Abstract
The Movin scoring system and its validated modifications and the Bonar scoring system are used to classify the histopathological findings of tendinopathy. We compared the reliability of these two different histopathological evaluation scores of tendon tissue. Tendon samples were harvested from 88 individuals (49 men, 39 women; mean age, 58.2 years) who underwent arthroscopic repair of a rotator cuff tear, and from five male patients who died of cardiovascular events (mean age, 69.6 years). A piece of supraspinatus tendon that was not directly involved in the tear was harvested en bloc within the intact middle portion of the tendon. Using hematoxylin and eosin staining and Alcian blue, slides were assessed using Bonar and Movin scores. The intraclass correlation was 0.921 (confidence interval 95% 0.790–0.963). Movin’s and Bonar’s scores have a high correlation and assess similar characteristics and variables of tendon abnormalities.
Introduction
Painful tendon disorders are a major problem in competitive and recreational sports [10, 13, 14, 18]. Over the last decade, several studies have provided the opportunity to develop novel therapeutic strategies [4–9, 17, 19, 23, 26]. While many of the epidemiological and imaging difficulties have been addressed [2, 11], relatively few studies have tried to quantify the histopathological findings of tendinopathy [18, 20, 21], and the histopathological changes are currently described in a subjective or at best semiquantitative fashion [1, 12, 20, 25, 27]. This may result in uncertainty about the histopathological findings of tendinopathy, and has produced a lack of diagnostic uniformity among surgical pathologists. Ideally, the pathological diagnoses in different studies should follow an accepted classification scheme, thus allowing data comparison and combination.
We are aware of two scoring systems that can be used for classification of the histopathological findings of tendinopathy: the Movin score [27] and its validated modifications [17, 20, 25] and the Bonar score [3].
We asked whether these two scores of abnormal tendon tissue were comparable. We also described the appearances of control and abnormal tendon.
Materials and Methods
In a prospective multicenter study we obtained 98 tendon samples to evaluate the two available scoring systems that quantify the changes associated with the process of tendinopathy. Eighty-eight of the 98 samples were ruptured supraspinatus tendons obtained from patients (49 men, 39 women; mean age, 58.2 years) who had sustained a rotator cuff tear and underwent arthroscopic repair of the lesion at our center between January 2004 and September 2006. The remaining 10 samples were nonruptured rotator cuff tendons from deceased patients. The tendon samples used in the present study have already been described in previous publications from our group [17, 18]. All procedures described in this study were approved by our respective Ethics Committees. All patients or their relatives gave written informed consent for participation in the study.
For the patients with tendon rupture, nonoperative treatment (including nonsteroidal antiinflammatory drugs, physiotherapy and rest) failed in all patients, and they continued to experience unacceptable pain and weakness in the affected shoulder. None of the patients had received injections of corticosteroids and none had undergone prior surgery on the affected shoulder. All patients fulfilled the following criteria: (1) positive cuff signs on preoperative examination; (2) no episodes of shoulder instability; (3) no radiographic sign of fracture of the glenoid or the tuberosities; (4) MRI evidence of cuff tear; (5) rotator cuff tear of one or more tendons at arthroscopic examination; and (6) no lesion of the glenoid labrum or of the capsule at arthroscopic examination. At arthroscopy, a piece of supraspinatus tendon that was not directly involved in the tear (about 4 mm × 4 mm in size) was harvested en bloc within the arthroscopically intact middle portion of the tendon between the lateral edge of the tendon tear and the muscle-tendon junction.
For the 10 nonruptured rotator cuff tendons from deceased patients, the right and left supraspinatus tendons were obtained from each of five male patients who died of cardiovascular events (mean age, 69.6 years). The tendons were harvested in the post-mortem room under sterile conditions. The rotator cuff tendon was freed from surrounding tissue, and as much muscle and fat as possible were removed. According to the patients’ relatives and from consultation of the hospital notes, no patient had sustained an acute or overuse injury to the rotator cuff tendon, and no patient had taken corticosteroids during the past 5 years.
All the tendon samples were placed in 20 mL of sterile 10% formalin in a universal container for transportation to the pathology department. Once fixed with buffered 10% formalin, the pieces were dehydrated, embedded in paraffin, and cut in 4 μm sections. Finally, five sections were stained with hematoxylin and eosin and the other five sections with Alcian blue (pH 2.5) methods for the detection of the areas rich in glycosaminoglycan (GAG), and examined under white light microscopy and under polarized light microscopy.
For each tendon and each staining technique, three slides were randomly selected and two authors (UGL and LR) examined each using a light microscope. We used a computer generated random-numbers table to allocate slides. The identification number on each slide was covered with a removable sticker, and each slide was numbered using randomly generated numbers. The area of each specimen showing the most advanced pathologic changes was selected, and the worst possible results for each slide were used in this study. The slides were interpreted using the semiquantitative grading scale of Movin [20, 25, 27] and the Bonar score [3], which assess various aspects of tendon tissue. The whole slide was assessed for areas of increased cellularity.
The variables included in the Movin scale are (1) fiber structure; (2) fiber arrangement; (3) rounding of the nuclei; (4) regional variations in cellularity; (5) increased vascularity; (6) decreased collagen stainability; (7) hyalinization; and (8) GAG content. Each variable is scored between 0 and 3, with 0 being normal, 1 slightly abnormal, 2 abnormal, and 3 markedly abnormal. The hematoxylin and eosin stained slides were used to assess the first seven variables and the Alcian blue stained slides were used to assess GAG content. The total semiquantitative histologic score for a given slide could vary between 0 (normal tendon) and 24 (the most severe abnormality detectable).
The variables included in the Bonar scale are (1) tenocytes; (2) ground substance; (3) collagen; and (4) vascularity. A four-point scoring system is used, where 0 indicates a normal appearance and 3 a markedly abnormal appearance (Table 1). Overall, the total score for a given slide could vary between 0 (normal tendon) and 12 (most severe abnormality detectable).
Table 1.
Bonar score
| Variables | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| Tenocytes | Inconspicuous elongated spindle shaped nuclei with no obvious cytoplasm at light microscopy | Increased roundness: nucleus becomes more ovoid to round in shape without conspicuous cytoplasm | Increased roundness and size: the nucleus is round, slightly enlarged and a small amount of cytoplasm is visible | Nucleus is round, large with abundant cytoplasm and lacuna formation (chondroid change) |
| Ground substance | No stainable ground substance | Stainable mucin between fibers but bundles still discrete | Stainable mucin between fibers with loss of clear demarcation of bundles | Abundant mucin throughout with inconspicuous collagen staining |
| Collagen | Collagen arranged in tightly cohesive well-demarcated bundles with a smooth dense bright homogeneous polarization pattern with normal crimping | Diminished fiber polarization: separation of individual fibers with maintenance of demarcated bundles | Bundle changes: separation of fibers with loss of demarcation of bundles giving rise to expansion of the tissue overall and clear loss of normal polarization pattern | Marked separation of fibers with complete loss of architecture |
| Vascularity | Inconspicuous blood vessels coursing between bundles | Occasional cluster of capillaries, less than one per 10 high-power fields | 1–2 clusters of capillaries per 10 high power fields | Greater than two clusters per 10 high-power fields |
We used the chi square test was used to ascertain the association between the types of tendon (control or ruptured) and the histologic score. The Mann-Whitney U-test was used to determine whether the sum-score difference between the two tendon groups was noteworthy. We used an intraclass correlation coefficient to evaluate the correlation between Movin score and Bonar score (p < 0.05).
Results
The intraclass correlation between the Movin and Bonar scores was 0.921 (p < 0.0001) (confidence interval 95%, 0.790–0.963).
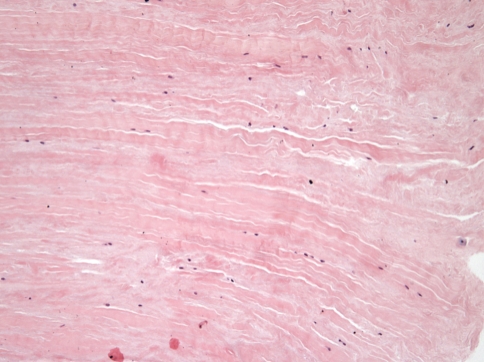
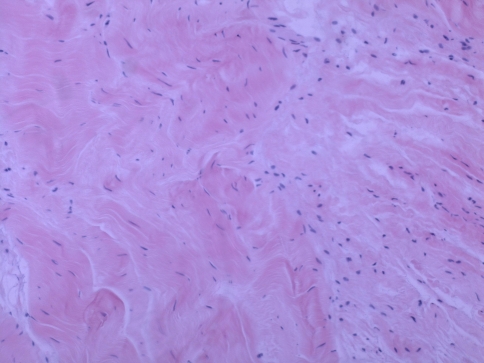
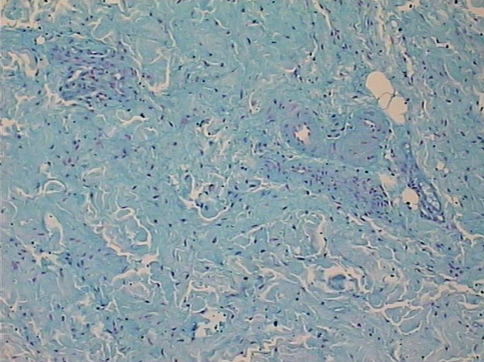
The mean Movin sum-score of ruptured tendons was greater (p = 0.001) than the mean score of control tendons (17 ± 1.88 versus 3.9 ± 2.42) (Table 2). Within each specific category of tendon abnormalities, the chi square test showed substantial differences (p < 0.05) between the control and ruptured tendons (Table 3). In the control specimens, the fibers were arranged close and parallel to each other with slight waviness (Figs. 1, 2). Increased waviness and separation of the fibers accompany slight and moderate changes. Markedly abnormal specimens showed loss of the finer fiber structure (Fig. 3). The median histologic score for the control tendons was 0, compared with 3 for the ruptured tendons. In ruptured and tendinopathic samples, this parallel arrangement was lost and haphazard. The median for the control tendons was 0, and for the ruptured tendons it was 3. Normally, the tenocyte nuclei were flattened and spindle shaped, sometimes arranged in rows (Figs. 1, 2). In the ruptured and tendinopathic samples, the tenocytes first decreased in number; then, as the pathologic changes progressed, the nuclei became progressively rounded. In some instances, these tenocytes resembled chondrocytes (Fig. 4). The median for the control tendons was 0.5, and for the ruptured tendons it was 3. The median cellularity score for the control tendons was 0, and for the ruptured tendons it was 3. Vascular bundles usually run parallel alongside the collagen fibers. The number of these vascular bundles increases with degeneration of the tendon (Fig. 5). The median for the control tendons was 0, and for the ruptured tendons it was 3. Normal collagen colors a deep pink-red when hematoxylin and eosin stain is added. However, with degenerated collagen, the section stainability is reduced and appears paler. This pallor was graded. The median value for the control tendons was 0, and for the ruptured tendons it was 3. Very few specimens showed any evidence of hyalinization, and analytical statistics showed this histopathological criterion was poorly reproducible. Normally, ground substance was not stainable. In the ruptured and tendinopathic samples, abundant mucin was stainable throughout with inconspicuous collagen staining. The median value for the control tendons was 0, and for the ruptured tendons it was 2.
Table 2.
Summary of pathologic scores of control and ruptured tendons (Movin score)
| Total tendon pathologic score | Control tendons | Ruptured tendons |
|---|---|---|
| Mean | 3.9 | 17 |
| Median | 3 | 18 |
| SD | 2.42 | 1.88 |
| Range | 1–7 | 11–23 |
Table 3.
Distribution of tendon pathologic scores (Movin score)
| Variable | Control tendons (N = 10) | Ruptured tendons (N = 88 ) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |
| Fiber structure | 6 | 3 | 1 | 0 | 0 | 8 | 32 | 48 |
| Fiber arrangement | 6 | 2 | 2 | 0 | 0 | 11 | 31 | 46 |
| Rounding of the nuclei | 5 | 4 | 1 | 0 | 0 | 1 | 36 | 51 |
| Regional variations in cellularity | 6 | 2 | 2 | 0 | 0 | 0 | 35 | 53 |
| Increased vascularity | 6 | 3 | 1 | 0 | 0 | 0 | 35 | 53 |
| Decreased collagen stainability | 7 | 2 | 1 | 0 | 0 | 13 | 24 | 51 |
| Hyalinization | 6 | 3 | 1 | 0 | 54 | 20 | 10 | 4 |
| GAG | 8 | 2 | 0 | 0 | 10 | 16 | 34 | 28 |
Fig. 1.
The control supraspinatus tendon in a 68-year-old man is shown. Collagen is arranged in tightly cohesive well-demarcated bundles. (Stain: hematoxylin and eosin; original magnification, ×150).
Fig. 2.
The supraspinatus tendon harvested from a control supraspinatus tendon in a 72-year-old man is shown. Note the closely packed, lightly stained parallel bundles of collagen fibers that contain flattened nuclei of tenocytes. (Stain: Alcian blue; original magnification, ×200).
Fig. 3.
The supraspinatus tendon harvested from the intact middle portion of the tendon between the lateral edge of the tendon tear and the muscle-tendon junction in a 57-year-old man is shown. The collagen fibers have an undulating distribution, and the area is hypercellular. (Stain: hematoxylin and eosin; original magnification, ×200).
Fig. 4.
The supraspinatus tendon harvested from the intact middle portion of the tendon between the lateral edge of the tendon tear and the muscle-tendon junction in a 55-year-old woman is shown. Nuclei are round, enlarged and cytoplasm is visible. (Stain: Alcian blue; original magnification, ×150).
Fig. 5.
The supraspinatus tendon harvested from the intact middle portion of the tendon between the lateral edge of the tendon tear and the muscle-tendon junction in a 60-year-old woman is shown. The collagen fibers have an undulating distribution, and the whole area is hypercellular. Clusters of capillaries are present. (Stain: Alcian blue; original magnification, ×150).
The mean Bonar sum-score of ruptured tendons was greater (p = 0.001) than the mean histologic score of control tendons (9.53 ± 1.55 versus 1.9 ± 1.29) (Table 4). Within each specific category of tendon abnormalities, the chi square test showed association between the control and ruptured tendons; all the variables were substantially different (Mann-Whitney U-test, 0.05; p = 0.001) (Table 5). In the control specimens, tenocyte nuclei were inconspicuous elongated and spindle shaped with no obvious cytoplasm at light microscopy (Figs. 1, 2). Increased roundness and size accompany slight and moderate changes. Markedly abnormal specimens showed nuclei were round and large with abundant cytoplasm and lacuna formation (chondroid change) (Fig. 4). The median score for the control tendons was 3, compared with 0.5 for the ruptured tendons. In the control tendons, after staining there was rarely evidence of ground substance. Stainable mucin between fibers with or without loss of clear demarcation of bundles accompanied slight and moderate changes. Markedly abnormal specimens showed abundant mucin throughout with inconspicuous collagen staining. The median score for the control tendons was 0, compared with 2 for the ruptured tendons. In the control specimens collagen was arranged in tightly cohesive well-demarcated bundles with a smooth dense bright homogeneous polarization pattern with normal crimping (Figs. 1, 2). Separation of fibers with or without loss of demarcation of bundles accompanied slight and moderate changes. Markedly abnormal specimens showed marked separation of fibers with complete loss of architecture (Fig. 3). The median score for the control tendons was 0, compared with 3 for the ruptured tendons. In the control specimens, inconspicuous blood vessels coursing between bundles were present. Clusters of capillaries accompanied slight and moderate changes. Markedly abnormal specimens showed greater than two clusters per 10 high-power fields (Fig. 5). The median score for the control tendons was 0, compared with 3 for the ruptured tendons.
Table 4.
Summary of pathologic scores of control and ruptured tendons (Bonar score)
| Total tendon pathologic score | Control tendons | Ruptured tendons |
|---|---|---|
| Mean | 1.9 | 9.53 |
| Median | 2 | 10 |
| SD | 1.29 | 1.55 |
| Range | 0–4 | 6–12 |
Table 5.
Distribution of tendon pathologic scores (Bonar score)
| Variable | Control tendons (N = 10) | Ruptured tendons (N = 88 ) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |
| Tenocytes | 5 | 4 | 1 | 0 | 0 | 1 | 36 | 51 |
| Ground substance | 8 | 2 | 0 | 0 | 10 | 16 | 34 | 28 |
| Collagen | 6 | 2 | 2 | 0 | 0 | 5 | 38 | 45 |
| Vascularity | 6 | 3 | 1 | 0 | 0 | 0 | 35 | 53 |
Discussion
Two scoring systems can be used for classification of the histopathological findings of tendinopathy: the Movin score [27] and its validated modifications [17, 20, 25] and the Bonar score [3].
We wished to ascertain whether these two scores of abnormal tendon tissue were comparable. In our hands, Movin and Bonar scores assess the same characteristics of tendon pathology.
The assessment system used was semiquantitative. We are conscious of the limitations of this assessment system, as we categorize in four classes (from 0, normal, to 3, markedly abnormal) a qualitative evaluation of several aspects of the histopathological appearance of the tendon section examined. It is likely the fully automated image analysis systems used in other fields of musculoskeletal medicine will be used in this field as well, and thus allow a more objective quantification of the abnormal appearance of tendinopathic tendons. Both the Movin [27] and Bonar scores [3] are based on semiquantitative criteria to assess the changes associated with the process of tendinopathy on a four-point scale ranging from 0 to 3. A formal assessment of their validity and reliability has not yet been performed. In this study, both scores were used simultaneously, and then compared in a large group of patients with rotator cuff tears and in controls. However, we recognize convergent validity (i.e., comparing two scales that have not been validated against some independent gold standard) does not constitute complete validation.
The fact that there are two semiquantitative scores to choose from when trying to quantify the degree of tendinopathy could engender confusion in researchers. Also, the two scales were originally developed for the Achilles tendon (Movin) [27] and the patellar tendon (Bonar) [3]. We have subsequently used the Movin scale for the patellar tendon [25], and to assess the degree of tendinopathy in the rotator cuff [17], and the long head of the biceps tendon [16].
We have demonstrated the Bonar semiquantitative scale [3] can be used in the upper limb as well, and the scale is simple and reproducible. Also, the two scales have a high correlation and essentially assess the same characteristics and variables of tendon pathology. A minor difference between these two scoring systems is the grading and definition of hyalinization. Hyalinization is assigned as Grade 0 to 3 in the Movin system, and it is not present in the Bonar score. The Movin scoring system [27] defines the grading of hyalinization as normal 0, slightly abnormal 1, abnormal 2, and markedly abnormal 3. In the present investigation, only a few specimens showed any evidence of hyalinization. In our previous use of Movin’s scale in tendinopathy of the lower limb [20, 22, 25], we came to realize that hyalinization is seldom present, and it is a poorly reproducible histopathological criterion in tendinopathic samples. For this reason, we proposed to remove this criterion from the assessment scale.
In both the Bonar [3] and Movin scales [27], special staining (GAG) is also used. We acknowledge it is important to assess the stainability of ground substance but, for practical purposes, this staining technique is costly, time consuming and does not seem to convey any further information on tendon histopathology than those reached from standard hematoxylin and eosin staining. Modified Movin score does not include an extra portion regarding ground substance. We recommend using it rather than the Movin score or the Bonar score.
Obviously, the fact that more advanced histochemical and immunohistochemical techniques and electron microscopy—to detect, for example, extra lipids, calcium deposits, collagen denaturation, pathologic tenocyte metabolism, collagen types, and foreign materials—were not used may have resulted in an underestimation of tendon abnormalities in the control group. However, our aim was to perform an easily reproducible study. Hematoxylin and eosin staining is widely available, is cost-effective, and requires little technical ability. Also, most pathologists are familiar with hematoxylin and eosin staining, and are used to interpreting a variety of specimens stained in this fashion.
Specific patterns in tendon abnormalities are difficult to distinguish even for well-trained pathologists with a special interest in this field [15]. We propose a simple and reproducible semiquantitative scale employing a commonly used staining technique that is easily accessible to the orthopaedic and pathology community at large.
We are aware of the fact that we could count the number of the cells but, again, we preferred to perform an easily reproducible study. In line with previous studies in the Achilles [20, 22, 27], patellar [25], and supraspinatus tendon [17] performed by our group and others using the semiquantitative scale employed in the present study, the evaluation of the number of cells is performed in a semiquantitative, not quantitative, fashion. We agree it is possible to actually count the number of cells per each microscopic field [30], but this would have changed the criteria used in calculating the pathological score.
Because several centers are undertaking studies on tendinopathy [24, 28, 29], the individual studies are unlikely to be large enough to result in adequate power for reliable evaluation. Therefore, combining the data from those studies with a similar study design will be essential. Consistent high-quality histologic data are thus critical for the success of the studies.
Acknowledgments
We thank the Department of Surgical Pathology of the University Campus Biomedico of Rome, Italy.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article. One of the authors (UGL) was partly supported by the 2006 British Association of Sports Medicine Aircast Prize.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Astrom M, Rausing A. Chronic Achilles tendinopathy: a survey of surgical and histopathologic findings. Clin Orthop Relat Res. 1995;316:151–164. [PubMed]
- 2.Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10:2–11. [DOI] [PubMed]
- 3.Cook J, Feller J, Bonar S, Khan K. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes’ patellar tendons. J Orthop Res. 2004;22:334–338. [DOI] [PubMed]
- 4.Franceschi F, Longo UG, Ruzzini L, Denaro V. Isolated tuberculosis of the patellar tendon. J Bone Joint Surg Br. 2007;89:1525–1526. [DOI] [PubMed]
- 5.Franceschi F, Longo UG, Ruzzini L, Papalia R, Rizzello G, Denaro V. To detach the long head of the biceps tendon after tenodesis or not: outcome analysis at the 4-year follow-up of two different techniques. Int Orthop. 2007;31:537–545. [DOI] [PMC free article] [PubMed]
- 6.Franceschi F, Longo UG, Ruzzini L, Rizzello G, Denaro V. Arthroscopic management of calcific tendinitis of the subscapularis tendon. Knee Surg Sports Traumatol Arthrosc. 2007;15:1482–485. [DOI] [PubMed]
- 7.Franceschi F, Longo UG, Ruzzini L, Rizzello G, Maffulli N, Denaro V. No advantages in repairing a type II superior labrum anterior and posterior (SLAP) lesion when associated with rotator cuff repair in patients over age 50: a randomized controlled trial. Am J Sports Med. 2008;36:247–253. [DOI] [PubMed]
- 8.Franceschi F, Longo UG, Ruzzini L, Rizzello G, Maffulli N, Denaro V. The Roman Bridge: a “double pulley - suture bridges” technique for rotator cuff repair. BMC Musculoskelet Disord. 2007;8:123. [DOI] [PMC free article] [PubMed]
- 9.Franceschi F, Ruzzini L, Longo UG, Martina FM, Beomonte Zobel B, Maffulli N, Denaro V. Equivalent clinical results of arthroscopic single-row and double-row suture anchor repair for rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2007;35:1254–1260. [DOI] [PubMed]
- 10.Kader D, Saxena A, Movin T, Maffulli N. Achilles tendinopathy: some aspects of basic science and clinical management. Br J Sports Med. 2002;36:239–249. [DOI] [PMC free article] [PubMed]
- 11.Khan K, Cook J. The painful nonruptured tendon: clinical aspects. Clin Sports Med. 2003;22:711–725. [DOI] [PubMed]
- 12.Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27:393–408. [DOI] [PubMed]
- 13.Khan KM, Cook JL, Kannus P, Maffulli N, Bonar SF. Time to abandon the “tendinitis” myth. BMJ. 2002;324:626–627. [DOI] [PMC free article] [PubMed]
- 14.Khan KM, Cook JL, Maffulli N, Kannus P. Where is the pain coming from in tendinopathy? It may be biochemical, not only structural, in origin. Br J Sports Med. 2000;34:81–83. [DOI] [PMC free article] [PubMed]
- 15.Longo G, Ripalda P, Denaro V, Forriol F. Morphologic comparison of cervical, thoracic, lumbar intervertebral discs of cynomolgus monkey (Macaca fascicularis). Eur Spine J. 2006;15:1845–1851. [DOI] [PMC free article] [PubMed]
- 16.Longo UG, Franceschi F, Ruzzini L, Rabitti C, Morini S, Maffulli N, Denaro V. Characteristics at Haematoxylin and Eosin staining of ruptures of the long head of the biceps tendon. Br J Sports Med. 2007; Dec 10; [Epub ahead of print]. [DOI] [PubMed]
- 17.Longo UG, Franceschi F, Ruzzini L, Rabitti C, Morini S, Maffulli N, Denaro V. Histopathology of the supraspinatus tendon in rotator cuff tears. Am J Sports Med. 2008;36:533–538. [DOI] [PubMed]
- 18.Longo UG, Franceschi F, Ruzzini L, Rabitti C, Morini S, Maffulli N, Forriol F, Denaro V. Light microscopic histology of supraspinatus tendon ruptures. Knee Surg Sports Traumatol Arthrosc. 2007;15:1390–1394. [DOI] [PubMed]
- 19.Maffulli N, Ajis A, Longo UG, Denaro V. Chronic rupture of tendo achillis. Foot Ankle Clin. 2007;12:583–596. [DOI] [PubMed]
- 20.Maffulli N, Barrass V, Ewen SW. Light microscopic histology of achilles tendon ruptures. A comparison with unruptured tendons. Am J Sports Med. 2000;28:857–863. [DOI] [PubMed]
- 21.Maffulli N, Ewen SW, Waterston SW, Reaper J, Barrass V. Tenocytes from ruptured and tendinopathic achilles tendons produce greater quantities of type III collagen than tenocytes from normal achilles tendons. An in vitro model of human tendon healing. Am J Sports Med. 200;28:499–505. [DOI] [PubMed]
- 22.Maffulli N, Reaper J, Ewen SW, Waterston SW, Barrass V. Chondral metaplasia in calcific insertional tendinopathy of the Achilles tendon. Clin J Sport Med. 2006;16:329–334. [DOI] [PubMed]
- 23.Maffulli N, Sharma P, Luscombe KL. Achilles tendinopathy: aetiology and management. J R Soc Med. 2004;97:472–476. [DOI] [PMC free article] [PubMed]
- 24.Maffulli N, Testa V, Capasso G, Bifulco G, Binfield PM. Results of percutaneous longitudinal tenotomy for Achilles tendinopathy in middle- and long-distance runners. Am J Sports Med. 1997;25:835–840. [DOI] [PubMed]
- 25.Maffulli N, Testa V, Capasso G, Ewen SW, Sullo A, Benazzo F, King JB. Similar histopathological picture in males with Achilles and patellar tendinopathy. Med Sci Sports Exerc. 2004;36:1470–1475. [DOI] [PubMed]
- 26.Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22:675–692. [DOI] [PubMed]
- 27.Movin T, Gad A, Reinholt F, Rolf C. Tendon pathology in long-standing achillodynia. Biopsy findings in 40 patients. Acta Orthop Scand. 1997;68:170–175. [DOI] [PubMed]
- 28.Murrell GA. Oxygen free radicals and tendon healing. J Shoulder Elbow Surg. 2007;16:S208–214. [DOI] [PubMed]
- 29.Murrell GA. Using nitric oxide to treat tendinopathy. Br J Sports Med. 2007;41:227–231. [DOI] [PMC free article] [PubMed]
- 30.Scott A, Cook JL, Hart DA, Walker DC, Duronio V, Khan KM. Tenocyte responses to mechanical loading in vivo: a role for local insulin-like growth factor 1 signaling in early tendinosis in rats. Arthritis Rheum. 2007;56:871–881. [DOI] [PubMed]