Abstract
Limb salvage in tumor surgery has encouraged the development of megaprostheses. However, reattaching the ligamentum patellae poses a particular problem: avulsion and/or extensor lag may lead to poor function. We describe a new technique of patellar ligament reconstruction. The technique involves reattachment of the patellar ligament to the tibial tuberosity of the proximal tibial megaprosthesis, which has a porous surface created, and the repair is protected with a cerclage wire through the patella and the prosthesis. In 10 consecutive patients, the range of motion averaged 95° (median, 90°; range, 70°–120°), and the mean extension lag averaged 4° (median, 0°; range, 0°–20°). We had one case of patellar ligament avulsion. This technique resulted in good quadriceps function and a low incidence of complications.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Resection of musculoskeletal tumors may result in a considerable bony defect. Limb salvage has become standard practice along with the development of megaprostheses. Megaprostheses typically involve the joint, with reconstruction around the knee most common [3]. The functional status after megaprosthetic reconstruction around the knee is strongly dependent on the competence of the extensor mechanism [5, 16]. Kendall et al. [16] reported functional deficits were mainly the result of a compromised extensor mechanism. Avulsion of the patellar ligament is one mechanism of compromising the extensor mechanism, with a reported incidence of 10.5% to 100% [1, 13]. In the absence of patellar ligament avulsion, attenuation may occur without complete avulsion. Attenuation will result in an extension lag while retaining some extension. In a study of 11 proximal tibial reconstructions, Abboud et al. [1] reported a mean extension lag of 7.5°. In contrast, Jaureguito et al. [15] reported seven patients using a medial gastrocnemius flap to replace the patellar ligament, resulting in an extension lag of 24° to 53°. Reconstruction of the patellar ligament therefore is an important consideration after resection of the proximal tibia. Few studies describe function after a proximal tibial megaprosthesis [8, 10, 19], and only one has reported functional scores [19].
We describe a simple technique to reconstruct the patellar ligament to a proximal tibial megaprosthesis and report (1) the incidence of failure of the quadriceps mechanism, (2) the ranges of motion (ROM), and (3) the functional results. Finally we asked whether this simple procedure has at least equivalent results in terms of continuity of the quadriceps mechanism as historical controls.
Materials and Methods
We retrospectively reviewed 10 patients (six males and four females) who underwent proximal tibial resection for a tumor. Their mean age was 48 years (range, 15–80 years). The most common diagnoses were osteosarcoma (three patients) and giant cell tumor (three patients) (Table 1). Key demographic information (Table 1) was recorded. Three patients died from their disease less than 6 months after surgery. The minimum followup was 2 years for surviving patients (mean, 4 years; range, 2–7.5 years).
Table 1.
Patients’ demographics
| Age (years) | Gender | Disease | Prosthesis | Review period (months) | Extension | Flexion | Lag | Status |
|---|---|---|---|---|---|---|---|---|
| 80 | F | Metastasis renal cell cancer | Finn | 44 | 0° | 90° | 20° | Dead |
| 15 | M | Osteosarcoma | Finn | 89 | 0° | 110° | 0° | NED |
| 71 | F | Chondrosarcoma | Finn | 63 | 0° | 80° | 10° | NED |
| 56 | M | Metastasis melanoma | Finn | 2 | 0° | 90° | 0° | Dead |
| 30 | M | Giant cell tumor | Finn | 62 | 0° | 110° | 0° | NED |
| 80 | M | Angiosarcoma | Finn | 2 | 0° | 70° | 0° | Dead |
| 53 | M | Giant cell tumor | Finn | 46 | 0° | 90° | 0° | NED |
| 33 | F | Giant cell tumor | Finn | 44 | 0° | 100° | 0° | NED |
| 15 | F | Osteosarcoma | OSS | 27 | 0° | 120° | 10° | NED |
| 46 | M | Osteosarcoma | OSS | 6 | 0° | 90° | 0° | Dead |
M = male; F = female; NED, no evidence of disease.
We used the Finn (Biomet, Warsaw, IN) in eight patients and subsequently its replacement, the OSS (Biomet), in two patients. In two patients, a medial gastrocnemius flap was used to cover a soft tissue defect at the time of the index procedure (both had infected biopsy wounds), and in one patient during the early postoperative period for skin loss secondary to wound infection. We reattached the patellar ligament to the prosthetic tibial tuberosity, which was porous-coated, using nonabsorbable Number 5 Ticron (Tyco Healthcare, Norwalk, CT) sutures around the ligament and Number 1 Ethibond (Ethicon, Somerville, NJ) sutures through the ligament. The ligament was sutured to the prosthetic tibial tuberosity at a level that was considered the appropriate height relative to the trochlear groove when the prosthesis was put through a ROM from 0° to 120°. We then reduced tension on the repair by placing a double 18-gauge cerclage wire horizontally through the patella and through the holes in the prosthetic tibial tuberosity, which then was tightened to take all strain from the patellar ligament resulting in a temporary patella baja (Fig. 1). In one patient, the tibial tuberosity was preserved and reattached to the prosthetic tibial tuberosity. We did not routinely use flaps, but rather only when skin loss precluded primary closure. Flaps were not used to augment the patellar ligament reconstruction.
Fig. 1.
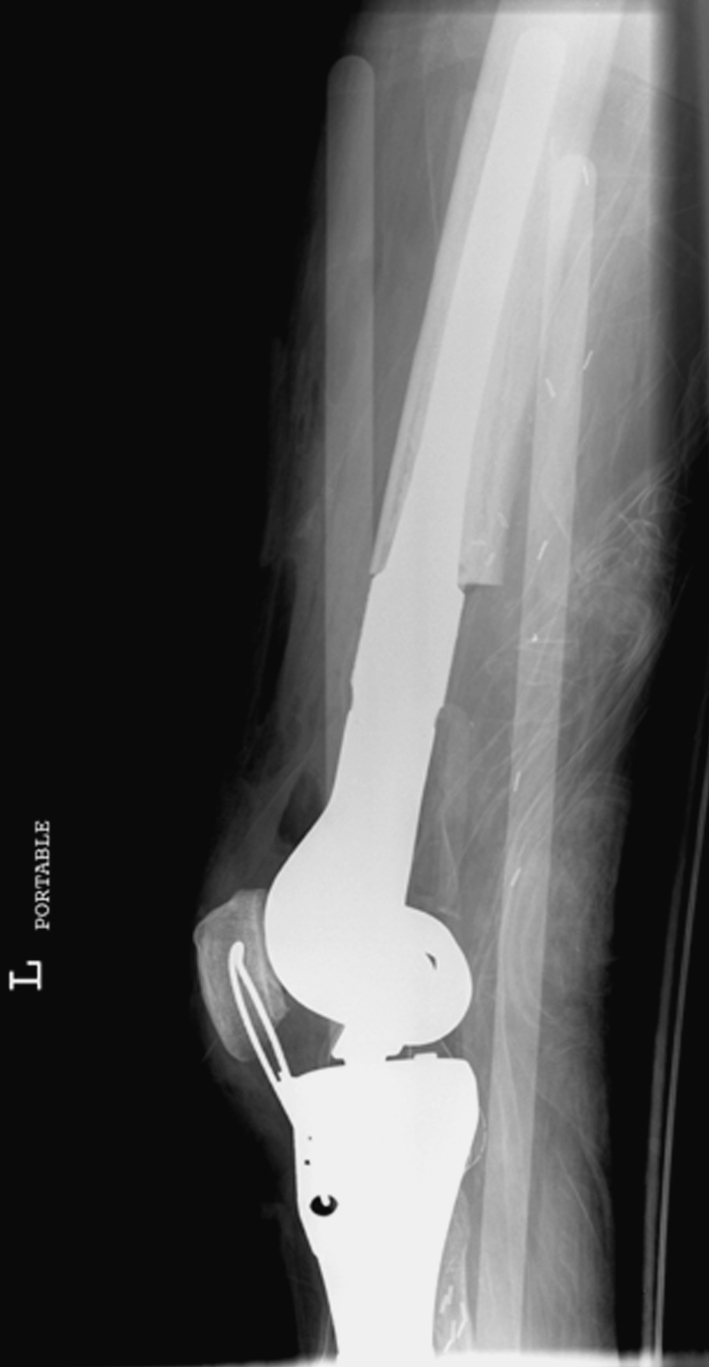
An immediate postoperative radiograph shows the initial patella baja created by tensioning the cerclage wire.
Postoperatively, the patients wore a knee extension splint for 6 weeks to prevent knee flexion. Patients used crutches to assist in balance only and were allowed full weightbearing on the surgically treated limb. Physical therapy was started after 6 weeks and initially consisted of formal therapy at a physiotherapy unit with an active assisted program of muscle strengthening in knee extension and flexion. The program generally consisted of 6 weeks of intensive physical therapy consisting of outpatient visits to a physical therapist once or twice a week for supervised quadriceps strengthening, ROM, and gait reeducation combined with a daily home exercise program.
One author (MC) followed up patients every 3 to 6 months depending on the grade of the original disease. The patients were followed indefinitely or until death. At each followup, the clinical examination included measurement of ROM using a goniometer and assessment of any extension lag or flexion contracture. One author (MC) assessed patients with the Musculoskeletal Tumor Society [11] scoring system, and from 2005, the Toronto Extremity Salvage Score (TESS) [8] at annual reviews.
Radiographs were obtained 6 months after surgery and at subsequent yearly followups; the Insall-Salvati ratio was calculated from the latest radiograph and compared with the preoperative ratio to determine if the position of the patella had changed. The presence of avulsion or attenuation was diagnosed from the radiographs.
Results
The quadriceps mechanism remained functional in all patients although avulsion developed in one patient and attenuation in another. We observed an extension lag in three patients (Table 1). In one, it was secondary to avulsion and proximal migration of the host tibial tuberosity (see subsequent). A second patient also had attenuation of the reattached patellar ligament. In the third, we believed it was the result of poor quadriceps strength secondary to avoiding exercise during the postoperative period (see subsequent). The mean Insall-Salvati ratio at last followup was similar (p = 0.48) to preoperatively (mean, 0.99, median 100, range, 0.91–1.03 versus mean, 1.15, median, 1.00; range, 0.52–2.91, respectively) (Table 2).
Table 2.
Patients’ results
| Age at surgery (years) | Insall-Salvati preoperatively | Insall-Salvati postoperatively | MSTS/TESS (%) | Status |
|---|---|---|---|---|
| 80 | 1.00 | 1.77 | 86.7/ | Dead |
| 15 | 1.02 | 0.94 | 90.0/90.5 | NED |
| 71 | 0.95 | 2.91 | 46.7/ | NED |
| 56 | 1.00 | 0.52 | NA | Dead |
| 30 | 1.00 | 1.11 | 93.3/75.8 | NED |
| 80 | 0.91 | 0.62 | NA | Dead |
| 53 | 0.95 | 1.00 | 93.3/80.8 | NED |
| 33 | 0.98 | 1.05 | 77.7/81.0 | NED |
| 15 | 1.03 | 1.00 | 86.7/87.0 | NED |
| 46 | 1.05 | 0.59 | NA | Dead |
MSTS = Musculoskeletal Tumor Society; TESS = Toronto Extremity Salvage Score; NA = not available; NED = no evidence of disease.
All patients had full passive extension. The mean flexion was 96° (median, 90°; range, 70°–110°) (Table 1).
The mean Musculoskeletal Tumor Society score was 82.1% (range, 46.7%–93.3%) and mean TESS score was 83.0% (range, 75.8%–90.5%) (Table 2).
Complications developed in six of the 10 patients. These included temporary peroneal nerve palsy in one patient, and permanent peroneal nerve palsy in another secondary to involvement of the peroneal nerve by the malignancy. Two patients had postoperative infections—one of which was a preoperative biopsy infection. Two patients also had quadriceps adhesions and underwent subsequent quadriceps releases (quadricepsplasty) to achieve flexion. In one of these patients, the prosthesis was too large resulting in a considerable overhang of the prosthetic condyles and it was at this point that the adhesion developed (Fig. 2). In the other patient, quadriceps adhesion developed after the patient refused to participate in physical therapy. After quadricepsplasty, both patients achieved a minimum of 100° flexion. A 10° extension lag remained after quadricepsplasty in the patient who declined physical therapy. The lag was exacerbated partly by her continued refusal to do physical therapy after quadricepsplasty, but her patella remains at the appropriate level relative to the trochlea.
Fig. 2.
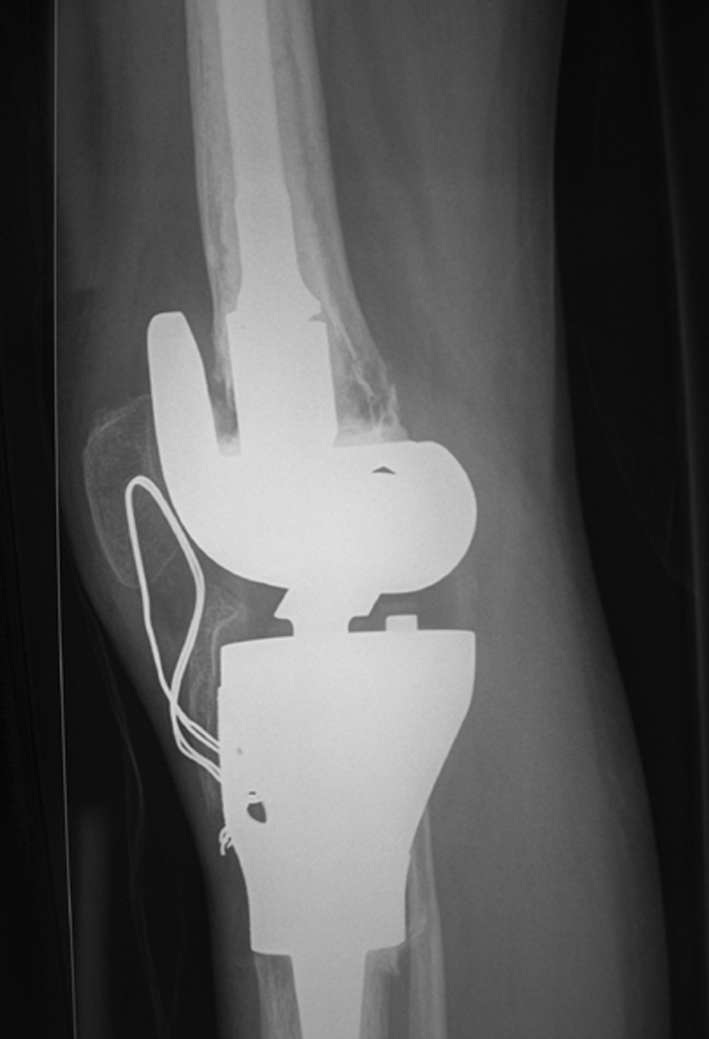
A postoperative radiograph shows the size mismatch between the femoral condyles and the underlying femur resulting in overstuffing of the patella. Partial regrowth of the proximal tibia adjacent to the insertion of the patellar ligament can be seen.
The cerclage wire broke in six patients (usually observed 3 months after the procedure), but was noticed only incidentally on reviewing the radiographs in five of the six patients. Breakage of the cerclage wire resulted in the patella returning to an appropriate level in relation to the trochlea in all but one patient. In one patient, wire breakage was associated with proximal migration of the attached tibial tuberosity and development of an extension lag (Fig. 3); this was the only patient who had the tibial tuberosity preserved. Subsequent to recognizing this complication we did not attempt to preserve the host tibial tuberosity. Rather, we directly attached the patellar ligament to the porous surface of the prosthetic tibial tuberosity. No additional proximal migrations of the patella have occurred in the seven patients since then.
Fig. 3.
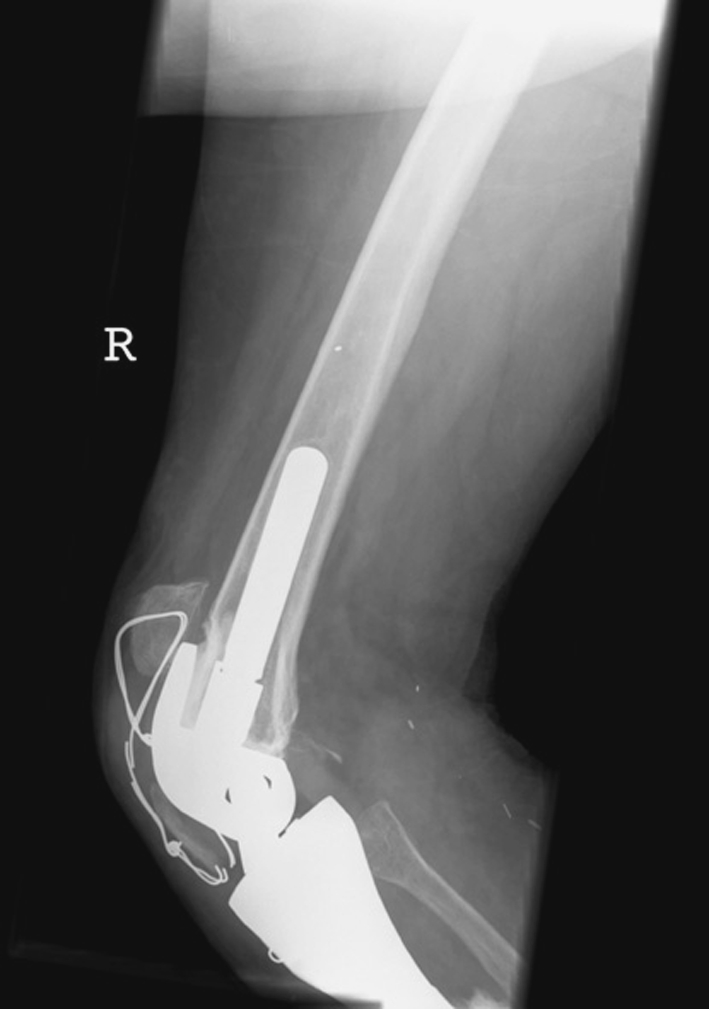
Avulsion of the tibial tuberosity after breakage of the cerclage wire resulted in proximal migration.
Discussion
Reattachment of the quadriceps mechanism to maintain quadriceps function can be difficult. We report a new technique that does not require the use of flaps, allograft, or synthetic ligaments and compared the results with those of historical controls, which have used these modalities, to determine if comparable results were at least equivalent in terms of quadriceps function, patellar height, complication rate, and general function.
Although the data were collected prospectively, the numbers are small and greater numbers are necessary to determine if the outcomes can be maintained. We had no concurrent controls and rather compared (below) with literature controls. The prostheses used are among the first with a porous surface specifically to encourage growth of the patellar ligament onto the prosthesis, whereas the historical controls used a loop in which the ligament is sutured around with no attempt at actual tissue ongrowth. It may be the reattachment to a porous surface is sufficient and the tension wire used to protect the repair is unnecessary.
An intact and well functioning extensor apparatus is mandatory for the success of any TKA. Extensor tendon rupture in TKA has been described [6, 14]. Numerous reconstruction techniques have been described with variable and often discouraging results [14]. Gracilis and semitendinosus tendons were used by Jarvela et al. [14], whereas others preferred tendo-Achilles allograft [7], allograft extensor mechanism (tibial tubercle, patellar ligament, patella and quadriceps tendon) [6], or a medial gastrocnemius flap [15]. In proximal tibial tumors, the tibial tubercle is excised and therefore, it is more difficult to achieve structural continuity and maintain function of the extensor apparatus. The competence of the extensor mechanism is the major determinant of functional outcome [5, 12]. In their study of 151 patients who had proximal tibial resections and megaprosthetic arthroplasties, Grimer et al. reported a 70% chance of needing additional surgical procedures and a 25% chance of amputation [13]. Of the original 151 patients, 50 were assessed for extension lag at a minimum of 2 years after surgery; the mean extensor lag was 30°. Their early prostheses relied on ligament attachment to surrounding fascia or a medial gastrocnemius flap rather than solid prosthetic attachment. Grimer et al. used a technique similar to that reported by Eckardt et al. [10] in 20 patients with endoprosthetic arthroplasties for malignant tumors involving the proximal tibia, although they reported their results only as “very adequate.” They did not mention either ROM or extensor lag.
Techniques using megaprostheses have been reported by others [3, 9, 12, 17, 18]. A technique using a Trevira tube (polyethylene terephthalate; Mutars, Implantcast Corp, Buxtehude, Germany) wrapped around a proximal tibial megaprosthesis in addition to a medial gastrocnemius flap was described and had a mean extensor lag of 7.5° (range, 0°–30°) [12]. Despite the size of the lag in some patients, the authors stated no extensor tendon avulsed, although their flexion ROM was restricted to a mean of 85°. Kollender et al. [17] described using the middle third of the quadriceps tendon and the patellar retinaculum and augmentation with Gore-Tex strips (Gore, Flagstaff, AZ) and a medial gastrocnemius flap. An extensor lag developed in all patients, but it was less than 20°. Others also have augmented the reconstruction with a medial gastrocnemius flap with less success [3, 18]. Polyester ligament reconstruction was used after resection of a malignant tumor [9]. The reconstruction failed in four patients, three had extensor lags of 30°, and nine had lags less than 20°. Kendall et al. [16] reconstructed the ligament with a bone block from the tibial tuberosity to the prosthesis and turndown of the quadriceps expansion. Two patients had extensor lags greater than 20° and two other patients had lags between 10° and 20°. Flexion contracture of the knee also was common (Table 3).
Table 3.
Comparison of historical results
| Authors | Type of reconstruction | Augment | Number of patients | Extension lag (mean) | Avulsion (%) |
|---|---|---|---|---|---|
| Eckardt et al. [10] | Megaprosthesis | Flap | 20 | NA | 0% |
| Grimer et al. [13] | Megaprosthesis | None | 50 | 30° | NA |
| Gosheger et al. [12] | Megaprosthesis | Trevira tube and flap | 7 | 7.5° | 0% |
| Kollender et al. [17] | Megaprosthesis | Gore-Tex and flap | 7 | < 20° | 0% |
| Shimose et al. [18] | Megaprosthesis | LAD and flap | 7 | 26° | 42% |
| Biau et al. [3] | Megaprosthesis | LAD and flap | 35 | NA | 26% |
| Dominkus et al. [9] | Megaprosthesis | LAD | 11 | 25° | 27% |
| Kendall et al. [16] | Megaprosthesis | Quadriceps turndown and flap | 8 | 4.5° | 0% |
| Ayerza et al. [2] | Allograft | None | 34 | 2° | 0% |
| Biau et al. [4] | Allograft-prosthesis | Flap | 26 | 7.7° | 23% |
| Current study | Megaprosthesis | Cerclage wire | 10 | 4° | 10% |
LAD = Ligament augmentation device; NA = not available.
In contrast to published studies, Ayerza et al. [2] reported their exceptional results using a proximal tibial allograft after proximal tibial resection for a tumor. Their series consisted of 34 patients of a potential 42 consecutive patients, and they reported no substantial lags or variation in the Insall-Salvati ratio, although three patients were excluded for failure of the allograft. In contrast, Biau et al. [4] and Wunder et al. [19] reported a high failure rate using an allograft-prosthesis composite. Biau et al. [4] reported failure of 73% of their allograft-prosthetic composites resulting from fracture, partial resorption of the graft, and extensor mechanism failure. Wunder et al. [19] reported reconstructive failure in 55% resulting from fracture or infection (Table 3).
The literature suggests successful reconstruction of the extensor mechanism may be difficult after proximal tibial resection despite major augmentation. Our technique uses a proximal tibial megaprosthesis, which has the reconstructive option of a porous-coated tibial tuberosity to encourage fibrous ingrowth of the reattached patellar ligament and does not rely on secondary augmentation such as a gastrocnemius flap. Using this relatively simple approach and temporarily reducing tension on the tendon reattachment by taking the strain through the cerclage wire has resulted in a low incidence of patellar ligament avulsion or attenuation. Function has been restored with a low incidence of extensor lag or flexion contracture and high functional scores. This technique avoids the need for augmentation using a synthetic ligament or flaps.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the reporting of these case reports, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Abboud J, Patel R, Donthineni-Rao R, Lackman R. Proximal tibial segmental prosthetic replacement without the use of muscle flaps. Clin Orthop Relat Res. 2003;414:189–196. [DOI] [PubMed]
- 2.Ayerza M, Aponte-Tinao L, Abalo E, Muscolo D. Continuity and function of patellar tendon host-donor suture in tibial allograft. Clin Orthop Relat Res. 2006;450:33–38. [DOI] [PubMed]
- 3.Biau D, Faure F, Katsahian S, Jeanrot C, Tomeno B, Anract P. Survival of total knee replacement after bone tumor resection. J Bone Joint Surg Am. 2006;88:1285–1293. [DOI] [PubMed]
- 4.Biau DJ, Dumaine V, Babinet A, Tomeno B, Anract P. Allograft-prosthesis composites after bone tumor resection at the proximal tibia. Clin Orthop Relat Res. 2007;456:211–217. [DOI] [PubMed]
- 5.Bickels J, Wittig JC, Kollender Y, Neff RS, Kellar-Graney K, Meller I, Malawer MM. Reconstruction of the extensor mechanism after proximal tibia endoprosthetic replacement. J Arthroplasty. 2001;16:856–862. [DOI] [PubMed]
- 6.Burnett RS, Berger RA, Paprosky WG, Della Valle CJ, Jacobs JJ, Rosenberg AG. Extensor mechanism allograft reconstruction after total knee arthroplasty: a comparison of two techniques. J Bone Joint Surg Am. 2004;86:2694–2699. [DOI] [PubMed]
- 7.Crossett LS, Sinha RK, Sechriest VF, Rubash HE. Reconstruction of a ruptured patellar tendon with Achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84:1354–1361. [DOI] [PubMed]
- 8.Davis AM, O’Sullivan R, Bell RS, Turcotte R, Catton CN, Wunder JS, Chabot P, Hammond A, Benk V, Isler M, Freeman C, Goddard K, Bezjak A, Kandel RA, Sadura A, Day A, James K, Tu D, Pater J, Zee B. Function and health status outcomes in a randomized trial comparing preoperative and postoperative radiotherapy in extremity soft tissue sarcoma. J Clin Oncol. 2002;20:4472–4477. [DOI] [PubMed]
- 9.Dominkus M, Sabeti M, Toma C, Abdolvahab F, Trieb K, Kotz RI. Reconstructing the extensor apparatus with a new polyester ligament. Clin Orthop Relat Res. 2006;453:328–334. [DOI] [PubMed]
- 10.Eckardt JJ, Matthews JG 2nd, Eilber FR. Endoprosthetic reconstruction after bone tumor resections of the proximal tibia. Orthop Clin North Am. 1991;22:149–160. [PubMed]
- 11.Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. 1986;204:9–24. [PubMed]
- 12.Gosheger G, Hillmann A, Lindner N, Rodl R, Hoffmann C, Burger H, Winkelmann W. Soft tissue reconstruction of megaprostheses using a trevira tube. Clin Orthop Relat Res. 2001;393:264–271. [DOI] [PubMed]
- 13.Grimer RJ, Carter SR, Tillman RM, Sneath RS, Walker PS, Unwin PS, Shewell PC. Endoprosthetic replacement of the proximal tibia. J Bone Joint Surg Br. 2000;81:488–494. [DOI] [PubMed]
- 14.Jarvela T, Halonen P, Jarvela K, Moilanen T. Reconstruction of ruptured patellar tendon after total knee arthroplasty: a case report and a description of an alternative fixation method. Knee. 2005;12:139–143. [DOI] [PubMed]
- 15.Jaureguito JW, Dubois CM, Smith SR, Gottlieb LJ, Finn HA. Medial gastrocnemius transposition flap for the treatment of disruption of the extensor mechanism after total knee arthroplasty. J Bone Joint Surg Am. 1997;79:866–873. [DOI] [PubMed]
- 16.Kendall SJ, Singer GC, Briggs TW, Cannon SR. A functional analysis of massive knee replacement after extra-articular resections of primary bone tumors. J Arthroplasty. 2000;15:754–760. [DOI] [PubMed]
- 17.Kollender Y, Bender B, Weinbroum AA, Nirkin A, Meller I, Bickels J. Secondary reconstruction of the extensor mechanism using part of the quadriceps tendon, patellar retinaculum, and Gore-Tex strips after proximal tibial resection. J Arthroplasty. 2004:19;354–360. [DOI] [PubMed]
- 18.Shimose S, Sugita T, Kubo T, Matsuo T, Ochi M. Reconstructed patellar tendon length after proximal tibia prosthetic replacement. Clin Orthop Relat Res. 2005;439:176–180. [DOI] [PubMed]
- 19.Wunder JS, Leitch K, Griffin AM, Davis AM, Bell RS. Comparison of two methods of reconstruction for primary malignant tumors at the knee: a sequential cohort study. J Surg Oncol. 2001;77:89–99, discussion 100. [DOI] [PubMed]


