Abstract
Apoptosis (programmed cell death) has been identified as a histopathologic feature of tendinopathy. While the precise mechanism(s) that triggers the apoptotic cascade in tendon cells has not been identified, it has been theorized that loss of cellular homeostatic tension following microscopic damage to individual tendon fibrils could be the stimulus for initiating the pathologic events associated with tendinopathy. To determine if loss of homeostatic tension following stress deprivation could induce apoptosis in tendon cells, rat tail tendons were stress-deprived or cyclically loaded (3% strain at 0.17 Hz) for 24 hours under tissue culture conditions. Caspase-3 (an upstream mediator of apoptosis) mRNA expression was evaluated using quantitative polymerase chain reaction and caspase-3 protein synthesis was identified using immunohistochemistry. Apoptotic cells were identified histologically using an antibody for single-stranded DNA. Stress deprivation for 24 hours resulted in an increase in caspase-3 mRNA expression when compared to fresh controls or cyclically loaded tendons. Stress deprivation also increased the percentage of apoptotic cells (10.59% ± 2.80) compared to controls (1.87% ± 1.07) or cyclically loaded tendons (3.73% ± 0.87). These data suggest loss of homeostatic tension following stress deprivation induces apoptosis in rat tail tendon cells.
Introduction
Tendinopathy remains one of the most common injuries encountered in sports or at the workplace, accounting for 30% to 50% of all sports injuries and more than 48% of reported occupational maladies [37, 48]. However, in spite of its high incidence, the precise etiopathogenesis of tendinopathy is still a topic of debate [9].
Numerous studies have examined the histopathologic features of tendinopathy in an effort to provide some insight into its root cause [8, 22, 23, 25, 31, 33, 51, 52]. These histologic changes include early alteration in tenocyte morphology [8], collagen degeneration and disarray [22], hypocellularity [22], and apoptosis [19, 31, 44, 51, 52]. While apoptosis or “programmed cell death” has been associated with rotator cuff [44, 51, 52] and patellar tendinopathy [31] in humans as well as superficial digital flexor tendinopathy in the horse [19], the precise mechanism(s) by which apoptosis is induced in tendon cells is unclear.
Apoptosis can be triggered by a wide variety of physiological and stressful stimuli [34]. Several recent reports suggest there may be a connection between the loading patterns of tendons and the pathological changes, including apoptosis, seen in tendinopathy [1, 31]. However, the link between mechanical loading conditions and the pathophysiological response in tendinopathy remains obscure. Previous investigations have failed to provide compelling evidence for the possible connection between the loading pattern and the histopathologic response(s) seen in tendinopathy [32].
We have suggested following acute microscopic damage to individual tendon fibril(s), the mechanical understimulation of the affected tendon cells and the loss of cytoskeletal homeostatic tension, secondary to altered cell-matrix interactions, is the stimulus for tendinopathy [1, 27]. Our studies suggest loss of homeostatic tension in tendon cells in situ, secondary to stress deprivation, results in an upregulation of interstitial collagenase mRNA expression and protein synthesis [1, 27]. In addition, stress deprivation produces a histological picture of cell and matrix degeneration comparable with that seen in tendinopathy [1, 17]. This is thought to result from a loss of cytoskeletal tensional homeostasis following removal of extracellular matrix stress [1]. This same mechanism could be responsible for inducing apoptosis in tendon cells. Indeed, one study suggests that release of mechanical tension can induce apoptosis in human dermal fibroblasts seeded into a collagen gel [16].
We hypothesized loss of homeostatic cellular tension, secondary to stress deprivation, upregulates caspase-3 mRNA expression and protein synthesis in tendon cells, thus inducing apoptosis. We also hypothesized in vitro cyclic loading of rat tail tendons would inhibit the induction of apoptosis in these tendon cells.
Materials and Methods
To determine the effect of stress deprivation and loss of cytoskeletal tension on the induction of apoptosis in tendon cells in vitro, we exposed rat tail tendons to 0 (fresh control) or 24 hours of stress deprivation or cyclically loaded at 3% strain (0.17 Hz). The relative expression of caspase-3 mRNA and the percentage of apoptotic cells were used as the dependent variables. In addition, the presence of caspase-3 protein was qualitatively examined using immunohistochemistry.
Following Institutional Animal Care and Use Committee approval, rat tail tendons were harvested from adult Sprague-Dawley rats immediately after euthanasia. Using a sterile scalpel blade, the tail was cut between coccygeal vertebrae at both the base and at the distal tip of the tail for a total length of approximately 120 mm. Tendons were gently teased from the distal portion of each tail with forceps and maintained in DMEM media supplemented with 10% FBS, 1% penicillin-streptomycin-amphotericin B, 0.02 mg/ml gentocin, and 7.5 mg/ml ascorbate (GIBCO, Grand Island, NY) incubated at 37°C and 10% CO2.
Rat tail tendons (RTTs) were divided into three groups: Group 1 - zero time controls; Group 2 - stress deprived (SD) for 24 hours; and Group 3 - cyclically loaded at 3% strain at 0.17 Hz for 24 hours using a previously described cyclic-loading apparatus [29]. Ten tendons per group were examined and the experiment was repeated three times. At the end of each experimental period, a 1.5 to 2 cm portion of each tendon was fixed in 4% paraformaldehyde for immunohistochemistry (IHC). The remaining portions of the tendons in each group were pooled and processed for quantitative polymerase chain reaction (Q-PCR).
To determine the prevalence of apoptosis and caspase-3 protein in the tendon cells, paraffin sections of RTT fascicles were stained with an antibody to single-stranded DNA (ssDNA) (F7-26; Chemicon Int., CA) according to the manufacturer’s protocol or an antibody to cleaved caspase-3 (Cell Signaling Technology Inc., Danvers, MA) followed by FITC or TR conjugated secondary antibodies. All sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) to label cell nuclei. As a positive control for caspase-3 and apoptosis, fresh RTTs were treated with 1 μM staurosporine (Calbiochem, San Diego, CA) in DMEM. To determine the percentage of apoptosis in each RTT, five sections from each tendon were examined. All cells were counted in 10 fields in each section at a magnification of 40× and the percentage of apoptosis expressed as the number of F7-26 positive cells divided by the total cell number × 100.
To determine the relative level of caspase-3 mRNA expression in each of the above groups, RTTs were placed in RNAlater™ (Qiagen, Valencia, CA) at 4°C for a period of at least 24 hours before processing. Total RNA was then extracted using the Qiagen RNEasy Kit with the protocol provided for fibrous tissues. Approximately 400 ng of RNA was then converted into cDNA using the Invitrogen SuperScript III Reverse Transcription system (Carlsbad, CA). Real-time quantitative PCR was performed using the TaqMan® Gene Expression Assay from Applied Biosystems (ABI, Foster City, CA) in the ABI 7500 Fast System. Rat caspase-3 primers and TaqMan® probes (ID # Rn00563902_m1) were obtained from the TaqMan® Gene Expression Assay database at ABI (http://allgenes.com). The endogenous control used for all Q-PCR experiments was 18s rRNA. The Q-PCR results were calculated and expressed as relative quantification to the fresh control sample.
To determine if stress deprivation caused an increase in caspase-3 mRNA expression compared to fresh controls or cyclically loaded tendons, the relative quantification values between groups were evaluated using an ANOVA and a Tukey’s post-hoc test. To determine if stress deprivation caused an increase in apoptosis compared to fresh controls or cyclically loaded tendons, the percentage of apoptosis between groups was evaluated using an ANOVA and a Tukey’s post-hoc test.
Results
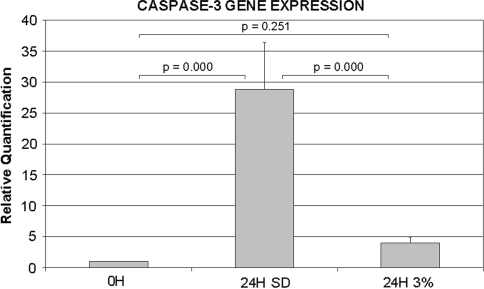
Stress deprivation of RTTs for 24 hours increased (p = 0.000) caspase-3 mRNA expression (Fig. 1). Cyclic loading (3% strain at 0.17 Hz) for 24 hours inhibited (p = 0.000) caspase-3 mRNA expression when compared to the stress-deprived tendons and was similar to (p = 0.251) fresh (0 hr) controls (Fig. 1).
Fig. 1.
The graph shows the relative Q-PCR expression of caspase-3 mRNA in fresh control tendons, tendons following 24 hours of stress deprivation, and tendons following 24 hours of 3% cyclic tensile loading at 0.17 Hz.
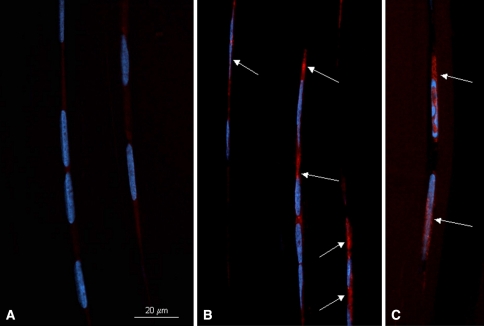
Stress deprivation resulted in positive staining of tendon cells with the antibody for caspase-3 protein when compared to an absence of positive staining in fresh (0 hr) tendons (Fig. 2). Staurosporine treatment of fresh RTTs also induced positive caspase-3 staining of the tendon cells (Fig. 2). Omitting the primary antibody as the negative control gave no specific staining.
Fig. 2A–C.
Fluorescent photomicrographs of rat tail tendons immunostained for caspase-3. Red staining within the cytoplasm (arrows) signifies a caspase-3 positive cell. Nuclei are stained blue (DAPI). Representative fields from fresh rat tail tendons (A), 24-hour stress-deprived rat tail tendons (B) and staurosporine-treated, positive control rat tail tendons (C) are shown. Bar = 20 μm.
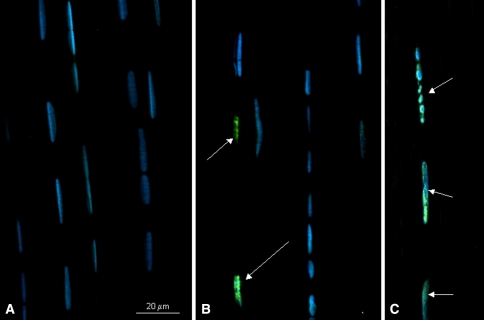
Stress deprivation induced apoptosis in the tendon cells as demonstrated by positive ssDNA staining (Fig. 3). Staurosporine treatment of fresh RTTs also induced positive ssDNA staining of the tendon cells (Fig. 3). Omitting the primary antibody as the negative control gave no specific staining.
Fig. 3A–C.
Fluorescent photomicrographs of rat tail tendons immunostained for single-stranded DNA using the F7-26 antibody. Cells undergoing apoptosis (arrows) stain positive for the F7-26 antibody. Nuclei are stained blue (DAPI). Representative fields from fresh rat tail tendons (A), 24-hour stress-deprived rat tail tendons (B) and staurosporine-treated, positive control rat tail tendons (C) are shown. Bar = 20 μm.
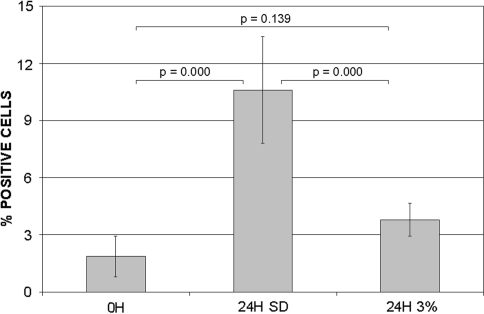
The percentage of apoptotic cells in the stress-deprived tendons was increased (p = 0.000) when compared to fresh (0 hr) tendons (10.59% ± 2.80 versus 1.87% ± 1.07, respectively) (Fig. 4). Cyclic loading decreased (p = 0.000) the percentage of apoptosis (3.79% ± 0.87) when compared to the 24 hours stress-deprived tendons. The apoptosis rate with cyclic loading for 24 hours was similar (p = 0.139) to that from fresh (0 hr) controls (Fig. 4).
Fig. 4.
The graph depicts the percentage (number of F7-26 positive cells/total cell count × 100) of apoptotic cells in fresh control tendons, tendons following 24 hours of stress deprivation, and tendons following 24 hours of 3% cyclic tensile loading at 0.17 Hz.
Discussion
Recent studies demonstrate apoptosis is an important histologic feature of tendinopathy [19, 31, 44, 51, 52]. The presence of large numbers of apoptotic cells in degenerative tendons has highlighted the potential role of programmed cell death in the pathogenesis of tendinopathy [19, 31, 44, 51, 52]. The loss of tendon cells through an apoptotic pathway could influence the rate of collagen synthesis and repair of damaged tendons, thus placing the tissue at risk from additional loading [51, 52]. In addition, cells lost via apoptosis rarely incite an inflammatory response [30, 51, 52]. Thus, the loss of tenocytes through an apoptotic pathway could explain the areas of hypocellularity reported in the histological picture of tendinopathy [22, 31, 33]. We, therefore, hypothesized loss of homeostatic tension secondary to stress deprivation upregulates caspase-3 mRNA expression and protein synthesis in tendon cells and results in an increase in apoptosis. We also hypothesized in vitro cyclic loading of rat tail tendons inhibits the induction of apoptosis in these tendon cells.
The stress-deprived, in vitro system used in the current study is intended to model the loss of homeostatic tendon cell strain that can occur as a result of isolated microscopic tendon fiber damage [1]. Previous studies from our lab have demonstrated the loss of homeostatic tendon cell strain in this model can induce many of the histologic and molecular features that have been reported in clinical cases of tendinopathy [1, 3, 8, 17, 19, 22, 23, 25, 27–29]. Thus, while this in vitro tendon model is not intended to replicate the entire gamut of complex abnormalities that may occur in clinical cases of tendinopathy, we believe it can provide a relevant and well-controlled system in which to examine the response of tendon cells following loss of homeostatic tendon cell strain.
While both an absence and an excess of force apparently induce apoptosis in connective tissue cells in vitro [7, 11, 14, 16, 20, 41], care must be taken when interpreting these results as it is possible that unforeseen physical forces (or the absence thereof) could complicate the experimental system [20]. In addition, the use of isolated cells on artificial substrates may alter the traction force responses of these cells, thus making them more or less susceptible to apoptotic induction [20]. Therefore, we believe the rat tail tendon model used in this study more closely maintains the normal cell-matrix relationship and is more reflective of the natural in vivo condition.
Programmed cell death plays a major role in embryogenesis, organogenesis and morphogenesis, as well as in the maintenance of homeostasis in healthy adult tissues [34, 38]. While the induction of apoptosis can occur through several different pathways, the final stages of the apoptotic process are mediated by the activation of a group of cysteine proteases called caspases [45]. Since caspase-3 is one of the terminal proteins in the caspase activation mechanism, its presence is indicative of a cell committed to the apoptotic pathway [5]. Apoptosis can be confirmed by the presence of both DNA fragmentation and single-stranded DNA fragments [45].
Our data demonstrate loss of homeostatic cellular tension in tendon cells stimulated an immediate increase in both caspase-3 mRNA expression and protein synthesis. Recent studies have also demonstrated increased staining for caspase-3 protein in tissue specimens from human patellar tendinopathy patients [31] and horses with superficial digital flexor tendinopathy [19]. These same studies also demonstrated an increase in the rate of apoptosis in pathological tissues when compared to normal control tissues.
Apoptosis can be triggered by numerous factors associated with stressful stimuli such as hypoxia, hyperthermia, oxidative stress, and nitric oxide [30]. Many of these same stimuli have also have been implicated as potential agents in the pathogenesis of tendinopathy [4, 35, 36, 42, 46, 47, 50]. However, apoptosis may also develop after the loss of various trophic signals that normally suppress the expression of the cell death program [13]. These so-called ‘survival factors’ can act through either cell surface or nuclear receptors [13]. Experimental studies have suggested that in a number of cell types, including fibroblasts and endothelial cells [10, 24], mechanical tension is necessary for cell survival. In these studies, loss of mechanical tension between cells and their extracellular matrix resulted in an immediate and extensive induction of apoptosis in response to the alteration in homeostatic cell tension [14–16]. While the precise molecular mechanism(s) by which the release of mechanical tension induces apoptosis in fibroblasts are still unknown, it has been suggested that the process involves alterations in the integrin-based cell-matrix connections [15, 20, 43]. These cell-matrix interactions are important in the regulation of apoptosis [43].
Previous experimental studies demonstrate loss of homeostatic cellular tension secondary to stress deprivation is associated with an immediate increase in interstitial collagenase mRNA expression and protein synthesis in rat tail tendon cells [3, 12, 28, 29]. This increase in interstitial collagenase protein synthesis is also associated with changes in cell shape as well as a loss of intimacy between the tendon cells and their pericellular matrix [12]. This alteration in cell-matrix connections, as well as the change in cell shape associated with loss of homeostatic tension, may have triggered the increase in apoptosis seen in the current study, as changes in cell shape induced by mechanical factors may also serve as a signal for apoptosis to occur [6].
The fact that only 10.6% of the cells in the stress-deprived tendons stained positive for apoptosis could be explained by the hierarchical structural organization of the rat tail tendon [26, 40]. The resulting heterogeneity in strain fields could allow for a differential amount of fibril relaxation to occur throughout the unloaded tendon and result in only a percentage of the cells initially losing their homeostatic tension [2, 18]. Additional research is needed to determine if longer durations of stress deprivation increase the rate of apoptosis.
Applying cyclic load to the tendons inhibited both the induction of caspase-3 expression and tendon cell apoptosis in this in vitro model. This suggests that, indeed, the loss of homeostatic cell tension, and not any factors related to the tissue-culture environment, was the stimulus for inducing apoptosis in these tendon cells. These results further support the supposition that tendon cells require a base-line level of cytoskeletal tension to maintain physiologic homeostasis [21].
Our data support the theory that the apoptosis seen in cases of tendinopathy may not be due to excessive strains per se, but rather to the loss of homeostatic cellular tension secondary to altered cell-matrix interactions resulting from isolated tendon fibril damage. While an experimental study has suggested apoptosis can be induced by high strain (20%), repetitive loading (1 Hz) of rat tibialis anterior tendons in vitro [39], the high strains utilized in this model were supraphysiological and most likely caused substantial damage to the tendon fibrils [49]. Indeed, a recent study has documented the mean failure strain of rat tibialis anterior tendons to be 15.8% ± 1.6% [49]. Thus, the disruption of collagen fibrils that would have occurred following 20% cyclic strain could have subsequently altered the normal mechanotransduction pathways between the damaged collagen fibrils and associated tendon cells [1, 27]. This would have then led to an understimulation of the tendon cells and the induction of apoptosis by the same mechanism seen in the current study. Further supporting the concept of the loss of homeostatic cellular tension as an etiologic stimulus for the degradative cascade seen in tendinopathy are the observations that besides inducing apoptosis, stress deprivation of tendons in vitro can also reproduce many of the major pathological findings reported in clinical cases of tendinopathy [8, 22, 23, 25, 31, 33, 51, 52]. These include alterations in tenocyte morphology, collagen degeneration and disarray [17, 22], loss of extracellular matrix properties [28], and an upregulation of degradative enzymes [3, 28, 29].
While the consequences of increased apoptosis seen in clinical cases of tendinopathy have yet to be elucidated, our data suggest loss of cellular homeostatic tension can induce apoptosis in tendon cells. The results further support the concept that it is the mechanical understimulation of tendon cells following microdamage to the extracellular matrix, rather than the overstimulation of these cells secondary to repetitive strain, that contributes to the etiopathogenesis of tendinopathy [1].
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Arnoczky SP, Lavagnino M, Egerbacher M. The mechanical etiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? Int J Exp Pathol. 2007;88:217–226. [DOI] [PMC free article] [PubMed]
- 2.Arnoczky SP, Lavagnino M, Whallon JH, Hoonjan A. In situ cell nucleus deformation in tendons under tensile load; a morphological analysis using confocal laser microscopy. J Orthop Res. 2002;20:29–35. [DOI] [PubMed]
- 3.Arnoczky SP, Tian T, Lavagnino M, Gardner K. Ex vivo static tensile loading inhibits MMP-1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J Orthop Res. 2004;22:328–333. [DOI] [PubMed]
- 4.Bestwick CS, Maffulli N. Reactive oxygen species and tendinopathy: do they matter? Br J Sports Med. 2004;38:672–674. [DOI] [PMC free article] [PubMed]
- 5.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. [DOI] [PubMed]
- 6.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. [DOI] [PubMed]
- 7.Cheng W, Li B, Kajstura J, Li P, Wolin MS, Sonnenblick EH, Hintze TH, Olivetti G, Anversa P. Stretch-induced programmed myocyte cell death. J Clin Invest. 1995;96:2247–2259. [DOI] [PMC free article] [PubMed]
- 8.Cook JL, Feller JA, Bonar SF, Khan KM. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes’ patellar tendons. J Orthop Res. 2004;22:334–338. [DOI] [PubMed]
- 9.Cook JL, Khan KM. Etiology of tendinopathy. In: Woo SL, Renstrom P, Arnoczky SP, eds. Tendinopathy in Athletes. Oxford, England: Blackwell Publishing; 2007:10–28.
- 10.Dimmeler S, Haendeler J, Rippmann V, Nehls M, Zeiher AM. Shear stress inhibits apoptosis of human endothelial cells. FEBS Lett. 1996;399:71–74. [DOI] [PubMed]
- 11.Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int. 1995;57:344–358. [DOI] [PubMed]
- 12.Egerbacher M, Arnoczky SP, Gardner K, Caballero O, Gartner J. Stress-deprivation of tendons results in alterations in the integrin profile and pericellular matrix of tendon cells. Trans Orthop Res Soc. 2006;31:1100.
- 13.Fesus L, Davies PJ, Piacentini M. Apoptosis: molecular mechanisms in programmed cell death. Eur J Cell Biol. 1991;56:170–177. [PubMed]
- 14.Fluck J, Querfeld C, Cremer A, Niland S, Krieg T, Sollberg S. Normal human primary fibroblasts undergo apoptosis in three-dimensional contractile collagen gels. J Invest Dermatol. 1998;110:153–157. [DOI] [PubMed]
- 15.Graf R, Freyberg M, Kaiser D, Friedl P. Mechanosensitive induction of apoptosis in fibroblasts is regulated by thrombospondin-1 and integrin associated protein (CD47). Apoptosis. 2002;7:493–498. [DOI] [PubMed]
- 16.Grinnell F, Zhu M, Carlson MA, Abrams JM. Release of mechanical tension triggers apoptosis of human fibroblasts in a model of regressing granulation tissue. Exp Cell Res. 1999;248:608–619. [DOI] [PubMed]
- 17.Hannafin JA, Arnoczky SP, Hoonjan A, Torzilli PA. Effect of stress deprivation and cyclic tensile loading on the material and morphologic properties of canine flexor digitorum profundus tendon: an in vitro study. J Orthop Res. 1995;13:907–914. [DOI] [PubMed]
- 18.Hansen KA, Weiss JA, Barton JK. Recruitment of tendon crimp with applied tensile strain. J Biomech Eng. 2002;124:72–77. [DOI] [PubMed]
- 19.Hosaka Y, Teraoka H, Yamamoto E, Ueda H, Takehana K. Mechanism of cell death in inflamed superficial digital flexor tendon in the horse. J Comp Pathol. 2005;132:51–58. [DOI] [PubMed]
- 20.Hsieh MH, Nguyen HT. Molecular mechanism of apoptosis induced by mechanical forces. Int Rev Cytol. 2005;245:45–90. [DOI] [PubMed]
- 21.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. [DOI] [PubMed]
- 22.Jarvinen M, Jozsa L, Kannus P, Jarvinen TL, Kvist M, Leadbetter W. Histopathological findings in chronic tendon disorders. Scand J Med Sci Sports. 1997;7:86–95. [DOI] [PubMed]
- 23.Jozsa L, Reffy A, Kannus P, Demel S, Elek E. Pathological alterations in human tendons. Arch Orthop Trauma Surg. 1990;110:15–21. [DOI] [PubMed]
- 24.Kaiser D, Freyberg MA, Friedl P. Lack of hemodynamic forces triggers apoptosis in vascular endothelial cells. Biochem Biophys Res Commun. 1997;231:586–590. [DOI] [PubMed]
- 25.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed]
- 26.Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6:11–23. [DOI] [PubMed]
- 27.Lavagnino M, Arnoczky SP, Egerbacher M, Gardner KL, Burns ME. Isolated fibrillar damage in tendons stimulates local collagenase mRNA expression and protein synthesis. J Biomech. 2006;39:2355–2362. [DOI] [PubMed]
- 28.Lavagnino M, Arnoczky SP, Frank K, Tian T. Collagen fibril diameter distribution does not reflect changes in the mechanical properties of in vitro stress-deprived tendons. J Biomech. 2005;38:69–75. [DOI] [PubMed]
- 29.Lavagnino M, Arnoczky SP, Tian T, Vaupel Z. Effect of amplitude and frequency of cyclic tensile strain on the inhibition of MMP-1 mRNA expression in tendon cells: an in vitro study. Connect Tissue Res. 2003;44:181–187. [DOI] [PubMed]
- 30.Lawen A. Apoptosis-an introduction. Bioessays. 2003;25:888–896. [DOI] [PubMed]
- 31.Lian O, Scott A, Engebretsen L, Bahr R, Duronio V, Khan K. Excessive apoptosis in patellar tendinopathy in athletes. Am J Sports Med. 2007;35:605–611. [DOI] [PubMed]
- 32.Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33:561–567. [DOI] [PubMed]
- 33.Maffulli N, Testa V, Capasso G, Ewen SW, Sullo A, Benazzo F, King JB. Similar histopathological picture in males with Achilles and patellar tendinopathy. Med Sci Sports Exerc. 2004;36:1470–1475. [DOI] [PubMed]
- 34.McConkey DJ, Orrenius S. Signal transduction pathways in apoptosis. Stem Cells. 1996;14:619–631. [DOI] [PubMed]
- 35.Murakami H, Shinomiya N, Kikuchi T, Fujikawa K, Nemoto K. Differential sensitivity to NO-induced apoptosis between anterior cruciate and medial collateral ligament cells. J Orthop Sci. 2005;10:84–90. [DOI] [PubMed]
- 36.Murakami H, Shinomiya N, Kikuchi T, Yoshihara Y, Nemoto K. Upregulated expression of inducible nitric oxide synthase plays a key role in early apoptosis after anterior cruciate ligament injury. J Orthop Res. 2006;24:1521–1534. [DOI] [PubMed]
- 37.Renstrom P. Sports traumatology today. A review of common current sports injury problems. Ann Chir Gynaecol. 1991;80:81–93. [PubMed]
- 38.Schwartzman RA, Cidlowski JA. Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr Rev. 1993;14:133–151. [DOI] [PubMed]
- 39.Scott A, Khan KM, Heer J, Cook JL, Lian O, Duronio V. High strain mechanical loading rapidly induces tendon apoptosis: an ex vivo rat tibialis anterior model. Br J Sports Med. 2005;39:e25. [DOI] [PMC free article] [PubMed]
- 40.Screen HR, Lee DA, Bader DL, Shelton JC. An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. Proc Inst Mech Eng [H]. 2004;218:109–119. [DOI] [PubMed]
- 41.Skutek M, van Griensven M, Zeichen J, Brauer N, Bosch U. Cyclic mechanical stretching of human patellar tendon fibroblasts: activation of JNK and modulation of apoptosis. Knee Surg Sports Traumatol Arthrosc. 2003;11:122–129. [DOI] [PubMed]
- 42.Szomor ZL, Appleyard RC, Murrell GA. Overexpression of nitric oxide synthases in tendon overuse. J Orthop Res. 2006;24:80–86. [DOI] [PubMed]
- 43.Tidball J, Albrecht D. Regulation of apoptosis by cellular interactions with the extracellular matrix. In: Lockshin RA, Zakeri Z, Tilly JL, eds. When Cells Die. Wilmington, DE: Wiley-Liss, Inc; 1998:411–427.
- 44.Tuoheti Y, Itoi E, Pradhan RL, Wakabayashi I, Takahashi S, Minagawa H, Kobayashi M, Okada K, Shimada Y. Apoptosis in the supraspinatus tendon with stage II subacromial impingement. J Shoulder Elbow Surg. 2005;14:535–541. [DOI] [PubMed]
- 45.Van Cruchten S, Van Den Broeck W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol. 2002;31:214–223. [DOI] [PubMed]
- 46.Wang MX, Wei A, Yuan J, Clippe A, Bernard A, Knoops B, Murrell GA. Antioxidant enzyme peroxiredoxin 5 is upregulated in degenerative human tendon. Biochem Biophys Res Commun. 2001;284:667–673. [DOI] [PubMed]
- 47.Wilson AM, Goodship AE. Exercise-induced hyperthermia as a possible mechanism for tendon degeneration. J Biomech. 1994;27:899–905. [DOI] [PubMed]
- 48.Woo SL-Y, Renstrom PAFH, Arnoczky SP, eds. Tendinopathy in Athletes. Ames, IA: Blackwell Publishing; 2007, p. XI.
- 49.Wu JZ, Brumfield A, Miller GR, Metheny R, Cutlip RG. Comparison of mechanical properties of rat tibialis anterior tendon evaluated using two different approaches. Biomed Mater Eng. 2004;14:13–22. [PubMed]
- 50.Yuan J, Murrell GA, Trickett A, Wang MX. Involvement of cytochrome c release and caspase-3 activation in the oxidative stress-induced apoptosis in human tendon fibroblasts. Biochim Biophys Acta. 2003;1641:35–41. [DOI] [PubMed]
- 51.Yuan J, Murrell GA, Wei AQ, Wang MX. Apoptosis in rotator cuff tendonopathy. J Orthop Res. 2002;20:1372–1379. [DOI] [PubMed]
- 52.Yuan J, Wang MX, Murrell GA. Cell death and tendinopathy. Clin Sports Med. 2003;22:693–701. [DOI] [PubMed]