Abstract
Aprotinin is a broad spectrum proteinase inhibitor (including matrix metalloproteinase [MMP] inhibitor) used for treating patellar and Achilles tendinopathies. One previous randomized control trial demonstrated aprotinin injections superior to both corticosteroid and saline injections in patellar tendinopathy (Level II), whereas results reported for aprotinin treatment in Achilles tendinopathy have been mixed. We performed a case review and followup questionnaire for 430 consecutive patients with tendinopathy treated by 997 aprotinin injections (30,000 KIU). A response rate of 72% was achieved with a minimum followup of 3 months (average, 12.2 months; range, 3–54 months). Seventy-six percent of patients had improved, 22% of patients reported no change, and 2% were worse. Sixty-four percent of patients thought aprotinin injections were helpful, while 36% believed they had neither a positive nor negative effect. Mid-Achilles tendinopathy patients (84% improvement) were more successfully treated than patellar tendinopathy patients (69% improvement). Despite stronger published evidence of benefit in patellar tendinopathy, clinical outcomes appeared better with aprotinin use in Achilles tendinopathies.
Level of Evidence: Level IV, case series. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Many recent publications regarding tendinopathy have demonstrated an increase in matrix metalloproteinases (MMPs) in tendinopathic tissue [1, 17, 21, 26, 29], particularly the collagenases (MMP-1, MMP-8, and MMP-13) and gelatinases (MMP-2 and MMP-9). We reasoned if an excess of collagenases (enzymes which break down collagen) represents an important part of the pathologic process of tendinopathy it may be a reason for delayed recovery in certain patients [31]. With this presumption, the previously reported [9, 10] local injection of a collagenase inhibitor seemed a sensible option for treating chronic tendinopathy. Aprotinin (pronounced a-PRO-tin-in) is a broad spectrum serine protease inhibitor, with particular inhibition of plasmin (along with trypsin and kallikrein) [19]. It is a strongly basic polypeptide with a half life of approximately 7 hours [19]. In vitro it is a strong inhibitor of MMPs, including the collagenases, with a likely mechanism of inhibition of the plasmin-activation pathway of the MMPs [5, 12, 14, 16, 28].
Aprotinin was first manufactured in the 1960s by Bayer (Leverkusen, Germany) and has primarily been used in medicine for preventing blood loss during major surgery [6, 13] and promoting soft tissue healing after surgery (as a component of “fibrin glue”) [27]. The doses used in anesthetics (for limiting blood loss) are high, up to 980 mg (7 million kallikrein inhibitor units [KIU]), with well-described actual and potential side effects [13, 32]. The major side effect is anaphylaxis, which is particularly seen after repeated use of the drug [4, 18, 36].
Aprotinin has also been used for over 35 years as an off-label injection for treatment of chronic tendinopathy. This was first described by Genety and Pernin [22] and Geudj in the early 1970s in France (where it is still used [3, 38]) and more recently has been popularized by Maffulli and colleagues [9, 10, 30, 31, 39]. The doses used to inject tendons are far smaller than those used in anesthesia, typically 4.2 to 8.5 mg (30,000–62,500 KIU) (which compares to the “test dose” in anesthesia) [36]. Anaphylaxis is definitely a potential side effect when aprotinin is used to treat tendinopathy [36, 39]. However, it is not known whether any of the other reported side effects are likely or possible in the far smaller doses used for tendinopathy management, with most side effects reportedly dose-related [32]. Although the side effect profile when using aprotinin in small doses to treat tendinopathy is almost certainly less pronounced, whether aprotinin actually acts as a collagenase-inhibitor in these low doses in vivo has not been studied.
Because aprotinin is still a relatively novel treatment for tendinopathy, our study objective was to describe results from a large clinical case series. We hypothesized aprotinin injection treatments for the common forms of tendinopathy would lead to good clinical improvement.
Materials and Methods
This retrospective cohort study is an extension of a previous investigation [35, 36] followed up with identical methods using a standard questionnaire. The dose used in the published studies and the current one was 3 mL of Trasylol (Bayer, Leverkusen, Germany) containing 30,000 KIU (4.2 mg) aprotinin, combined with 2 mL of the local anesthetic lignocaine 2%. This was the same volume of fluid injected (5 mL), but a lower dose of active agent, by Capasso et al. [10] (62,500 KIU), because of the more diluted concentration of the available aprotinin brand. The injections were made without ultrasound guidance in the clinic and we attempted to use a peri- rather than intratendinous technique.
The first published study followed up 155 cases of tendinopathy for an average of 9 months (minimum of 3 months) with an improvement rate of 69%, the majority of whom were given multiple aprotinin injections over a few weeks [35]. This study also reported a 6% case rate of systemic allergic reaction [35]. As a result, we recommended to subsequent patients that if multiple injections were to be used, there should be a delay between them to minimize the risk of allergy. A reduced rate of allergy was observed in 119 cases which were followed up for an average of 10 months (minimum of 3 months) [36].
In the current study we attempted followup for all patients who had been treated for a tendon injury with aprotinin from February 2003 to December 2006 by the first author (JO) at either of two clinics. We mailed out questionnaires (Appendix I) in three distinct blocks (July 2004, July 2006, and July 2007). The primary unit of analysis was cases; some patients were included as more than one case because they were treated for bilateral or multiple tendinopathies. There were 343 individual patients treated between February 2003 and December 2006 with aprotinin injections for 438 distinct cases of tendinopathy (therefore including 95 cases where patients had bilateral and/or multiple tendinopathies). The most commonly treated conditions were Achilles tendinopathies (149 cases of midsubstance and 48 cases of insertional Achilles tendinopathy), patellar tendinopathy (94 cases) and hamstring tendinopathy (38 proximal [origin] and 17 distal hamstring tendinopathy cases). The most common other diagnoses were lateral epicondylopathy (25 cases) and adductor tendinopathy (15 cases). All patients were considered as a single cohort and then four major subcohorts were classified (Table 1). Patients with insertional Achilles tendinopathy tended to be older patients, the patellar tendinopathy cases tended to be in younger patients, and the hamstring-origin cases were often in elite athletes with a long duration of symptoms. The minimum duration between initial injection and followup was 3 months (average, 12.2 months; range, 3–54 months). The study was performed in accordance with the National Statement on Ethical Conduct in Human Research, with the subjects at low risk due to the study itself not involving intervention, with questionnaire followup method only [34]. On returning the anonymous questionnaire forms, patients gave consent for their results to be included in the study.
Table 1.
Characteristics of each subpopulation
| Characteristic | All conditions | Achilles body | Achilles insertion | Patella tendon | Hamstring tendon |
|---|---|---|---|---|---|
| Number of cases | 438 | 149 | 48 | 94 | 55 |
| Number of aprotinin injections used | 997 | 323 | 135 | 215 | 130 |
| Average number of aprotinin injections used/case | 2.3 | 2.2 | 2.8 | 2.3 | 2.3 |
| Average age (years) | 37.6 | 38.5 | 49.1 | 29.8 | 34.1 |
| Average duration of symptoms (months) | 20.1 | 21.6 | 18.4 | 14.9 | 25.3 |
| Percentage male | 74% | 68% | 70% | 95% | 58% |
| Percentage elite athlete | 22% | 22% | 10% | 29% | 34% |
| Percentage followup | 72% | 72% | 79% | 62% | 78% |
| Minimum followup (months) | 3 (average, 12.2; range, 3–54) | 3 (average, 11.6; range, 3–42) | 3 (average, 11.5; range, 3–25) | 3 (average, 11.7; range, 3–23) | 3 (average, 14.4; range, 3–54) |
Our results are primarily descriptive. Where comparisons between groups were required, Pearson chi-squared tests were used to determine differences between groups.
Results
A response rate of 72% was achieved with a minimum followup of 3 months (average, 12.2 months; range, 3–54 months). Seventy-six percent of patients were improved, 22% of patients reported no change, and 2% were worse (Table 2). Sixty-four percent of patients thought aprotinin injections were helpful, with 36% feeling that they had neither a positive nor negative effect (Table 3). The conditions leading to the best results were mid-Achilles and hamstring tendinopathies (84% of patients improved in each group), with slightly inferior results for insertional Achilles and patellar tendinopathies (69% of patients improved in each group). The Achilles mid-substance group had greater rates (p < 0.02, χ2 = 5.16) of improvement than the patellar tendinopathy group. A small group of 19 cases in nonathletes (mainly worker’s compensation patients) had worse (p < 0.04, χ2 = 4.35) outcomes than the exercising patients (who made up the majority of this cohort) (Table 4). We observed little difference (p > 0.10) between men and women and between younger patients (35 years) and older patients (> 35 years) (Table 4).
Table 2.
Patient assessment of progress of condition at the time of followup
| Characteristic | All conditions | Achilles body | Achilles insertion | Patella tendon | Hamstring tendon |
|---|---|---|---|---|---|
| Completely cured | 11% | 18% | 11% | 7% | 7% |
| Much better | 42% | 43% | 48% | 34% | 49% |
| Slightly better | 24% | 23% | 11% | 31% | 28% |
| Similar | 22% | 13% | 32% | 28% | 16% |
| Slightly worse | 2% | 2% | 0% | 3% | 0% |
| Much worse | <1% | 1% | 0% | 0% | 0% |
Table 3.
Patient impression of injection(s)
| Characteristic | All conditions | Achilles body | Achilles insertion | Patella tendon | Hamstring tendon |
|---|---|---|---|---|---|
| I am sure that the treatment completely cured my condition | 5% | 11% | 8% | 0% | 2% |
| I am sure that the treatment made my condition better | 29% | 28% | 29% | 31% | 37% |
| I think that the treatment may have made my condition better | 30% | 29% | 34% | 34% | 28% |
| I am unsure whether the treatment did anything | 36% | 32% | 29% | 34% | 33% |
| I think that the treatment may have made my condition worse | 0% | 0% | 0% | 0% | 0% |
| I am sure that the treatment made my condition worse | 0% | 0% | 0% | 0% | 0% |
Table 4.
Impression of injection(s) by patient subgroup
| Characteristic | Gender | Elite athletes | Social athletes | Non-athletes | Younger patients | Older patients | |
|---|---|---|---|---|---|---|---|
| Male | Female | ||||||
| I am sure that the treatment completely cured my condition | 5% | 8% | 8% | 4% | 11% | 4% | 6% |
| I am sure that the treatment made my condition better | 30% | 32% | 32% | 30% | 11% | 32% | 27% |
| I think that the treatment may have made my condition better | 33% | 25% | 34% | 29% | 21% | 29% | 30% |
| I am unsure whether the treatment did anything | 32% | 36% | 25% | 37% | 58% | 35% | 36% |
Side effects of injections (perceived or actual) were uncommon (Table 5), except for itch and rash. Twenty-six percent of patients experienced an itching sensation at the injection site. Most patients were warned prior to injection about this risk and itch is a symptom that may be exaggerated by the power of suggestion. There were 13 probable systemic allergic reactions in this case series which have been previously described in detail [36]. Seven patients were treated within 30 minutes of the aprotinin injection with subcutaneous adrenaline (epinephrine), which resulted in successful reversal of the allergic symptoms. No patient required hospitalization or further management other than a single adrenaline injection although more serious reactions have been described [39]. Allergic reaction was more common in the early patients of the cohort, when aprotinin was typically given in multiple doses with short periods between injections [36].
Table 5.
Frequency of associated side effects
| Side effects | Cases |
|---|---|
| Common (> 5%) | |
| Itch | 81 (25%) |
| Rash | 22 (7%) |
| Uncommon (1%−5%) | |
| Sweating | 14 (4%) |
| Nausea | 13 (4%) |
| Allergic reaction | 12 (4%) |
| Postinjection pain | 11 (4%) |
| Headache | 8 (3%) |
| Tendon damage | 2 (1%) |
Two patients experienced a rupture of the Achilles tendon. One partial rupture occurred many months after the use of aprotinin in an elite hurdler, who had subsequently sought treatment elsewhere with a cortisone injection for a recurrence of the condition. The second patient was a high-level rugby league player, who underwent a course of three aprotinin injections over 3 weeks for long-standing Achilles tendinopathy, and suffered a complete rupture of the Achilles tendon in a game 5 days after the third injection. He also had a past history of panhypopituitism that was being treated at the time with anabolic steroid supplementation. He is included in this cohort twice as two distinct cases occurring on different sides as he subsequently sought aprotinin treatment for the contra-lateral side.
Of the other treatments used, strengthening and nitrate patches were the most common, with strengthening rated as the most likely helpful and cortisone injections the most likely to be unhelpful (Table 6).
Table 6.
Other treatments used
| Treatment | Times used | Patient opinion | ||
|---|---|---|---|---|
| Helpful (%) | Not sure (%) | Unhelpful (%) | ||
| Strengthening | 128 | 65 | 23 | 13 |
| Stretching | 97 | 58 | 30 | 12 |
| Manual therapy | 97 | 46 | 37 | 16 |
| Orthotic/brace | 49 | 55 | 33 | 12 |
| Cortisone injection | 73 | 40 | 14 | 47 |
| Nitrate patches | 102 | 27 | 45 | 27 |
| ESWT | 34 | 35 | 32 | 32 |
| Surgery | 25 | 56 | 24 | 20 |
ESWT = extracorporeal shock wave therapy.
Discussion
In one trial aprotinin injections appeared superior to both corticosteroid and saline injections in patellar tendinopathy, but the results reported for similar treatment in Achilles tendinopathy have been mixed. We therefore retrospectively reviewed a large clinical case series and hypothesized aprotinin injection treatments for the common forms of tendinopathy would lead to good clinical improvement.
The major limitation of this study is a lack of control group, resulting in a low level of evidence (Level 4). The followup is relatively short with a minimum of 3 months and an average of 12 months. Given patients with chronic tendinopathy can have recurrence after many months, we would not able to ascertain the rate of recurrence. Our questionnaire for assessing patient impressions has not been validated against some standard and accepted instrument measuring patient status or function, but we presume the responses are representative.
This study reports rates of patient improvement and patient impression of aprotinin injection treatment which compare well with previous published studies [3, 9, 23, 38]. The results are in concert with those described in previous published controlled trials [7, 9, 10]. For treatment of Achilles tendinopathy, there has previously been one semicontrolled study (Level 3) showing promising results with aprotinin injections [9] and multiple other published case series (Level 4) all with good clinical results in Achilles tendinopathy [3, 22, 38] (Table 7). By comparison, for corticosteroid injection around the Achilles tendon there has only been one case series of notable size [23]. While 40% of patients reported improvement in the study of Gill et al. [23], 7% reported being worse 2 years after the corticosteroid injection. Although the quality of followup was superior to those series published for aprotinin [23], the results of the corticosteroid injections for Achilles tendinopathy were inferior (Table 7). There has been one randomized controlled trial for the use of corticosteroid injections in Achilles tendinopathy with disappointing results [15].
Table 7.
Case series comparison for injection treatment of Achilles tendinopathy
| Study | Aubin et al. [3] | Capasso et al. [9] | Rochcongar et al. [38] | Gill et al. [23] | Current study |
|---|---|---|---|---|---|
| Patients | 62 Achilles cases treated with aprotinin | 77 Achilles cases treated with aprotinin | 209 Achilles cases treated with aprotinin | 83 Achilles cases treated with cortisone sheath injections | 107 mid-Achilles cases followed up after 1 or more aprotinin injections |
| 4 × 20,000 KIU | 4+ × 62,500 KIU | 5 × 20,000 KIU | |||
| Results | 74% Good | 78% Good-Excellent | 82% Good-Excellent | 40% Improved | 84% Improved |
| 10% Average | 14% Fair | 18% Poor | 53% Similar | 13% Similar | |
| 16% Failure | 8% Poor | 7% Worse | 3% Worse | ||
| Followup | Chart review | Not well described | 2–3 months | Minimum 2 years | Minimum 3 months (average 12 months) |
The best quality trials to assess efficacy are intervention trials, particularly randomized control double-blind trials, as this form of study design minimizes bias and confounding. Two such studies have been performed for aprotinin in tendinopathy. The study of Capasso et al. [10] involved athletes being injected every other week with two to four injections for patellar tendinopathy, and reported superior results for aprotinin at 12 month followup (72% good or excellent) compared to both cortisone (59%) and saline injections (28%). On the other hand, a study by Brown et al. [7] using aprotinin injections for Achilles tendinopathy reported no improvement over saline and local anesthetic injection. However the power of the study was low, with the aprotinin group achieving generally greater improvement on raw values [7]. Possible explanations of the findings in the Brown et al. [7] study are that: (1) aprotinin injections have no beneficial effect in the treatment of Achilles tendinopathy; (2) saline injections and aprotinin injections both have a beneficial prolotherapeutic effect in Achilles tendinopathy; or (3) aprotinin injections are slightly more efficacious than saline for Achilles tendinopathy but the study was underpowered to determine differences.
Our study included over 300 patients with a minimum followup of 3 months demonstrating a rate of patient improvement of 75%, with 64% of patients considering aprotinin injections helpful and none believing aprotinin injections were harmful for their tendinopathy. However, it is uncertain to what extent these results can be attributed to the drug itself. In such an uncontrolled clinical study, both the placebo effect and natural improvement of the condition almost certainly contribute to the group of beneficial results. In addition, there is a school of thought that any injection with an irritant agent can be beneficial in treating the condition [37].
The rate of allergic reaction when using repeat injections of aprotinin (bovine-derived) is higher than for most medications and this represents a major factor to consider when choosing this drug [36]. If aprotinin works simply as a form of prolotherapy, it would be a better choice to use dextrose or autologous blood for treatment of tendinopathy. However, if aprotinin works specifically as a collagenase inhibitor, then it may have advantages over more inert substances. This is an important question with mixed results demonstrated to date in the RCTs [7, 10].
We were surprised to find superior clinical results for treating mid-substance Achilles tendinopathy compared to patellar tendinopathy, given the superior results for aprotinin in the RCT for patellar tendinopathy than that involving Achilles tendinopathy. In clinical practice, midsubstance Achilles tendinopathy generally has a more benign prognosis than patellar tendinopathy. However, compared to patellar tendinopathy, we found comparable results for insertional Achilles tendinopathy and proximal hamstring tendinopathy, both of which have been resistant to conventional treatments [8]. It is generally easier to inject the Achilles tendon as a tendon sheath is usually present (which it is generally not for the patella tendon). It is not known in vivo whether aprotinin remains at the site of injection long enough to act as a collagenase inhibitor, but this may be more likely if the drug is injected into a tendon sheath. It is also likely the current Level 1–2 evidence for aprotinin in tendinopathy is incomplete and therefore it should not be concluded, simply based on Level 1–2 evidence, that aprotinin injections are effective in patellar tendinopathy but not in Achilles tendinopathy.
A disadvantage of using polidocanol sclerotherapy, inert agents, or dry needling to treat tendinopathy is these therapies probably or certainly require tendon penetration in order to get a beneficial effect, which theoretically increases the risk of tendon damage. In the belief that aprotinin is a therapeutic agent itself, it can be injected around the tendon (in the same fashion as cortisone) so the risk of iatrogenic tendon damage is reduced. This large case series supports the notion that the risk of tendon damage with aprotinin is very low, despite one Achilles tendon rupture that occurred soon after the use of aprotinin.
Because aprotinin is derived from bovine lungs, a further proposed complication of aprotinin treatment is the potential to contract bovine spongiform encephalopathy (BSE) [11]. Since this potential complication was raised, Bayer (Germany), the major manufacturer of bovine aprotinin, has outlined the precautions taken to ensure aprotinin does not contain viral prions [24]. These steps include verifying the product is only sourced from countries with no BSE, the tissue used is in the lung (rather than neural tissue), and a purification process is undertaken. There have been no suspected cases of BSE transmission in over 40 years of aprotinin use [4].
A further disadvantage of aprotinin is its image as an off-label treatment for tendinopathy, with the manufacturer marketing the drug for high-dose use in surgery. The primary indication is itself under threat with recent vigorous debate about whether the known benefits of aprotinin in terms of preventing blood loss during surgery outweigh the possible risks [25, 33]. Because of recent controversies, some countries have restricted the availability of aprotinin as a treatment. In Australia, for example, aprotinin is only available in 50- or 100-mL vials, which are not recommended for multidose use due to potential risk of contamination [20]. Although smaller dose presentations are available in other countries, these are generally more diluted and hence also less suitable for use as local injections. Either aprotinin vials should be used, as recommended, for single-use (which is very expensive), or great care must be taken to avoid contamination (ie, only fresh needles should ever penetrate the seal). A similar dilemma applies to the use of botulinum toxin in clinical practice, and many practitioners (and patients) choose to multidose from a single vial in order to keep the treatment costs down.
There have been recent attempts to manufacture aprotinin-like polypeptides in a recombinant fashion that could potentially give similar clinical effects yet not lead to nearly the same degree of allergic reactions [2]. If recombinant aprotinin is successfully introduced to the market at a competitive price (and hence allergy becomes a far less likely complication), aprotinin may become a first-line treatment for tendinopathy. On the other hand, if surgical complications related to its primary use cause aprotinin to be withdrawn from the worldwide market, other collagenase inhibitors may need to be tested in injection form to ascertain whether they may be safe and effective treatments for tendinopathy.
We believe patients must be warned of the risk of allergy from aprotinin injections for tendinopathy and be prepared to remain under medical surveillance (where anaphylaxis can be managed) for 30 to 60 minutes after injection. Because of this risk, aprotinin should be used as second-line therapy only, for chronic conditions where more basic measures (eccentric exercise, topical glyceryl trinitrate) have failed. In vitro, aprotinin acts as a collagenase inhibitor (via inhibition of the plasmin-activation pathway of matrix metalloproteases) and therefore it may theoretically assist in managing chronic tendinopathy when collagenase excess has been consistently demonstrated. For major load-bearing tendons (eg, Achilles, patella, hamstring tendons) in active individuals, aprotinin is a more appropriate second-line injection option than cortisone preparations.
Appendix I
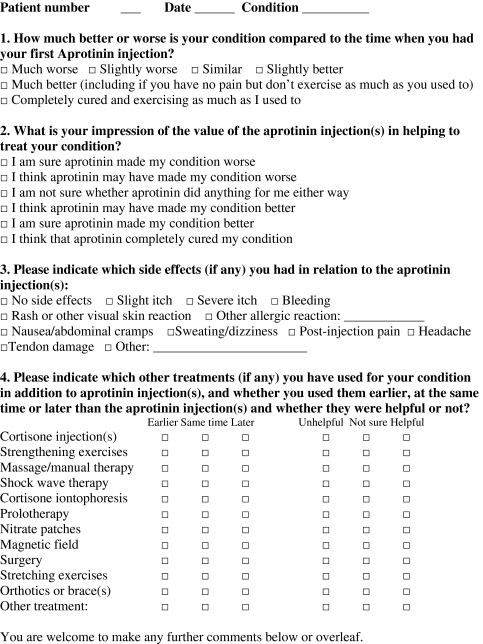
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
The study was performed in accordance with the National Statement on Ethical Conduct in Human Research (Australian National Health and Medical Research Council).
References
- 1.Alfredson H, Lorentzon M, Backman S, Backman A, Lerner U. cDNA-arrays and real time quantitative PCR techniques in the investigation of chronic human Achilles tendinopathy. J Orthop Res. 2003;21:970–975. [DOI] [PubMed]
- 2.Apeler H, Peters J, Schröder W, Schneider KH, Lemm G, Hinz V, Rossouw GW, Dembowsky K. Expression, purification, biochemical and pharmacological characterization of a recombinant aprotinin variant. Arzneimittelforschung. 2004;54:483–497. [DOI] [PubMed]
- 3.Aubin F, Javaudin L, Rochcongar P. Case report of aprotinin in Achilles tendinopathies with athletes [in French]. J Pharmacie Clinique. 1997;16:270–273.
- 4.Beierlein W, Scheule A, Dietrich W, Ziemer G. Forty years of clinical aprotinin use: a review of 124 hypersensitivity reactions. Annals of Thoracic Surgery. 2005;79:741–748. [DOI] [PubMed]
- 5.Berton A, Lorimier S, Emonard H, Laurent-Maquin D, Hornebeck W, Bellon G. Contribution of the plasmin/matrix metallopoteinase cascade to the retraction of human fibroblast populated collagen lattices. Mol Cell Biol Res Commun. 2000;3:173–180. [DOI] [PubMed]
- 6.Bojanov G, Belani KG. Aprotinin - an update for the perioperative physician. Ann Card Anaesth. 2005;8:75–80. [PubMed]
- 7.Brown R, Orchard J, Kinchington M, Hooper A, Nalder G. Aprotinin in the management of Achilles tendinopathy: a randomised controlled trial. Br J Sports Med. 2006;40:275–279. [DOI] [PMC free article] [PubMed]
- 8.Brukner P, Khan K. Clinical Sports Medicine, 3rd ed. McGraw-Hill: Sydney; 2006.
- 9.Capasso G, Maffulli N, Testa V, Sgambato A. Preliminary results with peritendinous potease inhibitor injections in the management of Achilles tendinitis. J Sports Traumatol Rel Res. 1993;15:37–40.
- 10.Capasso G, Testa V, Maffulli N, Bifulco G. Aprotinin, corticosteroids and normosaline in the management of patellar tendinopathy in athletes: a prospective randomized study. Sports Exerc Injury. 1997;3:111–115.
- 11.Cederholm-Williams SA. Fibrin glue [letter]. BMJ. 1994;308:1570. [DOI] [PMC free article] [PubMed]
- 12.Chu SC, Yang SF, Lue KH, Hsieh YS, Wu CL, Lu KH. Regulation of gelatinases expression by cytokines, endotoxin, and pharmacological agents in the human osteoarthritic knee. Connect Tissue Res. 2004;45:142–150. [DOI] [PubMed]
- 13.Coleman C, Rigali V, Hammond J, Kluger J, Jeleniowski K, White C. Evaluating the safety implications of aprotinin use: the Retrospective Evaluation of Aprotinin in Cardio Thoracic Surgery (REACTS). J Thorac Cardiovasc Surg. 2007;133:1547–1552. [DOI] [PubMed]
- 14.Collen A, Hanemaaijer R, Lupu F, Quax P, van Lent N, Grimbergen J, Peters E, Koolwijk P, van Hinsbergh V. Membrane-type maxtrix metelloproteinase-mediated angiogenesis in a fibrin-collagen matrix. Blood. 2003;101:1810–1817. [DOI] [PubMed]
- 15.DaCruz D, Geeson M, Allen M, Phair I. Achilles paratendonitis: an evaluation of steroid injection. Br J Sports Med. 1988;22:64–65. [DOI] [PMC free article] [PubMed]
- 16.Davis G, Pintar Allen KA, Salazar R, Maxwell SA. Matrix metalloproteinase-1 and -9 activation by plasmin regulates a novel endothelial cell-mediated mechanism of collagen gel contraction and capillary tube regression in three-dimensional collagen matrices. J Cell Sci. 2001;114:917–930. [DOI] [PubMed]
- 17.de Mos M, van El B, DeGroot J, Jahr H, van Schie H, van Arkel E, Tol H, KHeijboer R, van Osch G, Verhaar J. Achilles tendinosis: changes in biochemical composition and collagen turnover rate. Am J Sports Med. 2007;35:1549–1556. [DOI] [PubMed]
- 18.Dietrich W, Späth P, Zühlsdorf M, Dalichau H, Kirchhoff PG, Kuppe H, Preiss DU, Mayer G. Anaphylactic reactions to aprotinin reexposure in cardiac surgery: relation to antiaprotinin immunoglobulin G and E antibodies. Anesthesiology. 2001;95:64–71. [DOI] [PubMed]
- 19.Dollery C, ed. Therapeutic Drugs. Edinburgh UK: Churchill Livingstone; 1991.
- 20.Druce J, Locarnini S, Birch C. Isolation of HIV-1 from experimentally contaminated multidose local anaesthetic vials. Med J Aus. 1995;162:513–515. [DOI] [PubMed]
- 21.Fu S, Chan B, Wang W, Pau H, Chan K, Rolf C. Increased expression of matrix metalloproteinase 1 (MMP1) in 11 patients with patellar tendinosis. Acta Orthop Scand. 2002;73:658–662. [DOI] [PubMed]
- 22.Genety J, Pernin E. Utilisation du Zymofren® dans le traitement des tendinites chez le sportif. Cahiers Méd Lyonnais. 1971;47:135–139.
- 23.Gill SS, Gelbke MK, Mattson SL, Anderson MW, Hurwitz SR. Fluoroscopically guided low-volume peritendinous corticosteroid injection for Achilles tendinopathy. A safety study. J Bone Joint Surg Am. 2004;86:802–806. [DOI] [PubMed]
- 24.Golker C, Whiteman M, Gugel K, Gilles R, Stadler P, Kovatch R, Lister D, Wisher M, Calcagni C, Hubner G. Reduction of the infectivity of scrapie agent as a model for BSE in the manufacturing process of Trasylol. Biologicals. 1996;24:103–111. [DOI] [PubMed]
- 25.Hausenloy D, Pagano D, Keogh B. Aprotinin—still courting controversy. Lancet. 2008;371:449–450. [DOI] [PubMed]
- 26.Ireland D, Harrall R, Curry V, Holloway G, Hackney R, Hazleman B, Riley G. Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol. 2001;20:159–169. [DOI] [PubMed]
- 27.Komurcu M, Akkus O, Basbozkurt M, Gur E, Akkas N. Reduction of restrictive adhesions by local aprotinin application and primary sheath repair in surgically traumatized flexor tendons of the rabbit. J Hand Surg Am. 1997;22:826–832. [DOI] [PubMed]
- 28.Lee E, Vaughan DE, Parikh SH, Grodzinsky AJ, Libby P, Lark MW, Lee RT. Regulation of matrix metalloproteinases and plasminogen activator inhibitor-1 synthesis by plasminogen in clutured human vascular smooth muscle cells. Circ Res. 1996;78:44–49. [DOI] [PubMed]
- 29.Lo I, Marchuk L, Hollinshead R, Hart D, Frank C. Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase mRNA levels are specifically altered in torn rotator cuff tendons. Am J Sports Med. 2004;32:1223–1229. [DOI] [PubMed]
- 30.Maffulli N, Testa V, Capasso G, Sullo A. Calcific insertional Achilles tendinopathy. Reattachment with bone anchors. Am J Sports Med. 2004;32:174–182. [DOI] [PubMed]
- 31.Magra M, Maffulli N. Matrix metalloproteases: a role in overuse tendinopathies. Br J Sports Med. 2005;39:789–791. [DOI] [PMC free article] [PubMed]
- 32.Mangano D, Tudor I, Dietzel C. Group MSoPIR, Foundation IRaE. The risk associated with aprotinin in cardiac surgery. New Engl J Med. 2006;354:353–365. [DOI] [PubMed]
- 33.Mouton R, Finch D, Davies I, Binks A, Zacharowski K. Effect of aprotinin on renal dysfunction in patients undergoing on-pump and off-pump cardiac surgery: a retrospective observational study. Lancet. 2008;371:475–482. [DOI] [PubMed]
- 34.National Health and Medical Research Council Web site. National Statement on Ethical Conduct in Human Research. Available at: http://www.nhmrc.gov.au/publications/synopses/_files/e72.pdf. Accessed June 4, 2007.
- 35.Orchard J, Hofman J, Brown R. The risks of local aprotinin injections for treating chronic tendinopathy. Sport Health. 2005;23:24–28.
- 36.Orchard J, Massey A, Rimmer J, Hofman J, Brown R. Delay of 6 weeks between aprotinin injections for tendinopathy reduces risk of allergic reaction. J Sci Med Sport. 2007: Aug 11 epub ahead of print. [DOI] [PubMed]
- 37.Rabago D, Best TM, Beamsley M, Patterson J. A systematic review of prolotherapy for chronic musculoskeletal pain. Clin J Sport Med. 2005;15:376–380. [DOI] [PubMed]
- 38.Rochcongar P, Thoribe B, Le Beux P, Jan J. Tendinopathie calcanéenne et sport : place des injections d’aprotinin. Sci Sports. 2005;20:261–267. [DOI]
- 39.Rukin NJ, Maffulli N. Systemic allergic reactions to aprotinin injection around the Achilles tendon. J Sci Med Sport. 2007;10:320–322. [DOI] [PubMed]


