Abstract
Tendon integrity depends on the extracellular matrix (ECM) metabolism which is regulated by proteolytic enzymes. However, it is unclear which enzymes play a role in tendon rupture. We studied the ECM of 19 ruptured human Achilles tendons, comparing the composition of specimens harvested close to the rupture with specimens harvested from an apparently healthy area in the same tendon. We compared gene expression of collagen Type I, decorin, and versican including enzymes involved in their metabolism as matrix metalloproteases (MMP-2 and -9) and tissue inhibitory of metalloproteinase (TIMP-1 and -2) using real-time PCR, zymography and FACE analysis. We found greater gene expression of proteoglycan core protein decorin and versican, collagen Type I, MMPs and TIMPs in the tendon rupture. Zymography analysis, reflecting expression of enzymatic activity, confirmed the gene expression data at protein level. Carbohydrate content was greater in the macroscopically healthy area than in the ruptured area. In the ruptured area, we found increased core protein synthesis but without the normal glycosaminoglycan production. The tissue in the area of rupture undergoes marked rearrangement at molecular levels and supports the role of MMPs in the pathology.
Introduction
The molecular structure of tendons is finely regulated, and involves several molecules. The smallest unit of tendon is the collagen fibril, which is mostly made up of collagen I. The fibrils aggregate together to form fibers, and a number of fibers make up a fascicle. Fascicles are predominantly aligned along the longitudinal axis of the tendon, and are responsible for their tensile strength [14].
The extracellular matrix (ECM) is constituted by a complex network of molecules interacting with each other, including, beside the collagen fibers, glycosaminoglycans, proteoglycans, glycoproteins, other noncollagenous proteins, and water [23]. Tenocytes lie in the ECM and contribute to its homeostasis. Matrix metalloproteases (MMPs) are involved in remodeling of the extracellular matrix of tendons, and the various MMPs can be up- or down-regulated in tendinopathy [17]. A balance between MMPs and tissue inhibitors of metalloproteinases (TIMPs) is required to maintain ECM homeostasis [24]. The mechanism of activation of MMPs is poorly understood. Their precise role in tendinopathy is still unclear, and it is conceivable that MMPs play a role in tendinopathies [16]. However, in other tissues mechanical stress is coupled with the activation of MMP and matrix degradation, which alters its capacity to react to tensile strength [18].
We hypothesized that the expression and metabolism of collagen Type I and proteoglycans (decorin and versican) is altered with low protein turnover in samples from Achilles tendon ruptures. We specifically ascertained levels of MMPs, collagen Type I, and proteoglycans in human Achilles tendon ruptures.
Materials and Methods
We identified 19 nineteen consecutive patients (14 men and five women; average age, 37.3 years; range, 25–58 years) undergoing an open repair of a spontaneous Achilles tendon rupture. We obtained two biopsies of each patient: one from the rupture area and one from a seemingly healthy area proximal to the injury. Six patients were smokers (> 10 cigarettes per day). Ten patients sustained a right-sided rupture. Eight of the 19 were athletes, six had taken part in recreational sports, and five were sedentary. In 16 patients, the lesion occurred during sport activities and in three patients during daily activities. The average time between trauma and surgery was 3.7 days (range, 1–19 days). The study was approved by the Local Ethics Committee. All patients gave written informed consent to participate and to have specimens from the torn Achilles tendon harvested at surgery.
After exposure of the lesion, we obtained two specimens from each patient, one from the area of rupture, and one 6 cm proximal to it in the apparently healthy area of the tendon. In all patients, the lesion was in the mid part of the tendon with an average distance from the insertion of calcaneus of 4.2 cm (range, 3.5–4.4 cm). Each specimen was 6 mm long, 2 mm wide, and 3 mm thick. The samples were frozen at −80° immediately after surgical harvest and kept at −80°C until analysis.
Proteinase K came from Finnzyme (Epoo, Finland). Hyaluronidase SD and Chondroitinase ABC were purchased from Seikagaku (Tokyo, Japan), biotinilated HABP was purchased from Akira Asari of Seikagaku (Tokyo, Japan). Standard disaccharides were obtained from Sigma (St. Louis, MO), 2-aminoacridone came from Molecular Probes (Eugene, OR). Water for aqueous solutions was from MilliQ™. All chemicals were of analytical reagent grade.
The human tendon specimens were carefully isolated from the connective tissue contaminants using microsurgery tools and a stereomicroscope. The tissue removed was amorphous connective tissue, with scanty fibrin clots.
Total RNA from each sample was extracted using the RNAqueus-4PCR kit from Ambion (Cambridgeshire, UK), followed by a DNase treatment, included in the kit. The concentration of purified RNA was determined fluorometrically using the RiboGreen® RNA Quantitation Reagent and Kit from Molecular Probes (Leiden, the Netherlands).
Equal amounts (80 ng) of total RNA from each tissue sample were reverse transcribed using random primers (hexamers), according to the protocol of High Capacity cDNA Archive Kit from Applied Biosystems (Foster City, CA). An RNase inhibitor (Applied Biosystems, Foster City, CA) at final concentration of 1.0 U/mL was also included in the reaction.
Real-time PCR was performed using TaqMan technology and ABI Prism 7700 apparatus (Applied Biosystems). The GAPDH, Col I, Col IX, Decorin, Versican, MMP-2, MMP-9, TIMP-1, and TIMP-2 gene expression was studied. GAPDH gene expression was used as a housekeeping gene. For these experiments, oligonucleotide primers and TaqMan® probes were Assays-on-Demand of Applied Biosystems. The melting temperature (Tm) for primers was 58 to 60°C and for probes approximately 10°C higher than the Tm of the primers. TaqMan® probes had either FAM or VIC reporters. Amplicon lengths were between 50 to 150 bp.
The PCR reaction mix contained 2.5 μL of cDNA, 12.5 μL of Universal Master Mix (Applied Biosystems), 1.25 μL of Assay-on-Demand primer and probe and 8.75 μL of nuclease-free water. The PCR program consisted of an initial hot start at 50°C for 2 minutes, followed by 95°C for 10 minutes and a 45 amplification cycles (95°C for 15 seconds and 60°C for 60 seconds).
For each sample, ABI PRISM 7000 Sequence Detection System (SDS) software plotted an amplification curve by relating the fluorescence signal intensity (ΔRn) to the cycle number. The ΔRn value corresponded to the variation in reporter fluorescence intensity during each PCR cycle, normalized to the fluorescence of an internal passive reference (ROX, which is included in the Universal Master Mix). A specific termed cycle threshold (Ct) was determined for each PCR. Ct was defined as the cycle number at which we judged an increase in the fluorescence signal was first detected. At this point, the sample signal shows an exponential increase from the basal line.
We performed a relative quantitative analysis, using the 2^(−ΔΔCt) value, where ΔCt = Ct (target) − Ct (endogenous control) and ΔΔCt = ΔCt (sample) − ΔCt (calibrator). GAPDH was used as an endogenous control and the sample with the lower expression of the target was used as calibrator.
The proteoglycans content was assessed evaluating their specific polysaccharide chains (glycosaminoglycans − GAG). We lyophylized the specimens, suspended them in 300 μL of 0.1 M ammonium-acetate pH 7, and digested with proteinase K at 60°C for 2 hours in order to free the glycosaminoglycans (GAG) from proteins. After enzyme inactivation, 1.2 mL ethanol 96% (or absolute ethanol) was added and the mixture was frozen at −20°C overnight to precipitate the GAG. After centrifugation at 10,000 g at 4°C for 15 minutes, the pellets were solubilized with 100 μL of 0.1 M ammonium-acetate at pH 7, and sonicated for 1 minute in an ultrasound bath. Polysaccharides were digested with hyaluronidase (100 mU/mL) at 37°C for 1 hour in a shaking bath (Eppendorf) and then digested with chondroitinase ABC (100 mU/mL) at 37°C for 3 hours. This treatment was able to produce disaccharides from GAG hyaluronan (ΔHA 0S) and chondroitin sulfate (ΔCS 0S, ΔCS 4S and ΔCS 6S) present in the solution. After enzyme inactivation, the resulting disaccharides were lyophilized and treated in order to obtain 2-aminoacridone (AMAC) derivatization. AMAC derivatization was carried out according to Calabro et al. [4].
FACE analysis (fluorophore-assisted carbohydrate electrophoresis) was performed. The AMAC-derived disaccharides from CS and HA were separated by polyacrylamide gel electrophoresis, as previously described [13]. The gels were visualized by exposure to UV light (BIO-RAD Gel Doc 2000), and the semiquantitative analysis was performed using a dedicated software (BIO-RAD Quantity One).
We also evaluated expression by zymography as a measure of enzymatic activity. To detect MMP-2 and MMP-9 activities, the tendons were homogenized in ice cold PBS using an Ultraturrax apparatus. After centrifugation the extracts were run on SDS polyacrylamide gels containing 1 mg/mL gelatin. Samples were loaded in the gels without denaturation in the presence of reducing agents. Each gel was washed at room temperature for 2 hours in 2.5% Triton X-100 and incubated overnight at 37°C in 10 mM CaCl2, 150 mM NaCl, and 50 mM Tris-HCl, pH 7.5 buffer. The gel was stained in 2% (v/v) Coomassie Blue G-250, and photographed on a light box after appropriate destaining. Proteolysis was detected as a white zone in a dark blue field.
Descriptive statistics were calculated. We determined differences in the means of gene expressions of all proteins for the injured and apparently healthy tissue using nonparametric χ-paired test. Alpha of 0.05 was applied.
Results
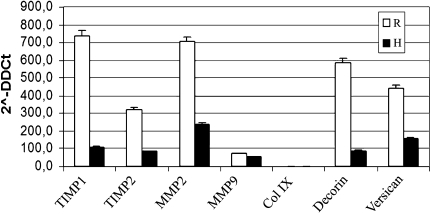
The real time RT-PCR data showed a similar trend in gene expression in all the samples analyzed. In the tendon rupture area, the gene expression and the synthesis of matrix protein were substantially altered. The gene expression of decorin, versican, TIMP-1 and TIMP-2, MMP-2 of the specimens, harvested from ruptured areas, was increased (p < 0.05) compared to control area (Fig. 1). Col IX expression was almost absent in both control and ruptured tendon. The expression of Col I A1 was also compared to other genes (Fig. 2). The expression of Col I A1 in the rupture area was increased (p < 0.05).
Fig. 1.
The gene expression pattern of tendon specimens harvested close and far from the rupture area is shown. The bars represent a mean of three different experiments on 19 different patients. R = gene expression of the ruptured area; H = gene expression of the control area.
Fig. 2.
The same genes reported in Fig. 1 are compared with the expression pattern of the Col I A1 gene. The bars represent a mean of three different experiments on 19 different patients. R = gene expression of the ruptured area; H = gene expression of the control area.
FACE analysis of disaccharides obtained from specimens from ruptured and control areas showed that the control area contained more (p < 0.05) ΔCSOS, and traces of ΔΗΑOS were more evident in the same area (Fig. 3A). The semiquantitative analysis for ΔCSOS indicated that the ruptured area contained a lower concentration (p < 0.05) than the intact area (3.22 ± 1.75 pmol/mg versus 7.66 ± 1.79 pmol/mg, respectively). For the ΔCS6S, the ruptured area also contained a lower concentration (p < 0.05) than the intact area (2.95 ± 1.48 pmol/mg versus 9.01 ± 3.12 pmol/mg, respectively) (Fig. 3B).
Fig. 3A–B.
These are examples of FACE analysis of the glycosaminoglycan disaccharides obtained from the human tendons: H = control area; R = rupture area. (A) The nonsulphated glycosaminoglycans are shown. (B) The sulphated glycosaminoglycans disaccharides are shown. FACE analysis was performed on 19 different samples, and the data showed the same experimental pattern. The data are reported in the text with standard deviation.
The zymography analysis was performed on different samples (Fig. 4). In the ruptured area, the tendon exhibited more evident gelatinolytic activity. In the area of rupture and control, the tendon showed the presence of an MMP-2 band (72 kDa) with activated band of smaller size (66 kDa). Bands of about 95 kDa were present in samples from ruptured area, and are identified as MMP-9. The bands above 100 kDa, evident in the samples from the ruptured area, could be identified as complex of MMP and lipocalin.
Fig. 4A–B.
Zymography of the extracts from human tendons is shown. The ladder indicates the molecular weight of the protein standards. H = control area; R = rupture area. The activity bands are the clear band on the dark background. The bands 72 kDa and 66 kDa represent the zymogen pro MMP2 and activated MMP-2 respectively. The bands with molecular weight above 100 kDa correspond to MMP-2 lipocalin complexes. Zymography was performed on 19 different samples, and the results showed the same experimental pattern (A) The 13th sample is shown. (B) The 16th sample is shown.
Discussion
The ECM in the main body of the tendon consists primarily of Type I collagen, while the predominant proteoglycan component is the small leucine-rich proteoglycan decorin, and the principal large aggregating proteoglycan is versican [17, 18, 19]. MMPs are involved in the remodeling of ECM through their broad proteolytic capability [22]. The activity of MMPs is inhibited reversibly by TIMPs. The balance between the activities of MMPs and TIMPs regulates tendon remodeling, and an imbalance produces collagen disturbances in tendons [7]. We therefore hypothesized that the expression and metabolism of collagen Type I and proteoglycans (decorin and versican) is altered with low protein turnover in samples from Achilles tendon ruptures. We specifically ascertained levels of MMPs, collagen Type I, and proteoglycans in human Achilles tendon ruptures.
One limitation of our study is the relatively small number of patients; we see relatively few of these injuries. Further studies are needed to increase the power of the studies compared to our work. We compared the gene expression of the most abundant ECM molecules (collagen Type I, decorin and versican), including the enzymes involved in their metabolism (MMP-2 and -9 and their inhibitors TIMP-1 and -2), in injured versus seemingly healthy tendon in the same patient. This approach reduces the biological variability between different subjects which can often affect data interpretation [16]. However, the apparently healthy tissue may not be normal since tendon degeneration might have been occurring despite the gross appearance: histopathological changes have been reported in 34% of normal (ie, asymptomatic) tendons [7, 12].
The presence of increased protein synthesis in the area of the rupture indicates the tendon reassembled the ECM. The ECM in the main body of the tendon consists primarily of Type I collagen, while the predominant proteoglycan component is the small leucine-rich proteoglycan decorin, and the principal large aggregating proteoglycan is versican [20–22]. MMPs are involved in the remodeling of ECM through their broad proteolytic capability [25]. The activity of MMPs is inhibited reversibly by TIMPs. The balance between the activities of MMPs and TIMPs regulates tendon remodeling, and an imbalance produces collagen disturbances in tendons [8].
The MMP activity is a typical sign of extracellular matrix reassembly [16]. This observation is confirmed not only by gene expression analysis but also by zymography which confirms the gene expression data at the protein level. Interestingly, in all samples analyzed it was evident the presence of a gelatinolytic activity with a molecular weight above 100 kDa. This could be justified considering these bands may contain lipocalin as reported in different tissues [19].
The changes of ECM are confirmed by the gene expression data on the core protein of proteoglycans. The polysaccharide side chains of PGs are glycosaminoglycans, which play a pivotal role in the correct function of PGs in ECM. In our study, the core protein synthesis of PGs is coupled with a very low level of glycosaminoglycan concentration in the ruptured tendon area. The ECM is therefore dramatically modified in terms of structural properties, and this is more evident observing the higher glycosaminoglycan concentration in the control area. These observations seem to indicate that in the ruptured area the tenocytes tried to restore the normal proteoglycan pattern increasing the core protein synthesis but without the normal glycosaminoglycan production. Nevertheless, a simpler explanation may be that the concentration of GAGs is relatively lower in the ruptured tendon due to the higher content of other reparative or exudative tissue proteins.
Moreover, the role of small proteoglycans in tendon development and in tendon biomechanics is well known. In fact, in decorin-deficient mouse tendons, fibril formation is altered at all stages of tendon development. The structural changes of the collagen fibrils in the absence of decorin include increased overall diameters and increased diameter variability with the progression through developmental stages. This observation indicates that decorin–fibril interactions are an important regulatory mechanism in fibrillogenesis during tendon development [26]. From our data, we infer that the increase of decorin expression may be related to the increase of collagen Type I expression as part of the process of tissue repair.
As far as the expression of versican is concerned, Ireland et al. [11] reported an increase in versican mRNA in three of four tendinopathic Achilles tendon samples (two painful and two ruptured) compared to a normal control, analyzed on a complementary deoxyribonucleic acid (cDNA) array. Otherwise, Corps et al. [6] observed, in a study comparing the gene expression of several proteoglycans of normal, painful, and ruptured Achilles tendons, a marked decrease in the expression levels of decorin mRNA in the last group. However, the levels of aggrecan, biglycan, and versican mRNA were unchanged compared to normal tendon samples. They thought that this decrease could reflect the activity of a tendon undergoing a repair response. The authors concluded that the contrast between the two studies could have arisen from the small number of samples screened in the earlier work, with the normal control being equivalent (in versican mRNA content) to the lower end of the range described in the second one. Moreover, it is important to determine whether the changes in versican mRNA expression are translated into changes at the protein level. In the lung, up-regulation of the versican gene is not followed by high concentration of protein as a result of the action of the MMPs [19]. Versican is important in soft tissue repair, and in this context its role could be interpreted as a tissue repair process.
The marked increased of expression of Col I A1 in ruptured and control areas was similar to that observed by Corps et al. [7] in ruptured and painful tendinopathic tendons. These findings are consistent with biochemical analyses, which showed changes in collagen synthesis after tendon rupture [10]. The increased gene expression of Col I A1 in the rupture area could be considered a tissue attempt to restore the normal tendon composition.
The presence of MMP-2 and -9 and lipocalin complex are typical signs of a marked reassembly process in the tendon which, if not carefully tuned, may affect matrix integrity [15]. The expression of MMP-2 can be up-regulated in Achilles tendinopathy [1]. The presence of MMP-9 synthesis in the rupture area is remarkable: this MMP is usually produced by inflammatory cells, and therefore its presence in the rupture area may be related to the presence of inflammatory cells in the area. The technique used for MMP determination, zymography, is extremely sensitive, and the activity detected is probably due to the presence of few cells. Interestingly, the presence of the activity of the MMP-9 and the absence of the real-time RT-PCR signal indicates that the MMP-9 is produced elsewhere from the tendon, and the molecule is probably secreted in the area by invading inflammatory cells [2]. The high gene expression of TIMPs could be considered a tissue reaction to overproduction of MMPs, in an effort to reduce their catalytic activity on the tendon matrix. In fact, TIMP-1 is not present in normal tendons, but, after acute tears of the supraspinatus tendon, in an animal model, it is expressed at the tendon edges for 2 weeks [5]. Thus, TIMP-1 may be down-regulated in chronic tendinopathy and up-regulated in acute tears [16].
A recent paper from de Mos et al. [9] reports the MMP expression and enzymatic activity in Achilles tendinopathy, and the data discussed are consistent with the findings in the present study. The unbalanced protease activity alters dramatically the ECM environment, affecting the viscoelastic properties of the tendon. Nevertheless, an excess of proteolytic activity can lead to a progressive degeneration and weakening of the extracellular matrix of tendons and a reduction on tendon biomechanical properties [3]. On this basis, the local balance of MMPS and TIMP proteins may play an important role in the correct maintenance of tendon ECM, and alterations to the synthetic–degradative equilibrium may induce the changes observed during the development of pathology in the tendon.
Our data support the hypothesis that in ruptured human Achilles tendons the tissue in the ruptured area undergoes marked rearrangement at molecular levels which involves MMP-2 activity and support the critical role of MMPs in tendon physiology.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the reporting of these cases, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Alfredson H, Lorentzon M, Backman S, Backman A, Lerner UH. cDNA-arrays and real-time quantitative PCR techniques in the investigation of chronic Achilles tendinosis. J Orthop Res. 2003;21:970–975. [DOI] [PubMed]
- 2.Arner O, Lindholm A, Orell SR. Histologic changes in subcutaneous rupture of the Achilles tendon; a study of 74 cases. Acta Chir Scand. 1959;116:484–490. [PubMed]
- 3.Arnoczky SP, Lavagnino M, Egerbacher M, Caballero O, Gardner K. Matrix metalloproteinase inhibitors prevent a decrease in the mechanical properties of stress-deprived tendons: an in vitro experimental study. Am J Sports Med. 2007;35:763–769. [DOI] [PubMed]
- 4.Calabro A, Hascall VC, Midura RJ. Adaptation of FACE methodology for microanalysis of total hyaluronan and chondroitin sulfate composition from cartilage. Glycobiology. 2000;10:283–293. [DOI] [PubMed]
- 5.Choi HR, Kondo S, Hirose K, Ishiguro N, Hasegawa Y, Iwata H. Expression and enzymatic activity of MMP-2 during healing process of the acute supraspinatus tendon tear in rabbits. J Orthop Res. 2002;20:927–933. [DOI] [PubMed]
- 6.Corps AN, Robinson AH, Movin T, Costa ML, Hazleman BL, Riley GP. Increased expression of aggrecan and biglycan mRNA in Achilles tendinopathy. Rheumatology (Oxford). 2006;45:291–294. [DOI] [PubMed]
- 7.Corps AN, Robinson AH, Movin T, Costa ML, Ireland DC, Hazleman BL, Riley GP. Versican splice variant messenger RNA expression in normal human Achilles tendon and tendinopathies. Rheumatology (Oxford). 2004;43:969–972. [DOI] [PubMed]
- 8.Dalton S, Cawston TE, Riley GP, Bayley IJ, Hazleman BL. Human shoulder tendon biopsy samples in organ culture produce procollagenase and tissue inhibitor of metalloproteinases. Ann Rheum Dis. 1995;54:571–577. [DOI] [PMC free article] [PubMed]
- 9.de Mos M, van El B, DeGroot J, Jahr H, van Schie HT, van Arkel ER, Tol H, Heijboer R, van Osch GJ, Verhaar JA. Achilles tendinosis: changes in biochemical composition and collagen turnover rate. Am J Sports Med. 2007;35:1549–1556. [DOI] [PubMed]
- 10.Eriksen HA, Pajala A, Leppilahti J, Risteli J. Increased content of type III collagen at the rupture site of human Achilles tendon. J Orthop Res. 2002;20:1352–1357. [DOI] [PubMed]
- 11.Ireland D, Harrall R, Curry V, Holloway G, Hackney R, Hazleman B, Riley G. Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol. 2001;20:159–169. [DOI] [PubMed]
- 12.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed]
- 13.Karousou EG, Porta G, De Luca G, Passi A. Analysis of fluorophore-labelled hyaluronan and chondroitin sulfate disaccharides in biological samples. J Pharm Biomed Anal. 2004;34:791–795. [DOI] [PubMed]
- 14.Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6:11–23. [DOI] [PubMed]
- 15.Koskinen SO, Hoyhtya M, Turpeenniemi-Hujanen T, Martikkala V, Makinen TT, Oksa J, Rintamaki H, Lofberg M, Somer H, Takala TE. Serum concentrations of collagen degrading enzymes and their inhibitors after downhill running. Scand J Med Sci Sports. 2001;11:9–15. [DOI] [PubMed]
- 16.Magra M, Maffulli N. Matrix metalloproteases: a role in overuse tendinopathies. Br J Sports Med. 2005;39:789–791. [DOI] [PMC free article] [PubMed]
- 17.Magra M, Maffulli N. Molecular events in tendinopathy: a role for metalloproteases. Foot Ankle Clin. 2005;10:267–277. [DOI] [PubMed]
- 18.Moriondo A, Pelosi P, Passi A, Viola M, Marcozzi C, Severgnini P, Ottani V, Quaranta M, Negrini D. Proteoglycan fragmentation and respiratory mechanics in mechanically ventilated healthy rats. J Appl Physiol. 2007;103:747–756. [DOI] [PubMed]
- 19.Passi A, Negrini D, Albertini R, Miserocchi G, De Luca G. The sensitivity of versican from rabbit lung to gelatinase A (MMP-2) and B (MMP-9) and its involvement in the development of hydraulic lung edema. FEBS Lett. 1999;456:93–96. [DOI] [PubMed]
- 20.Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford) 2004;43:131–142. [DOI] [PubMed]
- 21.Robbins JR, Vogel KG. Regional expression of mRNA for proteoglycans and collagen in tendon. Eur J Cell Biol. 1994;64:264–270. [PubMed]
- 22.Samiric T, Ilic MZ, Handley CJ. Characterisation of proteoglycans and their catabolic products in tendon and explant cultures of tendon. Matrix Biol. 2004;23:127–140. [DOI] [PubMed]
- 23.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005;87:187–202. [DOI] [PubMed]
- 24.Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6:181–190. [PubMed]
- 25.Somerville RP, Oblander SA, Apte SS. Matrix metalloproteinases: old dogs with new tricks. Genome Biol. 2003;4:216. [DOI] [PMC free article] [PubMed]
- 26.Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–1449. [DOI] [PubMed]