 0.0001) and tended to slightly increase GDF-5. All genes showed changes during the time of the healing process (p = 0.0001 for each one). The expression of GDF-5 and GDF-7 was low during healing compared to normal tendons. GDF-7 was back at the level of intact tendons at 21 days, but GDF-5 remained low. OP-1 and GDF-6 showed a reciprocal pattern with high expression during early healing. Both receptors also showed a slight increase during healing, with a peak at 14 days and then a decrease.
Discussion
Exogenous GDFs stimulate tendon healing [1, 6, 7, 15], and GDF-5-deficient mice have weaker tendons and slower tendon healing [2, 3, 11]. Tendon changes in GDF-7-deficient mice are more subtle, but show compensatory upregulation of both GDF-5 and -6 [10]. GDF-5 and -6 have been detected in human tendon by immunohistochemistry, especially in metabolically active cells [8, 18]. During tendon to bone healing in rats, immunohistochemistry demonstrates increased expression of GDF-5, -6, and -7 [19]. Healing of the rat Achilles tendon is sensitive to mechanical loading. The strength of the tendon callus is reduced by 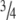 after 14 days if loading is prevented by Botox injections in the manner of our study [16]. Also the response to exogenous GDFs is influenced by loading: if loading is prevented, exogenous GDFs lead to more cartilage and bone formation [6]. Based on this information we believe BMP signaling is crucial during tendon healing and is influenced by mechanical loading. The questions we asked were: does mechanical loading influence the gene expression of the BMP signaling systems in intact and healing tendons? How does the BMP signaling system change during tendon healing?
after 14 days if loading is prevented by Botox injections in the manner of our study [16]. Also the response to exogenous GDFs is influenced by loading: if loading is prevented, exogenous GDFs lead to more cartilage and bone formation [6]. Based on this information we believe BMP signaling is crucial during tendon healing and is influenced by mechanical loading. The questions we asked were: does mechanical loading influence the gene expression of the BMP signaling systems in intact and healing tendons? How does the BMP signaling system change during tendon healing?
We note several limitations. Because we have not analyzed all BMPs, antagonists, and receptors, it is possible other factors within the BMP signaling system play important roles that would change our perception. Our choice of genes was governed by our previous finding of effects of exogenous GDFs and the fact that follistatin is expressed around the forming tendon in chicken embryos, where exogenous follistatin inhibits tendon formation [4]. BMPR1b was chosen because it is known to interact with GDF-5, which does not bind to BMPR1a [13].
We asked if loading activates the system by upregulating agonists or receptors, or downregulating antagonists. Since our idea was not more specific than that, this work is an intermediate between strict hypothesis testing and exploration. We therefore believe an effect of loading should have a very low p-value to be reliable. This is the case for the decreased expression of the antagonist follistatin. In addition, the ratio of agonist/follistatin was substantially increased for all agonists. This finding makes sense: loading would increase GDF signaling, thus mimicking the known effects of exogenous GDFs. Follistatin is expressed around forming chick tendons, and it seems reasonable to presume it serves to delimit their formation. With mechanical loading in our model, the tendon regenerates double their transverse area [16], suggesting delimitation is decreased in parallel with the decrease in follistatin. Follistatin downregulates GDF-6 in Xenopus, but we have found no data in the literature on binding to GDF-5, -6, and -7.
We found no effects of loading in intact tendons. This is not as surprising as it seems, because in previous experiments with similar rats, no or very minor effects on intact tendons were seen with unloading by Botox®; a large number of mechanical parameters were unaffected [5]. The tendons even continued to grow and mature normally in spite of dramatic muscle atrophy. Thus, the present results seem to confirm the intriguing observation that this way of unloading does not affect intact tendons in these animals.
Which is the best time point to look for effects of loading upon healing? The early tendon callus is really a granuloma consisting of many different cell types, of which only a small part might be fibroblasts or able to respond to mechanical stimulation. We have previously reported very early callus is not stimulated to better mechanical healing by loading [16]. At 14 days, most cells appear fibroblastic, and even more so on day 21. At this time the mechanical difference between loaded and unloaded tendons is substantial, which implies mechanosensitivity. Therefore, the groups which are most appropriate for a study of the mechanical influence on gene expression are 14 and 21 days. An ANOVA (with Bonferroni’s correction) with only these time points reveals major effects of loading on GDF-5 and follistatin and differences between the days for most variables. From histological appearance, the tissue at 21 days can be described as a well-organized scar, rather than a tendon (data not shown). It differs from normal tendon by a factor of ten in the expression of follistatin and GDF-5, but there is no difference for GDF-7. This suggests GDF-5 is specific for the mature tendon, and not much involved in healing. This contrasts to GDF-7, which is involved in both, with GDF-6 as an intermediate. OP-1 and GDF-6 seem involved in early healing.
Noggin was not expressed in any of our samples at any time point. It inhibits tendon-to-bone healing and is therefore more likely expressed in the enthesis rather than the midportion of the tendon we examined [9]. Loading decreased follistatin expression which could lead to increased signaling. Thus, the BMP system appears involved in tendon maintenance and healing, and may respond to mechanical loading.
Acknowledgments
We thank the group of Paul Ackermann, Department of Orthopaedics at the Karolinska Institute, Stockholm, who developed the RNA extraction routines, and Daniel Bring, who demonstrated them for us. We thank Maria Jenmalm, Department of Paediatrics at Linköping University, for assistance in creating primers for GDF-5 and her advice on PCR techniques. We thank Therese Andersson for assistance with surgery.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article. One or more of the authors (PE, PA) received grants from the Swedish Research Council, the Strategic Research Program “Materials in Medicine” by Östergötland County and the University of Linköping.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aspenberg P, Forslund C. Enhanced tendon healing with GDF 5 and 6. Acta Orthop Scand. 1999;70:51–54. [DOI] [PubMed]
- 2.Chhabra A, Tsou D, Clark RT, Gaschen V, Hunziker EB, Mikic B. GDF-5 deficiency in mice delays Achilles tendon healing. J Orthop Res. 2003;21:826–835. [DOI] [PubMed]
- 3.Clark RT, Johnson TL, Schalet BJ, Davis L, Gaschen V, Hunziker EB, Oldberg A, Mikic B. GDF-5 deficiency in mice leads to disruption of tail tendon form and function. Connect Tissue Res. 2001;42:175–186. [DOI] [PubMed]
- 4.D’Souza D, Patel K. Involvement of long- and short-range signalling during early tendon development. Anat Embryol (Berl). 1999;200:367–375. [DOI] [PubMed]
- 5.Eliasson P, Fahlgren A, Pasternak B, Aspenberg P. Unloaded rat Achilles tendons continue to grow, but lose viscoelasticity. J Appl Physiol. 2007;103:459–463. [DOI] [PubMed]
- 6.Forslund C, Aspenberg P. CDMP-2 induces bone or tendon-like tissue depending on mechanical stimulation. J Orthop Res. 2002;20:1170–1174. [DOI] [PubMed]
- 7.Forslund C, Rueger D, Aspenberg P. A comparative dose-response study of cartilage-derived morphogenetic protein (CDMP)-1, -2 and -3 for tendon healing in rats. J Orthop Res. 2003;21:617–621. [DOI] [PubMed]
- 8.Fu SC, Wong YP, Chan BP, Pau HM, Cheuk YC, Lee KM, Chan KM. The roles of bone morphogenetic protein (BMP) 12 in stimulating the proliferation and matrix production of human patellar tendon fibroblasts. Life Sci. 2003;72:2965–2974. [DOI] [PubMed]
- 9.Ma CB, Kawamura S, Deng XH, Ying L, Schneidkraut J, Hays P, Rodeo SA. Bone morphogenetic proteins-signaling plays a role in tendon-to-bone healing: a study of rhBMP-2 and noggin. Am J Sports Med. 2007;35:597–604. [DOI] [PubMed]
- 10.Mikic B, Bierwert L, Tsou D. Achilles Tendon characterization in GDF-7 deficient mice. J Orthop Res. 2006;24:831–841. [DOI] [PubMed]
- 11.Mikic B, Schalet BJ, Clark RT, Gaschen V, Hunziker EB. GDF-5 deficiency in mice alters the ultrastructure, mechanical properties and composition of the Achilles tendon. J Orthop Res. 2001;19:365–371. [DOI] [PubMed]
- 12.Murray DH, Kubiak EN, Jazrawi LM, Araghi A, Kummer F, Loebenberg MI, Zuckerman JD. The effect of cartilage-derived morphogenetic protein 2 on initial healing of a rotator cuff defect in a rat model. J Shoulder Elbow Surg. 2007;16:251–254. [DOI] [PubMed]
- 13.Nickel J, Kotzsch A, Sebald W, Mueller TD. A single residue of GDF-5 defines binding specificity to BMP receptor IB. J Mol Biol. 2005;349:933–947. [DOI] [PubMed]
- 14.Reno C, Marchuk L, Sciore P, Frank CB, Hart DA. Rapid isolation of total RNA from small samples of hypocellular, dense connective tissues. Biotechniques. 1997;22:1082–1086. [DOI] [PubMed]
- 15.Rickert M, Jung M, Adiyaman M, Richter W, Simank HG. A growth and differentiation factor-5 (GDF-5)-coated suture stimulates tendon healing in an Achilles tendon model in rats. Growth Factors. 2001;19:115–126. [DOI] [PubMed]
- 16.Virchenko O, Aspenberg P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthop. 2006;77:806–812. [DOI] [PubMed]
- 17.Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N, Dube JL, DiBlasio-Smith E, Nove J, Song JJ, Wozney JM, Rosen V. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321–330. [DOI] [PMC free article] [PubMed]
- 18.Wong YP, Fu SC, Cheuk YC, Lee KM, Wong MW, Chan KM. Bone morphogenetic protein 13 stimulates cell proliferation and production of collagen in human patellar tendon fibroblasts. Acta Orthop. 2005;76:421–427. [DOI] [PubMed]
- 19.Würgler-Hauri CC, Dourte LM, Baradet TC, Williams GR, Soslowsky LJ. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J Shoulder Elbow Surg. 2007;16 (5 Suppl):S198–203. [DOI] [PMC free article] [PubMed]