Abstract
Vascular function and angiogenesis are regulated by vascular endothelial growth factor-A (VEGF). The purpose of this preliminary study was to address the following questions: Is VEGF expression in the patellar tendon more prevalent in patients with patellar tendinopathy than in individuals with normal, pain-free patellar tendons? Which cell populations express VEGF in normal and tendinopathic tendon? Is there a difference in symptom duration between VEGF+ and VEGF− tendons? We collected patellar tendon tissue from 22 patients undergoing open débridement of the patellar tendon and from 10 patients undergoing intramedullary nailing of the tibia. VEGF expression was assessed immunohistochemically. Relevant inflammatory and repair cell types were immunolabeled. VEGF expression was absent from control tendons, but was present in a subset of patients with histopathological evidence of angiofibroblastic tendinosis. VEGF was expressed in the intimal layer of tendon vessels, but was absent in other cell types. Patients demonstrating VEGF expression in the patellar tendon had a shorter symptom duration (12 ± 7.8 months) than patients with no detectable VEGF (32.8 ± 23.5 months). VEGF may contribute to the vascular hyperplasia that is a cardinal feature of symptomatic tendinosis, particularly in cases with more recent onset.
Introduction
Tendon injuries may be chronic or acute in nature, and occur at a variety of common anatomic locations [24]. Acute tendon injuries include lacerations or ruptures, whereas chronic injuries (tendinopathies) are typically insidious in their onset. Achilles tendinopathy, patellar tendinopathy (jumper’s knee), lateral epicondylalgia, and rotator cuff tendinopathy are major causes of chronic pain and disability both in general and recreational and professional athletes [5, 17, 23]. Although many of these tendinopathies resolve spontaneously [7] or with nonoperative treatment including eccentric training [4, 18, 25, 45], a proportion of injuries remain chronically symptomatic (painful and edematous) and require surgery [10, 41]. One report suggests chronic tendon injuries pass through several overlapping stages, including an initial tendinitis (Stage I), followed by tendinosis (Stage II), complete rupture (Stage III), and tendinosis plus other changes including fibrosis or calcification (Stage IV) [21]. Tendinosis is characterized histopathologically in the chronic stage by the disruption of fibroblast arrays with proliferation and apoptosis, and the proliferation of vessels and nerves containing sensory, nociceptive, and autonomic elements [3, 11, 12, 20, 21, 37]. This histopathologic picture has been characterized as a “failed healing response” and has also been termed “angiofibroblastic tendinosis” to refer to the major finding of increased vascular and fibroblastic cellularity [28].
Modern imaging methods, color Doppler and power Doppler ultrasound, reveal vascular hyperplasia in and around painful tendons [34, 47]. Several authors report an association between the appearance of excessive vascular flow on power Doppler imaging and tendon pain and impaired function [13, 29]. Thus, both clinical and anatomic findings of regionally increased vascularity in tendinopathies have sparked interest in the possible role of angiogenesis in painful tendons [33, 34]. We previously reported increased microvascular density, proliferation of endothelial and smooth muscle cells, and increased perivascular mast cell density in patient biopsies [22, 38, 39].
Vascular endothelial growth factor (VEGF), a potent angiogenic cytokine and signaling peptide with seven molecularly diverse isoforms [42], may play a role in this process. VEGF regulates many genes that drive the adaptive and angiogenic response to hypoxia or inflammation in pathologies such as cancer and soft tissue repair [42], promoting endothelial cell proliferation, migration, and survival, as well as the permeabilization of microvessels leading to localized edema [1, 42].
VEGF is not highly expressed in adult tendon, but its expression is increased in several animal models of acute injury or mechanical loading, including an overuse model [30, 33]. VEGF mRNA levels are elevated in homogenized Achilles tendinosis biopsies [2] and could arise from tendon fibroblasts, endothelial cells, or extrinsic cell populations recruited to the site of the lesion. Further, VEGF mRNA can be detected in ruptured Achilles tendon, but not in normal adult tendon [32]. One study suggests tendon rupture is preceded by degenerative changes including evidence of hypoxia [19]. Thus, mechanical overload, injury and inflammation, hypoxic conditions, or some combination of the above could lead to increased expression of VEGF in tendon [33].
The purpose of our preliminary study was to address the following questions: (1) Is VEGF expression in the patellar tendon more prevalent in men and women with patellar tendinopathy than in individuals with normal, pain-free patellar tendons? (2) Which cell populations express VEGF in normal and tendinopathic tendon? (3) Is there a difference in symptom duration between VEGF+ and VEGF− tendons?
Materials and Methods
We obtained biopsies from the patellar tendons of 32 patients (mean age, 30 years; range, 21–42 years), who were divided into two groups. One group consisted of 22 patients with patellar tendinopathy (19 men and three women; mean age, 30.4 years; range, 22–40 years) with pain at the infrapatellar pole for at least 3 months severe enough to prevent them from participating in activities at the preinjury level. MRI confirmed high signal changes that corresponded to the area of pain (infrapatellar pole). The duration of current symptoms ranged from 5 to 81 months. These patients had been randomized to receive surgical treatment for tendon pain with an open procedure, as part of a clinical trial comparing the effects of eccentric exercise and surgery [6]. The control group was comprised of 10 patients (seven men and three women; mean age, 28.2 years; range, 21–42 years) with normal patellar tendons confirmed by clinical examination, with no current or prior history of patellar tendon pain. These individuals were being treated with intramedullary nailing for tibial fractures. Exclusion criteria included: (1) age less than 18 years; (2) previous knee surgery; (3) corticosteroid injections in or around the knee; (4) knee trauma requiring medical attention; and (5) rheumatic or degenerative knee conditions. All patients provided written, informed consent. The study was approved by the University and Hospital Research Ethics Committees, and was conducted in accordance with the World Medical Association Declaration of Helsinki. Patient confidentiality was protected according to the U.S. Health Insurance Portability and Accountability Act.
The surgical technique and biopsy handling were identical in the two groups. Biopsies were taken from the proximal bone-tendon junction, and the tendon tissue was excised using a full thickness wedge-shaped incision that was widest at the patellar pole (1 cm) and narrower distally (2–3 cm in length). A suture was passed through the proximal end of the tendon to aid orientation. Immediately after the procedure, biopsies were transferred to Zamboni’s fixative where they were stored for 4 to 24 hours, and then washed in 0.1 M phosphate-buffered NaCl, pH 7.2, with 15% sucrose (weight/volume) (PBS) and 0.1% natriumazide. The entire biopsies were then stored in PBS at 4°C for a minimum of 48 hours after which they were embedded in paraffin. From each biopsy, we stained one 5 μm tissue section for hematoxylin and eosin (H&E) (general morphology) and one for Alcian blue (sulphated glycosaminoglycans) and viewed them at 100 × to 630 × magnification on a Zeiss Axioplan upright microscope (Carl Zeiss Canada Ltd, Toronto, Canada). We (AS, RS) examined the entire longitudinal tissue section during analysis, avoiding areas of adipose or peritendinous tissue.
VEGF expression was examined using a well-characterized mouse monoclonal antibody raised against amino acids 1–140 of VEGF of human origin (Santa Cruz clone sc-7269). We performed immunohistochemistry using an autostainer (DAKO Diagnostics, Glostrup, Denmark). The sections were cleared in xylene (3 × 15 minutes), quenched for 15 minutes in 3% hydrogen peroxide, incubated in protein-free blocking solution (DAKO) for 15 minutes, then exposed to 100 μL of primary antibody diluted 1:50 in 0.1% bovine serum albumin in TBS for 1 hour. We used a secondary anti-mouse IgG antibody (DAKO), followed by incubation with APAAP (DAKO) for 30 minutes. The signal was visualized with New Fuschin as the substrate (Sigma-Aldritch, Oakville, Canada). Identically fixed and processed tonsil with or without the VEGF antibody was used as positive or negative control, respectively. In tonsil, scattered VEGF positive leukocytes and endothelial cells were seen only in the positive controls.
As in previous studies [22, 38, 39], we used the following mouse monoclonal antibodies to identify cell types potentially expressing VEGF: CD68 (Signet KP1 clone, Cedarlane Laboratories, Hornby, Canada) for macrophages, CD3 (DAKO, clone F7.2.38) for T-lymphocytes, mast cell tryptase (DAKO, clone AA1) for mast cells, CD31 (DAKO, clone JC70A) for endothelial cells, α- smooth muscle actin (Biomeda 1A4) for perivascular cells and myofibroblasts. For mast cell tryptase, sections were cleared in xylene (3 × 15 minutes), steamed in sodium citrate for 3 × 5 minutes, incubated at 37° with 0.1% trypsin in 0.1% calcium chloride pH 7.3 for 5 minutes, quenched for 15 minutes in 3% hydrogen peroxide, incubated in protein-free blocking solution (DAKO) for 15 minutes, then exposed to 100 μL of primary antibody diluted 1:50 in 0.1% bovine serum albumin in TBS for 1 hour. A secondary anti-mouse IgG antibody (DAKO) was used, followed by incubation with APAAP (DAKO) for 30 minutes. The signal was visualized with New Fuschin as the substrate. CD68 and α-SMA processing was identical to mast cells but without the trypsin pre-treatment. For T-lymphocytes and CD31, we used the CSA II detection system (DAKO) with 3,3′-diamino-benzidine as the chromogen (Vector Laboratories, Burlingame, U.S.A.). Identically fixed and processed tonsil with or without the primary antibody was used as positive or negative control, respectively. In tonsil, mast cells, lymphocytes, macrophages, and endothelial cells were labeled only in the positive controls.
We (AS, RS) examined slides with the patient/control identity code masked with black tape. Using a 40 × objective lens, the entire tissue section was scanned and all areas of nonartifactual, cellular VEGF staining were captured at 1392 × 1045 pixels with a digital camera (Retiga Exi 1394, Qimaging Corp, Burnaby, Canada). To be considered nonartifactual, the staining had to be red, intracellular and absent in negative controls. Based on the number of fields with positive staining, each section was considered VEGF-positive or VEGF-negative. To be considered positive, specific cellular staining had to be present in three or more captured viewing fields. This method ensured that sections with only one or two areas of staining were not considered positive.
We computed means and standard deviations (SD). We compared VEGF presence or absence between normal and tendinosis tissue using the Fisher exact probability test. We compared the mean symptom duration (in months) of tendinopathy patients with and without VEGF expression using an unpaired t-test. All statistical tests were carried out using SPSS 13.0 (SPSS Inc., Chicago, IL).
Results
Patellar tendon from control subjects was normal in all cases but one, which showed mild cell rounding. VEGF was more often (p = 0.03) observed in tendinopathy samples than normal samples. VEGF expression could be detected in eight of 22 patient samples, but was absent in all control tendons.
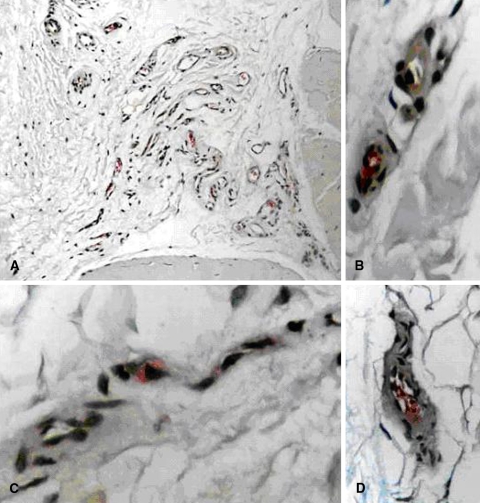
When present, VEGF was localized to endothelial cells, rather than fibroblasts, perivascular cells, or inflammatory cell types (Fig 1A–D).
Fig. 1A–D.
(A) VEGF is expressed in endothelial cells in an area of expanded endotendon (New Fuschin, original magnification × 200). (B) Higher-power views demonstrate endothelial cells with cytoplasmic VEGF staining (New Fuschin, original magnification × 600). Note the absence of staining in the tunica media. (C) Higher-power views demonstrate endothelial cells with cytoplasmic VEGF staining (New Fuschin, original magnification × 600). (D) A capillary is shown. Higher-power views demonstrate endothelial cells with cytoplasmic VEGF staining (New Fuschin, original magnification × 400). Staining is localized to the tunica intima of an arteriole.
The duration of symptoms in patients demonstrating VEGF expression (n = 8) was greater (p = 0.015) than that of patients without VEGF expression (n = 14) (12 ± 7.7 months versus 32.8 ± 23.5 months, respectively). The symptom duration range was 5 to 18 months for VEGF-positive patients, and 6 to 81 months for VEGF-negative patients.
Discussion
Vascular function and angiogenesis are regulated by VEGF. VEGF is not highly expressed in adult tendon but is increased in several animal models of acute injury or mechanical loading [30, 33]. VEGF mRNA levels are also elevated in homogenized Achilles tendinosis biopsies [2]. Further, VEGF mRNA is expressed in ruptured Achilles tendon, but not in normal adult tendon [32]. We therefore asked whether VEGF expression in the patellar tendon is more prevalent in individuals with patellar tendinopathy compared to those with asymptomatic patellar tendons, which cell populations are responsible for VEGF production in tendon, and whether patients with VEGF-positive or VEGF-negative patellar tendons differed in terms of symptom duration.
We note several limitations. VEGF expression was examined in a cross-sectional cohort of surgically treated tendinopathy patients with symptom duration greater than 3 months. Studies of the temporal expression of factors regulated by wound healing have generally been conducted using acute injury models [27]. These studies have demonstrated VEGF expression peaks early in the healing process, eg, in the first week after an acute injury [8, 43]. In our study VEGF expression may have reached maximal levels at an earlier time point in the course of tendinosis development. Nonetheless VEGF-positive and VEGF-negative patients had differing mean symptom durations. There was no detectable difference in age, gender distribution or VISA-A score between patients with VEGF-positive and VEGF-negative tendons. Another limitation is we did not measure other factors that may be involved in angiogenesis, including other isoforms of VEGF, as well as a host of other substances with well-known or emerging roles in angiogenesis [35]. VEGF is the most potent angiogenic peptide in humans, and its activity is largely produced by activation of the tyrosine kinase VEGF receptor 2, which stimulates endothelial cell proliferation, survival, migration and tube formation [42].
We found eight of 22 patellar tendinopathy patients demonstrated VEGF expression compared to 0/10 asymptomatic tendons. VEGF expression was localized to endothelial cells rather than to mast cells, fibroblasts, or inflammatory cells. Patients demonstrating VEGF expression in the patellar tendon had shorter symptom duration than those with no VEGF expression. Thus, the results are consistent with the hypothesis that VEGF may contribute to a temporally regulated angiogenic process as part of the injury response in patellar tendinopathy [30, 33].
Normal adult tendons demonstrate a relatively low level of vascularity due to their low metabolic rate, as well as their mechanical requirements [33, 40]. Following injury, the metabolic requirements increase and correspondingly tendons undergo an angiogenic response characterized by the expression of VEGF and IGF-I, both of which may contribute to the growth of vessels into the healing tendon [15, 32]. One study in an animal model of supraspinatus tendinopathy demonstrated upregulation of VEGF expression after 8 weeks of overuse [27].
We found VEGF expression absent in those patients with the longest duration of symptoms, yet these patients still demonstrated angiofibroblastic tendinosis with morphological evidence of increased vascularity. During the later stages of neovascularization and vessel maturation, VEGF expression declines and vessel survival and stability is promoted by the presence of perivascular and vascular smooth muscle-containing cells [14]. Whether mooth muscle-containing cells play a role in the chronicity and survival of neovessels in tendons will be a key point to examine in the future, as many neovessels appear to persist despite the absence of VEGF in the chronic stage. The increased presence of vessels in tendinopathies is reportedly an important aspect of the “failed healing response” in tendons [28].
In addition to promoting the proliferation, migration and survival of endothelial cells following an acute injury, VEGF plays a role in the increased permeability of immature vessels by influencing cell-cell contacts in the vessel walls, thus contributing to soft tissue edema [1, 42]. It is less clear whether VEGF modulates vascular function during overuse injuries. A recently proposed theoretical model suggests chronic tendon loading leads to mechanical trauma of the tissue with ensuing microruptures of the tendon microvasculature [29]. These microvascular ruptures initiate a VEGF-mediated vascular remodeling and edema that over time can become pathological [29]. According to this conceptual model, VEGF expression is nearly completely repressed in healthy adult tendon due to the presence of endogenous inhibitors, but is reexpressed following mechanical overload. Our data are in accordance with such a model, as we detected VEGF expression within the endothelial cell layer only in tendinopathy patients and always in association with other well-known features of angiofibroblastic tendinosis. However, the actual consequences of increased VEGF expression on endothelial function in tendinopathic tendon remain to be determined.
VEGF expression is a recognized element of the inflammatory response to soft tissue injury, being acutely induced in endothelial cells in the wound environment by hypoxia or by other cytokines and growth factors [40]. Alternatively, it has been proposed VEGF expression can be induced directly in tenocytes by mechanical loading via mechanotransduction pathways [31]. In support of the possible relevance of this alternate mechanism of VEGF upregulation in tendinopathy, the VEGF gene, along with its upstream transcriptional regulator HIF-1α, is expressed in cultured rat tendon cells in response to cyclic stretch at high (1 Hz) but not low (0.5 Hz) frequencies [31]. However, our data do not offer support for the hypothesis of tenocyte-derived VEGF expression, as VEGF expression was absent in tenocytes in both controls and patients.
Tendinosis lesions are characterized by increased numbers of fibroblasts and vascular cells (endothelial and smooth muscle cells) and abnormal extracellular matrix [9, 21, 46]. An increased prominence of tenocytes demonstrating proliferating cell nuclear antigen (PCNA) has been reported in patellar and supraspinatus tendinopathies, demonstrating proliferation of intrinsic cellularity may be an ongoing phenomenon in chronic injuries [26, 36]. VEGF, along with many other peptides, promotes the proliferation of fibroblasts [46] and endothelial cells [16]. VEGF treatment of tendon autografts led to an increase in the number of endothelial cells, and increased numbers of fibroblastic cells [44]. Therefore, it is possible VEGF contributes in some part to the hypercellularity that characterizes tendinosis lesions. Prior work with this set of biopsies has demonstrated increased vascular density, proliferation of endothelial and smooth muscle cells and increased mast cell density in patient tendons [29, 30]. It is likely any proliferative effect of VEGF would occur in concert with other growth factors and cytokines present in the lesion [27].
Our preliminary data are consistent with the concepts of Kraushaar and Nirschl [21], who postulated the majority of tendinopathies in athletes are due to angiofibroblastic tendinosis, rather than tendonitis. Our data provides evidence a potent angiogenic peptide, VEGF, may contribute to the vascular hyperplasia that is a cardinal feature of chronic tendinosis.
Acknowledgments
We thank the Oslo Sports Trauma Research Center established at the Norwegian University of Sport and Physical Education through generous grants from the Royal Norwegian Ministry of Culture, the Norwegian Olympic Committee and Confederation of Sport, Norsk Tipping, and Pfizer. We thank Rafat Sobouti for assistance viewing tissue sections.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
One of more of the authors received funding from the Canadian Institutes of Health Research (AS, DAH, VD), the Worker’s Compensation Board of British Columbia (AS, VD), the Oslo Sports Trauma Research Center (OL, RB); one of the authors (AS) has received funding through a CIHR Post-Doctoral Fellowship; one of the authors (DAH) is the Calgary Foundation-Grace Glaum Professor in Arthritis Research and supported by IGH of CIHR; one of the authors (VD) receives funding through a Michael Smith Foundation for Health Research Senior Scholar fellowship.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Abraham D, Taghavi S, Riml P, et al. VEGF-A and -C but not -B mediate increased vascular permeability in preserved lung grafts. Transplantation. 2002;73:1703–1706. [DOI] [PubMed]
- 2.Alfredson H, Lorentzon M, Backman S, Backman A, Lerner UH. cDNA-arrays and real-time quantitative PCR techniques in the investigation of chronic Achilles tendinosis. J Orthop Res. 2003;21:970–975. [DOI] [PubMed]
- 3.Alfredson H, Ohberg L, Forsgren S. Is vasculo-neural ingrowth the cause of pain in chronic Achilles tendinosis? An investigation using ultrasonography and colour Doppler, immunohistochemistry, and diagnostic injections. Knee Surg Sports Traumatol Arthrosc. 2003;11:334–338. [DOI] [PubMed]
- 4.Alfredson H, Pietila T, Jonsson P, Lorentzon R. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998;26:360–366. [DOI] [PubMed]
- 5.Andersson GB. Epidemiology of occupational neck, shoulder disorders. In: Gordon SL, Blair SJ, Fine LJ, eds. Repetitive Motion Disorders of the Upper Extremity. Bethesda, MD: American Academy of Orthopaedic Surgeons; 1994:31–42.
- 6.Bahr R, Fossan B, Loken S, Engebretsen L. Surgical treatment compared with eccentric training for patellar tendinopathy (Jumper’s Knee). A randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1689–1698. [DOI] [PubMed]
- 7.Bisset L, Paungmali A, Vicenzino B, Beller E. A systematic review and meta-analysis of clinical trials on physical interventions for lateral epicondylalgia. Br J Sports Med. 2005;39:411–422; discussion 411–422. [DOI] [PMC free article] [PubMed]
- 8.Boyer MI, Watson JT, Lou J, Manske PR, Gelberman RH, Cai SR. Quantitative variation in vascular endothelial growth factor mRNA expression during early flexor tendon healing: an investigation in a canine model. J Orthop Res. 2001;19:869–872. [DOI] [PubMed]
- 9.Budoff JE, Kraushaar BS, Ayala G. Flexor carpi ulnaris tendinopathy. J Hand Surg [Am]. 2005;30:125–129. [DOI] [PubMed]
- 10.Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10:2–11. [DOI] [PubMed]
- 11.Danielson P, Alfredson H, Forsgren S. Distribution of general (PGP 9.5) and sensory (substance P/CGRP) innervations in the human patellar tendon. Knee Surg Sports Traumatol Arthrosc. 2006;14:125–132. [DOI] [PubMed]
- 12.Danielson P, Alfredson H, Forsgren S. Studies on the importance of sympathetic innervation, adrenergic receptors, and a possible local catecholamine production in the development of patellar tendinopathy (tendinosis) in man. Microsc Res Tech. 2007;70:310–324. [DOI] [PubMed]
- 13.de Vos RJ, Weir A, Cobben LP, Tol JL. The value of power Doppler ultrasonography in Achilles tendinopathy: a prospective study. Am J Sports Med. 2007;35:1696–1701. [DOI] [PubMed]
- 14.Goh PP, Sze DM, Roufogalis BD. Molecular and cellular regulators of cancer angiogenesis. Curr Cancer Drug Targets. 2007;7:743–758. [DOI] [PubMed]
- 15.Hansson HA, Brandsten C, Lossing C, Petruson K. Transient expression of Insulin-like Growth Factor I immunoreactivity by vascular cells during angiogenesis. Exp Mol Path. 1989;50:125–138. [DOI] [PubMed]
- 16.Hardy B, Raiter A, Weiss C, Kaplan B, Tenenbaum A, Battler A. Angiogenesis induced by novel peptides selected from a phage display library by screening human vascular endothelial cells under different physiological conditions. Peptides. 2006;28:691–701. [DOI] [PubMed]
- 17.Jarvinen TA, Kannus P, Maffulli N, Khan KM. Achilles tendon disorders: etiology and epidemiology. Foot Ankle Clin. 2005;10:255–266. [DOI] [PubMed]
- 18.Jonsson P, Wahlstrom P, Ohberg L, Alfredson H. Eccentric training in chronic painful impingement syndrome of the shoulder: results of a pilot study. Knee Surg Sports Traumatol Arthrosc. 2006;14:76–81. [DOI] [PubMed]
- 19.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed]
- 20.Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393–408. [DOI] [PubMed]
- 21.Kraushaar BS, Nirschl RP. Tendinosis of the elbow (tennis elbow). Clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am. 1999;81:259–278. [DOI] [PubMed]
- 22.Lian O, Scott A, Engebretsen L, Bahr R, Duronio V, Khan K. Excessive apoptosis in patellar tendinopathy in athletes. Am J Sports Med. 2007;35:605–611. [DOI] [PubMed]
- 23.Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33:561–567. [DOI] [PubMed]
- 24.Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22:675–692. [DOI] [PubMed]
- 25.Martinez-Silvestrini JA, Newcomer KL, Gay RE, Schaefer MP, Kortebein P, Arendt KW. Chronic lateral epicondylitis: comparative effectiveness of a home exercise program including stretching alone versus stretching supplemented with eccentric or concentric strengthening. J Hand Ther. 2005;18:411–419, quiz 420. [DOI] [PubMed]
- 26.Matthews TJ, Hand GC, Rees JL, Athanasou NA, Carr AJ. Pathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increases. J Bone Joint Surg Br. 2006;88:489–495. [DOI] [PubMed]
- 27.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33:381–394. [DOI] [PubMed]
- 28.Nirschl RP. Patterns of failed healing in tendon injury. In: Leadbetter W, Buckwalter J, Gordon S, eds. Sports-Induced Inflammation: Clinical and Basic Science Concepts. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1990:577–585.
- 29.Peers KH, Brys PP, Lysens RJ. Correlation between power Doppler ultrasonography and clinical severity in Achilles tendinopathy. Int Orthop. 2003;27:180–183. [DOI] [PMC free article] [PubMed]
- 30.Perry SM, McIlhenny SE, Hoffman MC, Soslowsky LJ. Inflammatory and angiogenic mRNA levels are altered in a supraspinatus tendon overuse animal model. J Shoulder Elbow Surg. 2005;14:79S–83S. [DOI] [PubMed]
- 31.Petersen W, Varoga D, Zantop T, Hassenpflug J, Mentlein R, Pufe T. Cyclic strain influences the expression of the vascular endothelial growth factor (VEGF) and the hypoxia inducible factor 1 alpha (HIF-1alpha) in tendon fibroblasts. J Orthop Res. 2004;22:847–853. [DOI] [PubMed]
- 32.Pufe T, Petersen W, Tillmann B, Mentlein R. The angiogenic peptide vascular endothelial growth factor is expressed in foetal and ruptured tendons. Virchows Arch. 2001;439:579–585. [DOI] [PubMed]
- 33.Pufe T, Petersen WJ, Mentlein R, Tillmann BN. The role of vasculature and angiogenesis for the pathogenesis of degenerative tendons disease. Scand J Med Sci Sports. 2005;15:211–222. [DOI] [PubMed]
- 34.Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology (Oxford). 2006;45:508–521. [DOI] [PubMed]
- 35.Ribatti D, Conconi MT, Nussdorfer GG. Nonclassic endogenous novel [corrected] regulators of angiogenesis. Pharmacol Rev. 2007;59:185–205. [DOI] [PubMed]
- 36.Rolf CG, Fu BS, Pau A, Wang W, Chan B. Increased cell proliferation and associated expression of PDGFRbeta causing hypercellularity in patellar tendinosis. Rheumatology (Oxford). 2001;40:256–261. [DOI] [PubMed]
- 37.Scott A, Khan KM, Cook J, Duronio V. Human tendon overuse pathology: histopathologic, biochemical findings. In: Woo SL, Arnoczky SP, Renstrom P, eds. Tendinopathy in Athletes. Malden, MA: Blackwell Publishing Ltd; 2007:69–84.
- 38.Scott A, Lian O, Bahr R, Hart D, Duronio V. Elevated mast cell numbers in human patellar tendinosis: correlation with symptom duration and vascular hyperplasia Br J Sports Med. 2008 Mar 4 [Epub ahead of print]. [DOI] [PubMed]
- 39.Scott A, Lian O, Roberts CR, Cook JL, Handley CJ, Bahr R, Samiric T, Ilic MZ, Parkinson J, Hart DA, Duronio V, Khan KM. Increased versican content is associated with tendinosis pathology in the patellar tendon of athletes with jumper’s knee. Scand J Med Sci Sports. 2007 Dec 7 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 40.Stein V, Laprell H, Tinnemeyer S, Petersen W. Quantitative assessment of intravascular volume of the human Achilles tendon. Acta Orthop Scand. 2000;71:60–63. [DOI] [PubMed]
- 41.Tallon C, Coleman BD, Khan KM, Maffulli N. Outcome of surgery for chronic Achilles tendinopathy. A critical review. Am J Sports Med. 2001;29:315–320. [DOI] [PubMed]
- 42.Yamazaki Y, Morita T. Molecular and functional diversity of vascular endothelial growth factors. Mol Divers. 2006. [DOI] [PubMed]
- 43.Yoshikawa T, Tohyama H, Enomoto H, Matsumoto H, Toyama Y, Yasuda K. Expression of vascular endothelial growth factor and angiogenesis in patellar tendon grafts in the early phase after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2006;14:804–810. [DOI] [PubMed]
- 44.Yoshikawa T, Tohyama H, Katsura T, Kondo E, Kotani Y, Matsumoto H, Toyama Y, Yasuda K. Effects of local administration of vascular endothelial growth factor on mechanical characteristics of the semitendinosus tendon graft after anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2006;34:1918–1925. [DOI] [PubMed]
- 45.Young MA, Cook JL, Purdam CR, Kiss ZS, Alfredson H. Eccentric decline squat protocol offers superior results at 12 months compared with traditional eccentric protocol for patellar tendinopathy in volleyball players. Br J Sports Med. 2005;39:102–105. [DOI] [PMC free article] [PubMed]
- 46.Yuan J, Murrell GA, Wei AQ, Wang MX. Apoptosis in rotator cuff tendonopathy. J Orthop Res. 2002;20:1372–1379. [DOI] [PubMed]
- 47.Zanetti M, Metzdorf A, Kundert HP, et al. Achilles tendons: clinical relevance of neovascularization diagnosed with power Doppler US. Radiology. 2003;227:556–560. [DOI] [PubMed]