Abstract
Periprosthetic joint infection is one of the most challenging complications of joint arthroplasty. We identified current risk factors of periprosthetic joint infection after modern joint arthroplasty, and determined the incidence and timing of periprosthetic joint infection. We reviewed prospectively collected data from our database on 9245 patients undergoing primary hip or knee arthroplasty between January 2001 and April 2006. Periprosthetic joint infections developed in 63 patients (0.7%). Sixty-five percent of periprosthetic joint infections developed within the first year of the index arthroplasty. The infecting organism was isolated in 57 of 63 cases (91%). The most common organisms identified were Staphylococcus aureus and Staphylococcus epidermidis. We identified the following independent predictors for periprosthetic joint infection: higher American Society of Anesthesiologists score, morbid obesity, bilateral arthroplasty, knee arthroplasty, allogenic transfusion, postoperative atrial fibrillation, myocardial infarction, urinary tract infection, and longer hospitalization. This study confirmed some previously implicated factors and identified new variables that predispose patients to periprosthetic joint infection.
Level of Evidence: Level II, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Total joint arthroplasty is a safe and effective procedure improving the quality of life and restoring function to patients with arthritis of the hip and knee [4, 6, 8]. Although its general success is beyond dispute, postoperative complications such as periprosthetic joint infection (PJI) still occur. Currently, PJI is an important mechanism of implant failure and need for revision arthroplasty [3, 18, 20].
Numerous challenges may be associated with PJI that can include the need for multiple operations, a long period of disability for the patient, and, occasionally, suboptimal outcome [2, 17]. In the elderly, PJI may result in a higher incidence of mortality [9].
Although the use of prophylactic antibiotics and other strategies such as the use of body exhaust systems and laminar airflow in the operating room have helped reduce the incidence of PJI [12, 13], they have not eliminated the risk. It has been projected that the incidence of PJI may be increasing [7]. Because of the economic and psychologic burden of this complication, strategies to minimize or prevent PJI may be needed. Therefore, continuous effort and research are necessary to help reduce its incidence to a minimum. We believe identification of the current risk factors that predispose patients to deep periprosthetic joint infection after modern hip and knee arthroplasties will help establish simple strategies to avoid them. We presumed modern-day risk factors (Table 1) for PJI might differ from those previously described.
Table 1.
Continuous and categorical variables used to identify possible predictors of joint infection
| Preoperative variables |
|---|
| Demographic factors: Gender, age, ethnicity, height, weight, body mass index |
| Patient medical factors: American Society of Anesthesiologists score, alcohol abuse, hypertension, hyperlipidemia, diabetes mellitus, rheumatoid arthritis, cardiac arrhythmias, coronary heart disease, peripheral vascular disease, congestive heart failure, cardiac transplant, cardiac valvular disease, dementia, stroke, neurologic disease (paralysis, dyskinesia, Parkinson), renal insufficiency, renal failure and dialysis, anemia (aplastic, autoimmune, iron deficiency), coagulopathy, urinary tract infection, liver disease (hepatitis B, hepatitis C, hepatic insufficiency), malignancy (all visceral, metastatic and melanoma), tuberculosis, venous thromboembolic disease |
| Preoperative laboratory values: Hemoglobin, international normalized ratio (INR), leukocyte count, glucose, creatinine, albumin |
| Surgical and postoperative variables |
| Surgery: Joint operated (hip versus knee), side (unilateral, simultaneous bilateral), operative time (minutes) |
| Blood management: Transfusion (yes or no, number of units transfused, type of transfusion—allogenic versus autologous) |
| Postoperative laboratory values: Hemoglobin, INR, leukocyte count, glucose, creatinine, albumin |
| Postoperative medical complications: Urinary tract infection, pneumonia, Clostridium difficile-associated diarrhea, pulmonary embolism, acute myocardial infarction, arrhythmia, congestive heart failure, atrial fibrillation, stroke, deep venous thrombosis, lung aspiration, fever |
| Postoperative local complications: Cellulitis, hematoma, wound infection, wound drainage, wound dehiscence, blisters, vascular injury, compartment syndrome, dislocation |
We therefore had two objectives: First to identify current risk factors of periprosthetic joint infection after modern joint arthroplasty, and second to determine the incidence and timing of periprosthetic joint infection. We also report our experience with the current causative organisms and initial management of PJI.
Materials and Methods
We performed a retrospective cohort study using prospectively collected data from our arthroplasty database; patients were not recalled for this study. We identified 9245 patients undergoing primary hip or knee arthroplasty between January 2001 and April 2006. Of these, 7739 patients underwent unilateral joint arthroplasty, and 1496 had simultaneous bilateral surgery. Primary hip arthroplasty was performed in 5060 patients, including 4635 unilateral surgeries, and 425 simultaneous bilateral hip replacements. Primary knee arthroplasty was performed in 4185 patients, including 3114 unilateral procedures and 1071 bilateral total knee replacements. There were 3882 males with a mean age of 62 years (range, 15–94 years) and 5363 females with a mean age of 66 years (range, 14–97 years). The minimum followup was 12 months (mean, 43 months; range 12–76 months). We did not have information regarding the number of patients lost to followup before the 12-month minimum followup.
The protocols for infection prophylaxis were the same throughout the study period. This included administration of intravenous antibiotic within 1 hour of the arthroplasty and for 24 hours postoperatively. We performed all arthroplasties in operating rooms equipped with vertical laminar flow with all members of the surgical team wearing helmet aspirator suits. Neuraxial anesthesia was used for all surgeries unless contraindicated. We placed patients in the supine position for surgery. Skin preparation included the use of alcohol and iodine lavage plus an Incise adhesive drape (3M™ Ioban™, St. Paul, MN). We routinely used double gloves for all procedures.
We performed hip arthroplasty through a modified anterolateral approach using uncemented components in all cases. Knee arthroplasty was performed under tourniquet using a medial parapatellar arthrotomy approach. We used cemented fixation for all knees. No drains were used in any of the cases.
Postoperative wound management consisted of application of a sterile dressing, which was placed over the incision in the operating room and usually kept for 48 hours. We then inspected the wound and changed the sterile gauze twice daily until the patient was discharged from the hospital.
The prophylactic anticoagulation regimen consisted of administration of warfarin on postoperative Day 1 and continuing for 6 weeks, aiming for an international normalized ratio of 1.5 to 2.0. Unless contraindicated, patients also received 1000 units of intravenous heparin at the time of dislocation of the hip or before inflation of the tourniquet during knee arthroplasty. Patients’ comorbid conditions or postoperative cardiovascular complications altered the chemoprophylaxis protocol in some cases, which included administration of intravenous or subcutaneous heparin for patients with arrhythmia or cardiac disease.
We followed patients at 6 weeks, 6 months, 2 years, and 5 years after surgery. They are encouraged to contact their surgeon as needed or in the presence of fever, wound drainage, or any unexpected adverse event. We prescribed antibiotic prophylaxis before dental procedures (600 mg clindamycin orally) to all patients at their first followup, which was recommended during the first 2 years after arthroplasty.
The diagnosis of deep periprosthetic infection was made if at least three of the following five criteria were present: (1) abnormal serology (erythrocyte sedimentation rate > 30 mm/hour; C-reactive protein > 1 mg/dL); (2) strong clinical and radiographic suspicion for periprosthetic infection such as periosteal elevation, focal osteolysis, hot and swollen joint, draining sinus, (3) positive joint aspiration culture; (4) evidence of purulence during the subsequent surgical intervention; and (5) a positive intraoperative culture [11].
We performed descriptive analysis using univariate statistics on a large number of variables (Table 1). Patients were included only once in the analysis even though some patients received multiple surgeries. All continuous variables are reported as means and standard deviations. We compared potential predictive variables between the two groups of patients (with or without deep periprosthetic infection) using parametric (t statistics) or nonparametric statistics (Wilcoxon) as appropriate. We reported categorical variables as frequency distribution and comparisons were made using parametric (chi square statistics) and nonparametric statistics (Fisher’s exact). We kept variables significant at p = 0.10 in the final statistical model using stepwise logistic regression. Multivariate analysis then was performed using selection stepwise logistic regression after adjusting for the potential confounders to determine noteworthy predictors of periprosthetic infection. Confounders included the following: race, physical status score (ASA), body mass index, rheumatoid arthritis, venous thromboembolism, anemia, hypercholesterolemia, dementia, knee arthroplasty, simultaneous bilateral surgery, operative time, hospital length, postoperative creatinine, allogenic transfusion, postoperative atrial fibrillation, postoperative myocardial infarction, postoperative urinary tract infection, postoperative wound drainage, and postoperative hematoma. Variables were considered clinically important predictors of periprosthetic infection with a p < 0.05 after adjusting for the potential confounders. All analyses performed were two-tailed and at an alpha level of 0.05. All results have been reported as 95% confidence intervals and p values.
Results
Periprosthetic infection was diagnosed in 63 of 9245 patients, representing an overall incidence of 0.7% (Table 2). Infection developed after hip arthroplasties in 15 patients and after knee arthroplasties in 48 patients accounting for an incidence of 0.3% (15 of 5060) and 1.1% (48 of 4185), respectively (p < 0.0001). The majority of PJI (65% [41 of 63 infections]) were diagnosed within the first year after surgery. Twenty-seven percent of the infections were diagnosed during the first 30 days after arthroplasty (17 patients). The average time to diagnosis of PJI was 431 days after the index surgery (range, 11–1699 days). Infection was classified as chronic late in 35 patients (56%), acute or early in 20 patients (31%), and as acute hematogenous in eight patients (13%).
Table 2.
Details of periprosthetic joint infection in the cohort
| Description | Total joint | Knee arthroplasty | Hip arthroplasty |
|---|---|---|---|
| Number of patients | 9245 | 4185 | 5060 |
| Incidence (%) | 0.7 | 1.1 | 0.3 |
| Mean time to diagnosis (days) | 431 | 431 | 428 |
| Classification | |||
| I. Acute | 20 (31.7%) | 14 (30%) | 6 (40%) |
| II. Chronic | 35 (55.6%) | 29 (60%) | 6 (40%) |
| III. Acute hematogenous | 8 (12.7%) | 5 (10%) | 3 (20%) |
| Microorganism | 63 | 48 | 15 |
| Gram-positive | 46 | 32 | 14 |
| MRSA | 12 | 7 | 5 |
| MSSA | 12 | 8 | 4 |
| MRSE | 7 | 4 | 3 |
| MSSE | 5 | 4 | 1 |
| Staphylococcus lugdunensis | 1 | 1 | |
| Streptococcus agalactiae | 3 | 3 | |
| Streptococcus pyogenes | 1 | 1 | |
| Streptococcus mitis | 3 | 3 | |
| Streptococcus pneumoniae | 1 | 1 | |
| Coryneform striatum | 1 | 1 | |
| Gram-negative | 7 | 7 | |
| Escherichia coli | 2 | 2 | |
| Klebsiella pneumoniae | 2 | 2 | |
| Proteus mirabilis | 1 | 1 | |
| Pseudomonas aeruginosa | 1 | 1 | |
| Serratia marcescens | 1 | 1 | |
| Polymicrobial | 4 | 3 | 1 |
| P. aeruginosa + E. faecalis + K. pneumoniae | 1 | 1 | |
| Escherichia coli + Enterococcus faecalis | 1 | 1 | |
| MRSA + MSSA | 1 | 1 | |
| MRSE + Enterococcus faecalis | 1 | 1 | |
| No growth | 6 | 6 | 0 |
MRSA = Methicillin-resistant Staphylococcus aureus; MSSA = Methicillin-sensitive S. aureus; MRSE = Methicillin-resistant Staphylococcus epidermidis; Methicillin-sensitive S. epidermidis.
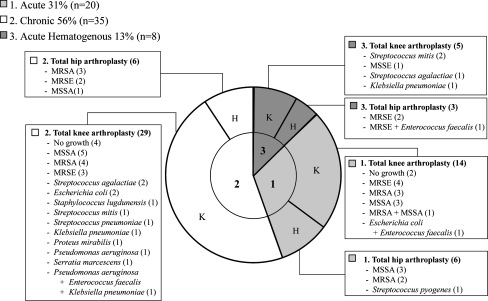
The organism was isolated in 91% of the cases (57 of 63). Clustered gram-positive cocci were the most common infecting organisms. The isolated organisms in the order of frequency were methicillin-resistant Staphylococcus aureus (19%), methicillin-sensitive S. aureus (19%), methicillin-resistant Staphylococcus epidermidis (11%), and methicillin-sensitive S. epidermidis (8%). Fifty-three percent of the Staphylococcus organisms were methicillin-resistant. Gram-negative bacteria were isolated in seven joints (11%); Escherichia coli and Klebsiella pneumoniae were the most prevalent organisms. Polymicrobial infection was diagnosed in four patients (Fig. 1).
Fig. 1.
Classification and infectious organisms in deep periprosthetic joint infections are shown. Methicillin-resistant Staphylococcus aureus (MRSA), methicillin-sensitive S. aureus (MSSA), methicillin-resistant Staphylococcus epidermidis (MRSE), methicillin-sensitive S. epidermidis (MSSE).
The majority (63%) of the PJIs initially were treated with two-stage exchange arthroplasty. We performed irrigation and débridement in 21 patients (33%). Two patients (4%) underwent one-stage reimplantation.
Unadjusted univariate analysis presented multiple variables associated with PJI (Table 3). After multivariable logistic regression analysis, we found the following independent predictors of PJI after primary joint arthroplasty: higher American Society of Anesthesiologists score (> 2), morbid obesity (body mass index > 40 kg/m2), simultaneous bilateral surgery (compared with unilateral procedures), TKA (compared with THA), transfusion of allogenic blood units, postoperative atrial fibrillation, myocardial infarction, urinary tract infection, and longer hospitalizations (mean of 5 days hospitalization) (Table 4).
Table 3.
Predisposing factors for periprosthetic joint infection*
| Variables | p Value |
|---|---|
| Patient preoperative factors | |
| Hispanic | 0.0001 |
| American Society of Anesthesiologists > 2 | 0.002 |
| Higher body mass index | 0.03 |
| Rheumatoid arthritis | 0.008 |
| Venous thromboembolism | 0.001 |
| Anemia | 0.004 |
| Hypercholesterolemia | 0.03 |
| Dementia | 0.02 |
| Surgical factors | |
| TKA | < 0.0001 |
| Simultaneous bilateral | 0.0008 |
| Longer operative time | < 0.0001 |
| Longer hospital stay | < 0.0001 |
| Postoperative factors | |
| Higher creatinine | 0.006 |
| Allogenic blood transfusion | < 0.0001 |
| Myocardial infarction | 0.03 |
| Atrial fibrillation | < 0.0001 |
| Urinary tract infection | < 0.0001 |
| Wound drainage | 0.002 |
| Hematoma | 0.0007 |
*univariate, unadjusted analysis.
Table 4.
Independent predisposing factors for periprosthetic joint infection*
| Independent factors | Slope | Standard error | Odds ratio | 95% Confidence interval | p Value |
|---|---|---|---|---|---|
| Body mass index > 40 kg/m2 | 1.17 | 0.36 | 3.23 | 1.6–6.5 | 0.001 |
| American Society of Anesthesiologists > 2 | 0.67 | 0.33 | 1.95 | 1.0–3.7 | 0.04 |
| Simultaneous bilateral surgery | 1.77 | 0.44 | 5.85 | 2.5–13.9 | < 0.0001 |
| TKA | 1.05 | 0.34 | 2.85 | 1.5–5.6 | 0.002 |
| Allogenic blood transfusion | 0.75 | 0.31 | 2.11 | 1.1–3.9 | 0.02 |
| Postoperative atrial fibrillation | 1.83 | 0.78 | 6.22 | 1.4–28.5 | 0.02 |
| Postoperative myocardial infarction | 3.02 | 1.16 | 20.4 | 2.1–199.9 | 0.009 |
| Postoperative urinary infection | 1.7 | 0.84 | 5.45 | 1.0–8.7 | 0.04 |
| Longer hospital stay | 0.09 | 0.02 | 1.09 | 1.0–1.1 | 0.0003 |
*multivariate, adjusted analysis.
Discussion
Deep periprosthetic infection is one of the most common causes of implant failure and revision surgery [3, 18, 20], with dramatic medical and socioeconomic implications. Although implementation of standardized strategies such as a clean air operating room, adequate administration of perioperative antibiotics, and body exhaust systems have contributed to decrease this complication, periprosthetic infection still continues to occur after total joint arthroplasties [3, 5, 13]. Identification of patients at high risk for PJI after modern joint replacement is necessary and would allow establishing adequate measurements to help prevent it.
There are some weaknesses of our study. First, this is a retrospective cohort study subject of variability in data collection. However, the prospective nature of our data collection probably helped reduce recall and selection bias to our analysis. Second, because of the relatively low incidence of events (PJI), the analysis may have failed to detect the significance of some factors. However, it is based on a relatively large cohort of patients undergoing primary arthroplasty in one institution and with all patients subjected to the same care protocols. Third, because it was not our intention to report the all-time incidence of PJI, but merely to follow the patients for a minimum of 1 year, the true incidence of PJI in this cohort is likely underestimated. Finally, we are aware the multivariate model is more appropriate of the two models. However, we provided the results of our initial unadjusted analysis (Table 3), because that provides all possible important variables associated with infection from among those we studied.
We confirmed some previously implicated risk factors such as wound drainage, hematoma formation, history of rheumatoid arthritis, longer operative time, and urinary tract infection [1, 12–14, 19, 21] remain as important predisposing factors for modern PJI. We also identified new risk factors that, to our knowledge, have not been linked to PJI previously [1, 12–14, 16, 19, 21]. However, to remove the influence of confounding variables, we performed multivariate analysis to identify the independent factors (Table 4). Among these independent factors was the development of postoperative atrial fibrillation and myocardial infarction. It is intriguing why such medical complications should predispose the patients to PJI. One plausible explanation is all patients with serious cardiac complications receive aggressive anticoagulation with heparin or similar agents. Use of aggressive anticoagulation has been reported as an independent risk factor for development of PJI [10]. Another explanation is these complications develop in patients who generally are sicker and older with preexistent medical conditions that would retard wound healing resulting in later infection.
Yet another important risk factor for development of PJI was longer hospital stay. Longer hospital stay was an independent risk factor even after adjusting for age, previous medical comorbidities, wound problems, and development of medical complications. One may hypothesize that with a longer hospital stay, patients were more likely exposed to nosocomial and virulent organisms that could result in later PJI. In fact, the large incidence of PJI by resistant organisms may relate partly to this factor.
Our data confirm transfusion with allogenic blood is an independent risk factor for having PJI develop. Patients receiving allogenic transfusion were 2.1 times more likely to have PJI develop compared with patients receiving no transfusion. The association between allogenic transfusion and infection has been reported and relates to the immunomodulation effect of the transfusion [15]. Among other important risk factors, and in line with a previous study by Wilson et al., the presence of urinary tract infection and obesity is also important [21].
Based on the findings of our study, we have instituted stringent preoperative protocols that aim to minimize these risk factors. For example, for patients with a history of urinary tract infection or at high risk, we have instituted preoperative screening. If urinary infection is confirmed, the patients will receive appropriate antibiotic therapy and the infection will be eradicated before proceeding with joint arthroplasty. Another important protocol relates to blood conservation. Autologous blood donation is offered to all of our patients undergoing hip arthroplasty and particularly encouraged for patients awaiting bilateral hip arthroplasties. We reserve bilateral joint arthroplasties for a select group of patients with minimal comorbidities. Knee arthroplasty is performed under tourniquet to minimize operative blood loss and all exposed tissue is cauterized gently to prevent postoperative hematoma formation. Other efforts used to prevent postoperative hematoma formation include meticulous hemostasis and the use of drain in cases with large dead space. In addition, we attempt to minimize the need for postoperative transfusion by screening and correcting, whenever possible, the preoperative anemia. Some patients may be candidates for erythropoietin therapy before surgery. We also refrain from administering blood transfusion based on hemoglobin values and proceed with transfusion for symptomatic patients only. Patients who have atrial fibrillation or myocardial infarction now receive controlled anticoagulation without boluses per new protocols. Patients also are discharged from the hospital as soon as it is possible and safe to do so. We continue to implement strict sterile techniques and other proven methods such as adequate antibiotic prophylaxis to minimize PJI.
We believe PJI will continue to present a major challenge for the medical community in the future. Effective strategies to minimize risk factors identified in this and previous studies should be implemented if the incidence of this complication is to be reduced.
Footnotes
One of the authors (JP) is a consultant for Stryker Orthopaedics.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Berbari EF, Hanssen AD, Duffy MC, Steckelberg JM, Ilstrup DM, Harmsen WS, Osmon DR. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis. 1998;27:1247–1254. [DOI] [PubMed]
- 2.Bozic KJ, Katz P, Cisternas M, Ono L, Ries MD, Showstack J. Hospital resource utilization for primary and revision total hip arthroplasty. J Bone Joint Surg Am. 2005;87:570–576. [DOI] [PubMed]
- 3.Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop Relat Res. 2004;429:188–192. [DOI] [PubMed]
- 4.Felson DT, Lawrence RC, Hochberg MC, McAlindon T, Dieppe PA, Minor MA, Blair SN, Berman BM, Fries JF, Weinberger M, Lorig KR, Jacobs JJ, Goldberg V. Osteoarthritis: new insights. Part 2: treatment approaches. Ann Intern Med. 2000;133:726–737. [DOI] [PubMed]
- 5.Garvin KL, Hanssen AD. Infection after total hip arthroplasty: past, present, and future. J Bone Joint Surg Am. 1995;77:1576–1588. [DOI] [PubMed]
- 6.Jones CA, Beaupre LA, Johnston DW, Suarez-Almazor ME. Total joint arthroplasties: current concepts of patient outcomes after surgery. Clin Geriatr Med. 2005;21:527–41, vi. [DOI] [PubMed]
- 7.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. [DOI] [PubMed]
- 8.Lavernia CJ, Guzman JF, Gachupin-Garcia A. Cost effectiveness and quality of life in knee arthroplasty. Clin Orthop Relat Res. 1997;345:134–139. [DOI] [PubMed]
- 9.McGarry SA, Engemann JJ, Schmader K, Sexton DJ, Kaye KS. Surgical-site infection due to Staphylococcus aureus among elderly patients: mortality, duration of hospitalization, and cost. Infect Control Hosp Epidemiol. 2004;25:461–467. [DOI] [PubMed]
- 10.Parvizi J, Ghanem E, Joshi A, Sharkey PF, Hozack WJ, Rothman RH. Does “excessive” anticoagulation predispose to periprosthetic infection? J Arthroplasty. 2007;22(suppl 2):24–28. [DOI] [PubMed]
- 11.Parvizi J, Ghanem E, Menashe S, Barrack RL, Bauer TW. Periprosthetic infection: what are the diagnostic challenges? J Bone Joint Surg Am. 2006;88(suppl 4):138–147. [DOI] [PubMed]
- 12.Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;392:15–23. [DOI] [PubMed]
- 13.Phillips JE, Crane TP, Noy M, Elliott TS, Grimer RJ. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg Br. 2006;88:943–948. [DOI] [PubMed]
- 14.Poss R, Thornhill TS, Ewald FC, Thomas WH, Batte NJ, Sledge CB. Factors influencing the incidence and outcome of infection following total joint arthroplasty. Clin Orthop Relat Res. 1984;182:117–126. [PubMed]
- 15.Raghavan M, Marik PE. Anemia, allogenic blood transfusion, and immunomodulation in the critically ill. Chest. 2005;127:295–307. [DOI] [PubMed]
- 16.Saleh K, Olson M, Resig S, Bershadsky B, Kuskowski M, Gioe T, Robinson H, Schmidt R, McElfresh E. Predictors of wound infection in hip and knee joint replacement: results from a 20 year surveillance program. J Orthop Res. 2002;20:506–515. [DOI] [PubMed]
- 17.Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty: a retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81:1434–1445. [DOI] [PubMed]
- 18.Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7–13. [DOI] [PubMed]
- 19.Surin VV, Sundholm K, Bäckman L. Infection after total hip replacement: with special reference to a discharge from the wound. J Bone Joint Surg Br. 1983;65:412–418. [DOI] [PubMed]
- 20.Vessely MB, Whaley AL, Harmsen WS, Schleck CD, Berry DJ. The Chitranjan Ranawat Award: Long-term survivorship and failure modes of 1000 cemented condylar total knee arthroplasties. Clin Orthop Relat Res. 2006;452:28–34. [DOI] [PubMed]
- 21.Wilson MG, Kelley K, Thornhill TS. Infection as a complication of total knee-replacement arthroplasty: risk factors and treatment in sixty-seven cases. J Bone Joint Surg Am. 1990;72:878–883. [PubMed]