Abstract
Tendon cells respond to mechanical loads. The character (anabolic or catabolic) and sensitivity of this response is determined by the mechanostat set point of the cell, which is governed by the cytoskeleton and its interaction with the extracellular matrix. To determine if loss of cytoskeletal tension following stress deprivation decreases the mechanoresponsiveness of tendon cells, we cultured rat tail tendons under stress-deprived conditions for 48 hours and then cyclically loaded them for 24 hours at 1%, 3%, or 6% strain at 0.17 Hz. Stress deprivation upregulated MMP-13 mRNA expression and caused progressive loss of cell-matrix contact compared to fresh controls. The application of 1% strain to fresh tendons for 24 hours inhibited MMP-13 mRNA expression compared to stress-deprived tendons over the same period. However, when tendons were stress-deprived for 48 hours and then subjected to the same loading regime, the inhibition of MMP-13 mRNA expression was decreased. In stress-deprived tendons, it was necessary to increase the strain magnitude to 3% to achieve the same level of MMP-13 mRNA inhibition seen in fresh tendons exercised at 1% strain. The data suggest loss of cytoskeletal tension alters the mechanostat set point and decreases the mechanoresponsiveness of tendon cells.
Introduction
The ability of connective tissue cells to “sense” and respond to changes in their mechanical environment is central to the concept of mechanotransduction and the subsequent maintenance of tissue homeostasis [6–8, 10, 22, 23, 59]. However, the precise threshold of mechanical ‘sensitivity’ for a given cell type has not been determined. It has been suggested that the cellular regulation of biological function lies in the ability of cells to sense, generate, and balance mechanical forces [10, 13]. This mechanoresponsiveness was reportedly mediated through a tensegrity apparatus comprised of the cell’s cytoskeleton as well as its attachment to its surrounding extracellular matrix [8, 12, 16, 18, 20, 22, 23, 25, 47, 49, 53, 55, 56, 59]. Previous studies demonstrate cells can generate an internal tension within their cytoskeleton allowing the cell to maintain a constant cytoskeletal tension in response to changing loads [10, 13, 16, 23]. This has been termed cytoskeletal homeostasis and is thought to be the mechanism by which cells maintain a preset level of sensitivity to external loading; it is also termed the mechanostat set point [10, 18].
Frost first proposed the concept of the mechanostat set point to explain the mechanoresponsiveness of bone cells in controlling bone mass [18]. He theorized bone cells are programmed to sense a certain level of strain-induced signals [18]. If a signal was below the set point, the cell would activate catabolic mechanisms that decreased bone mass [18]. Conversely, if the strain signal exceeded the set point, anabolic mechanisms would be activated to increase bone mass [18]. A similar response occurs in tendon cells [33].
A recent study from our lab has suggested tendon cells are also capable of establishing a cytoskeletal tensional homeostasis through interactions with their local extracellular environment [33]. This cytoskeletal tension appears to create a mechanostat set point that calibrates the response of the tendon cell to external forces and regulates gene expression [33]. In this study, loss of cytoskeletal tension in response to the absence of local extracellular matrix strain resulted in an immediate and profound catabolic response by the tendon cells [33].
It has been suggested that the destructive mechanism(s) that precede(s) the overt pathological development of tendinopathy may, in fact, be the catabolic response of tendon cells to the local loss of homeostatic strain as a result of isolated, microscopic collagen fiber damage [2, 28]. Indeed, in vitro experimental studies have demonstrated that loss of homeostatic tendon strain can produce a pattern of catabolic gene expression [5, 33–36, 41], apoptosis [3, 17], and degenerative histological changes [5, 21] similar to those seen in clinical cases of tendinopathy [1, 2, 5, 19, 24, 26, 28, 30, 31, 38, 39, 40, 45, 50, 54, 60, 61]. However, the effect of these catabolic changes on the subsequent mechanoresponsiveness of tendon cells is unknown.
Studies in bone have suggested the local, catabolic, cellular response to stress deprivation reflects the osteocyte’s autoregulation of its local mechanical environment and, ultimately, its sensitivity to strain [15, 48]. This concept would suggest that following loss of homeostatic strain, the mechanostat set point of bone or tendon cells could be influenced (recalibrated) by the cell in response to alterations in its pericellular environment. This could occur directly, through a change in the modulus of the cell, or indirectly, through an alteration in the cell’s interaction with its pericellular matrix or neighboring cells [7, 22, 25, 46, 47, 49, 53, 55, 56]. Such changes could determine how tendon cells might respond to rehabilitation following injury and may explain why some eccentric exercise regimes, which result in increased tendon strain, have proved superior to other exercise programs in the nonoperative treatment of tendinopathy [11, 27, 29, 32, 44, 57].
We hypothesized following 48 hours of stress deprivation and loss of cytoskeletal tensional homeostasis, the mechanoresponsiveness of rat tail tendon cells would be decreased. We hypothesized the documented [33] inhibitory effect of in vitro cyclic, tensile loading on MMP-13 mRNA expression would be decreased in previously stress-deprived cells and a higher level of cyclic tensile strain would be required to achieve the same level of MMP-13 mRNA inhibition seen in fresh (nonstress-deprived) tendons following cyclic loading. We hypothesized this change in mechanoresponsiveness was due to an alteration in the mechanostat set point of the cells and not because of any major change in the material properties of these stress-deprived tendons. Finally, we hypothesized the catabolic response of rat tail tendon cells to the loss of cytoskeletal homeostatic tendon strain following stress deprivation would result in a progressive change in cell morphology and a loss of intimate contact between the tendon cells and their extracellular matrix.
Materials and Methods
To determine the effect of stress deprivation and loss of homeostatic cytoskeletal tension on the mechanoresponsiveness of tendon cells in vitro, rat tail tendons were exposed to 24 hours of 1, 3, or 6% cyclic (0.17 Hz) strain following 0 or 48 hours of stress deprivation under tissue culture conditions. The relative expression of rat interstitial collagenase (MMP-13) mRNA was used as the dependent variable. Additional control groups (24 and 72 hour stress-deprived tendons) were also evaluated. To evaluate the effect of stress deprivation on cell-matrix contact and tendon cell morphology, control (0 time) and stress-deprived (24, 48, and 72 hour) rat tail tendons were examined using transmission electron microscopy. Nuclear aspect ratios were calculated from these images and used to evaluate changes in cell shape following stress deprivation. Finally, to determine if stress deprivation resulted in any substantial change in the material properties of the tendons, the maximum stress, tensile modulus, and strain at maximum stress of control (0 hour) and stress-deprived (48 hours and 72 hours) tendons were compared.
Following Institutional Animal Care and Use Committee approval, rat tail tendons were harvested from adult Sprague-Dawley rats immediately after euthanasia. Using a sterile scalpel blade, the tail was cut between coccygeal vertebrae at both the base and at the distal tip of the tail for a total length of approximately 120 mm. Tendons were gently teased from the distal portion of each tail with forceps and maintained in DMEM media supplemented with 10% FBS, 1% penicillin-streptomycin-amphotericin B, 0.02 mg/ml gentocin, and 7.5 mg/ml ascorbate (GIBCO, Grand Island, NY) incubated at 37°C and 10% CO2, with the media being changed every other day.
The rat tail tendons were divided into eight groups as follows: Group 1: fresh (0 hour control); Group 2: stress-deprived for 24 hours; Group 3: 1% cyclic strain at 0.17 Hz for 24 hours; Group 4: stress-deprived for 48 hours; Group 5: stress-deprived for 72 hours; Group 6: stress-deprived for 48 hours followed by 1% cyclic strain at 0.17 Hz for 24 hours; Group 7: stress-deprived for 48 hours followed by 3% cyclic strain at 0.17 Hz for 24 hours; Group 8: stress-deprived for 48 hours followed by 6% cyclic strain at 0.17 Hz for 24 hours. Stress-deprived tendons were maintained in a culture dish in media under the tissue culture conditions noted above. Cyclic strain was applied to tendons in complete media under tissue culture conditions using a custom made, computer controlled, motor driven device [36].
To determine the relative level of MMP-13 mRNA expression in each of the above groups, approximately 10 tendons from each group were placed in 1.0 mL of RNAlaterTM (Qiagen, Valencia, CA) for a period of at least 24 hours at 4°C before processing. Total RNA was then extracted using the Qiagen RNEasy Kit (Qiagen, Valencia, CA) with the protocol provided for fibrous tissues. RNA (200–400 ng) was converted into cDNA using the Invitrogen SuperScript III Reverse Transcription system (Invitrogen, Carlsbad, CA). Real Time Quantitative PCR was performed using the TaqMan Gene Expression Assay (Applied Biosystems, Foster City, CA). Samples were run in a 96-well plate (20 μl final volume per reaction) on an ABI 7500-Fast Q-PCR apparatus. The endogenous control used for all Q-PCR experiment was 18 s rRNA. Results were analyzed using Sequence Detection System software (Applied Biosystems, Foster City, CA). TaqMan probe and primer sets were obtained for MMP-13 (Rn01448197_m1) and 18 s rRNA (Hs99999901_s1) from Applied Biosystems’ Gene Expression Assay database (http://allgenes.com). The Q-PCR results were calculated as relative quantification to the fresh control (0 hours) sample. The effect of the various treatments on MMP-13 mRNA expression were evaluated among all groups using an ANOVA and Tukey’s post-hoc tests. Significance was set at p < 0.05. The entire experiment was repeated three times.
To evaluate changes in cell morphology following stress deprivation, five rat tail tendons from fresh and each of the stress-deprived groups were cut into 0.5 cm longitudinal segments and fixed in 4% paraformaldehyde. Six segments representing the entire length of the tendon were chosen and placed in 1% phosphate-buffered osmium tetroxide, dehydrated in graded alcohols, and embedded in Poly/Bed 812-Araldite resin (Polysciences, Inc., Warrington, PA). From semi-thin sections, a representative sector (1.5–2 mm) was selected and ultra-thin sections were cut and examined with a JEM-100CX II electron microscope (Japanese Electron Optics Laboratory Co., Ltd., Tokyo, Japan). The entire length of the tendon segments was examined and representative photomicrographs taken.
Nuclear measurements were performed in order to document nuclear shape changes over the time of stress deprivation. The semi-thin sections (n = 6) from fresh and each of the stress-deprived groups were examined and light microscopy pictures were taken with a ZEISS Axioscope (ZEISS, Oberkochen, Germany) with a 63x oil objective. The major and minor axis lengths of tendon cell nuclei (average n = 59 per group) were measured using SCION image software (SCION corp., Frederick, MD). The ratio of the major and minor axis length was determined and used to quantify cell rounding. Differences in cell shape change were evaluated using ANOVA with Tukey’s post-hoc test with significance set at p < 0.05.
To determine if any changes in the material properties of the tendons occurred as a result of stress deprivation, additional rat tail tendons were paired as fresh and stress-deprived for either 48 hours or 72 hours (n = 12 per paired group). Maximum stress, tensile modulus, and strain at maximum stress were determined as described previously [35]. Briefly, tendons from each group were frozen at −80°C in media until testing. At the time of testing, tendons were thawed to room temperature and a 10 mm portion of each tendon dissected and placed in a saline-filled, 12-well plate to measure initial tendon diameter with a calibrated microscope. The tendon diameter was determined by taking the mean of six measurements perpendicular to the long axis of the tendon and a circular cross section was assumed for the computation of initial area [35]. The remaining portion of the tendon was gripped at the ends with saw-tooth clamps for a 40 mm gauge length. The portion of the tendon under each clamp was first air-dried and placed between two pieces of emery board while the midsection (test area) was kept moistened with saline. The gripped tendon was then mounted onto a custom-made material testing system. The system was equipped with a 5 lb load cell (Sensotec, Columbus, OH), a linear variable differential transformer (LVDT) (Lucas Schaevitz, Pennsauken, NJ) to measure grip-to-grip tendon displacement, and a motion controller (Newport, Fountain Valley, CA) to strain the tendons at a constant rate of 0.17 mm/s (∼0.42% strain/s). Each tendon was preloaded to 5 g then loaded at the above rate to failure in a PBS bath at room temperature. The load and displacement values were recorded using an analog-to-digital computer data acquisition system. Ultimate stress, tensile modulus, and strain at ultimate stress were computed from the load-deformation data. Material properties of the groups were compared using paired t-tests with significance set at p < 0.05.
Results
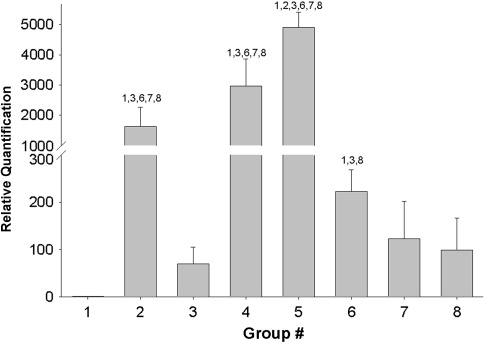
Stress deprivation and loss of homeostatic cytoskeletal tension resulted in an increase (p = 0.000) in MMP-13 mRNA expression when compared to fresh controls (Fig. 1).
Fig. 1.
The bar graph shows the change in the inhibitory effect of cyclic tensile loading on MMP-13 mRNA expression following 48 hours of stress deprivation. All experimental samples were quantified relative to the 0 hour fresh control. Group 1: fresh (0 hour control); Group 2: stress-deprived for 24 hours; Group 3: 1% cyclic strain at 0.17 Hz for 24 hours; Group 4: stress-deprived for 48 hours; Group 5: stress-deprived for 72 hours; Group 6: stress-deprived for 48 hours followed by 1% cyclic strain at 0.17 Hz for 24 hours; Group 7: stress-deprived for 48 hours followed by 3% cyclic strain at 0.17 Hz for 24 hours; Group 8: stress-deprived for 48 hours followed by 6% cyclic strain at 0.17 Hz for 24 hours. Numbers above each bar signify significant (p < 0.05) differences between specific groups.
The application of 1% cyclic strain at 0.17 Hz inhibited (p = 0.000) MMP-13 mRNA expression, as compared to tendons that were stress-deprived for the same period of time (24 hours). However, when tendons were stress-deprived for 48 hours and then exposed to a loading regime of 1% cyclic strain at 0.17 Hz for 24 hours, MMP-13 mRNA expression was higher (p = 0.000) than in those tendons immediately exercised (no prior stress deprivation) at the same loading regime. Increasing the strain magnitude to 3% or 6% at 0.17 Hz for 24 hours following 48 hours of stress deprivation inhibited MMP-13 mRNA expression to the same extent (p = 0.074 for 3% strain and p = 0.075 for 6% strain) as seen in tendons that were cyclically loaded at 1% strain at 0.17 Hz for 24 hours without any prior stress deprivation. We observed no difference (p = 0.305) in MMP-13 mRNA expression between tendons stress-deprived for 48 hours and then exercised at either 3% or 6% at 0.17 Hz for 24 hours. All loading regimes inhibited (p = 0.000) MMP-13 mRNA expression when compared to stress-deprived tendons over the same time periods (Fig. 1).
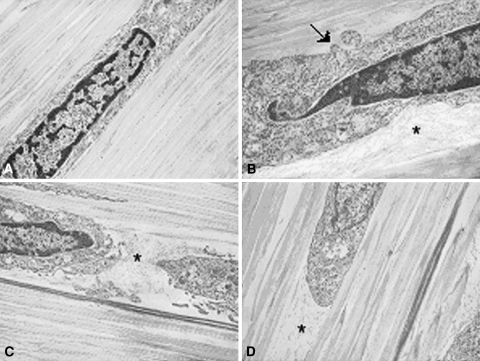
Following stress deprivation the rat tail tendons demonstrated striking changes in the immediate pericellular environment (Fig. 2A–D). Over the investigated time periods, there was a distinct loss of intimacy between the tendon cells and their adjacent pericellular matrix. The increasing space between the cells and their pericellular matrix was filled with loose, fine, fibrillar material typical of Type VI collagen (Fig. 2A–D). Additionally, the normally uniform cell borders developed irregular protrusions. Collagen Type I fibril packing in the tendons appeared less dense with time and distinct separations were visible between collagen fibrils.
Fig. 2A–D.
Transmission electron photomicrographs of (A) fresh, and (B) 24-hour, (C) 48-hour, and (D) 72-hour stress-deprived rat tail tendons demonstrating a progressive loss of contact between the cells and the extracellular matrix (ECM). Fine, fibrillar ECM components become prominent in the pericellular space (asterisk) and the normally uniform cell borders developed irregular protrusions (arrow). Collagen Type I fibril packing adjacent to the cells also appears less dense. (Stain, osmium tetroxide, uranyl acetate, and lead citrate; original magnification, ×10,000).
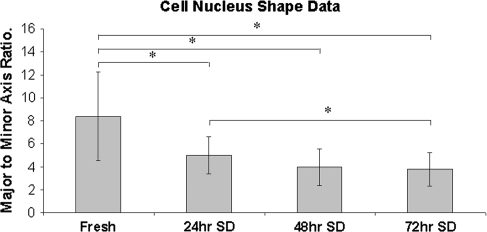
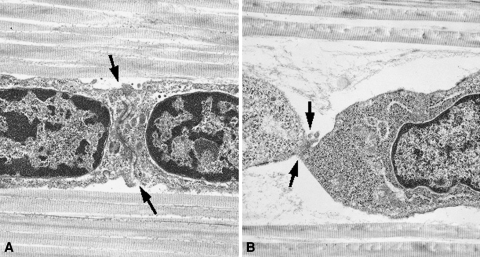
As the time of stress-deprivation increased, the shape of the tendon cells changed from their normal spindle shape to a more ovoid appearance: the nuclear aspect ratio decreased (p = 0.000) in the stress-deprived tendons when compared to fresh controls (Fig. 3). There was a decrease (p = 0.000) in the ratios of the major and minor axes of cell nuclei with increasing time of stress deprivation. The change in cell shape in the stress-deprived tendons coincided with an obvious decrease in cell-to-cell contact. Fresh tenocytes demonstrated intimate cell-cell contact along the entire width of the cell (Fig. 4A). However, following 48 hours of stress deprivation this cell-to-cell contact appears to be decreased (Fig. 4B).
Fig. 3.
The bar graph shows the decrease in cell nuclear aspect ratios seen in stress-deprived tendons as a function of time. The decrease in the ratio of major axis length to minor axis length reflects the “rounding” up of the cells in response to the loss of homeostatic strain. The symbol (*) signifies significant (p < 0.05) differences between specific groups.
Fig. 4A–B.
Transmission electron photomicrographs demonstrating representative cell-to-cell junctions (arrows) from (A) fresh and (B) a 48-hour stress-deprived rat tail tendon. The decrease in cell-to-cell contact area in the stress-deprived tendon could affect the extent of gap junctional intercellular communications. Loose, fine, fibrillar material is visible in the pericellular space. (Stain, osmium tetroxide, uranyl acetate, and lead citrate; original magnification, ×10,000).
There were no alterations in the maximum stress, strain at maximum stress, or modulus of either the 48-hour or 72-hour stress-deprived tendons when compared to fresh (0 hours) controls (Table 1).
Table 1.
Material properties of stress-deprived and control tendons
| Stress deprivation | Maximum stress (MPa) | Strain at maximum stress | Modulus (MPa) | |
|---|---|---|---|---|
| Set 1 (n = 12) | 0 hours | 29.53 ± 7.71 | 0.085 ± 0.017 | 450.25 ± 119.03 |
| 48 hours | 26.35 ± 6.03 | 0.081 ± 0.015 | 411.88 ± 93.90 | |
| p value | 0.27 | 0.54 | 0.39 | |
| Set 2 (n = 12) | 0 hours | 31.95 ± 8.21 | 0.073 ± 0.017 | 547.34 ± 133.40 |
| 72 hours | 30.84 ± 8.40 | 0.078 ± 0.015 | 516.66 ± 137.61 | |
| p value | 0.72 | 0.40 | 0.53 |
Discussion
The ability of tendon cells to alter the sensitivity of their responsiveness to perceived mechanical loads may play a major role in how these cells respond to rehabilitation following injury. Using a previously described in vitro test system [36], the current study examined the effect of short-term (48 hours) stress deprivation and loss of cytoskeletal tensional homeostasis on the morphology and the mechanoresponsiveness of rat tail tendon cells to cyclic load. We hypothesized the documented [36] inhibitory effect of in vitro cyclic, tensile loading on MMP-13 mRNA expression would be decreased in previously stress-deprived cells and a higher level of cyclic tensile strain would be required to achieve the same level of MMP-13 mRNA inhibition seen in fresh (nonstress-deprived) tendons following cyclic loading. We also hypothesized this change in mechanoresponsiveness was due to an alteration in the mechanostat set point of the cells and was not a result of any substantial change in the material properties of these stress-deprived tendons.
The stress-deprived in vitro system used in the current study is intended to model the destructive mechanism(s) that are thought to precede the overt pathological development of tendinopathy, namely, the catabolic response of tendon cells to the local loss of homeostatic strain as a result of isolated, microscopic collagen fiber damage [4, 28]. While the concept that understimulation, rather than overstimulation, of tendon cells may be at the heart of the etiopathogenesis of tendinopathy is a new concept [5], we believe our previous investigations [3, 4, 33, 34, 36] have validated this model as a useful tool to investigate the potential role of mechanobiology in the etiopathogenesis of tendinopathy. The increase in MMP-13 mRNA expression and protein synthesis seen in this model [2, 5, 33–36], as well as the histological features of cell shape change [21] and apoptosis [2, 12], which accompany loss of homeostatic strain, are very similar to those changes reported in clinical cases of tendinopathy [1, 2, 5, 19, 24, 26, 28, 30, 31, 38–40, 45, 50, 54, 60, 61]. Thus, while this in vitro tendon model is not intended to replicate the entire gamut of complex abnormalities that may occur in clinical cases of tendinopathy, we believe it can provide a relevant and well-controlled system in which to examine the response of normal and pathologic tendons to mechanobiological stimuli.
Our data confirm previous investigations reporting the stimulatory effect caused by the loss of homeostatic strain on MMP-13 mRNA expression in tendon cells and the inhibitory effect provided by cyclic strain [36, 41]. However, the increase in the amount of cyclic strain needed to elicit the same inhibitory effect after 48 hours of stress deprivation is a new and clinically relevant finding. These results suggest the observed change in the mechanobiological responsiveness of tendon cells following loss of homeostatic tendon strain represents an alteration in the mechanostat set point of the cells. This apparent change in the mechanostat set point was confirmed by the findings that following 48 hours of stress deprivation, increased extracellular matrix strain (3% or 6% cyclic strain) was required to achieve the same level of mechanotransduction signaling that was seen following the application of 1% cyclic strain to fresh tendons. The maintenance of material properties (maximum stress, strain at maximum strain, tensile modulus) in tendons following 24 or 48 hours of stress deprivation suggests the altered response of the tendon cells to strain was not due to any alterations in the physical properties of the tendon. We believe this finding important since it suggests local, pericellular changes rather than global extracellular matrix changes are responsible for the set point alteration observed in this study.
We found the loss of homeostatic strain also produced alterations in cell shape after only 24 hours. The cell nuclei became more ovoid and there was less intimate contact between adjacent cells. Similar alterations in tenocyte morphology have been reported in clinical cases of tendinopathy [26, 31, 37, 50]. In our study, these changes coincided with a progressive loss of intimate contact between the cells and their pericellular matrix. Experimental studies have demonstrated that whole tendon strain results in cellular (nuclear and cytoskeletal) deformation and, subsequently, mechanotransduction signaling through a calcium-based, second messenger pathway [3, 4, 52]. Mechanotransduction signals are mediated through the pericellular matrix to the cell nucleus via integrin-based cell-matrix connections [7, 12, 22, 23, 25, 46, 47, 49, 53, 55, 56, 59]. These connections are thought to interact with other pericellular matrix components, such as Type VI collagen, to aid in the integration of the tendon cell with its extracellular matrix environment [7, 12, 22, 23, 25, 46, 47, 49, 53, 55, 56, 59]. Type VI collagen is known to form an interconnecting meshwork between tendon cells and their extracellular matrix [9, 46]. In the current study, loosely bound, fine, fibrillar material, presumably Type VI collagen, was observed in the spaces that developed between the stress-deprived cells and their immediate pericellular matrix. This would suggest a disruption of the normal interconnecting meshwork between the cell and its pericellular matrix. While immunolocalization for Type VI collagen was not performed in the current study, previous investigations using electron microscopy have identified this loosely bound, fine, fibrillar material as Type VI collagen [43, 51]. Additional studies are required to confirm that the material observed in our stress-deprived system is Type VI collagen.
Alterations in tendon cell-matrix interactions influence cytoskeletal homeostasis and gene expression and have been implicated in the etiopathogenesis of tendinopathy [3, 5, 28, 33, 34]. Therefore, it is possible that, following loss of homeostatic tendon strain, the local degradative actions of catabolic enzymes on components of the pericellular matrix of tendon cells alters the cell-matrix interactions making them less sensitive to extracellular matrix strain. This would also suggest that, as was demonstrated in the current study, increased extracellular matrix strain is required to activate the mechanobiological signaling mechanism(s) in these altered cells.
Previous studies have demonstrated cell-to-cell communication (via gap junctions) in tendons [14, 42, 58]. Gap junctional intercellular communication (GJIC) has been postulated as a critical component of extracellular signal transduction, integration, and amplification in bone [15]. A recent study has suggested tendon cells may control and coordinate their response to mechanical signals, at least in part, through the actions of gap junctions [58]. It has been theorized that, in bone, a decrease in GJIC will result in an increase in the mechanostat set point and thus result in an increase in the mechanotransduction activation threshold of these cells [15]. In the current study, the loss of intimate cell-to-cell contact associated with stress deprivation could decrease the gap junction connections between tendon cells which could, in turn, contribute to the apparent increase in the activation threshold of the mechanostat set point required for the inhibition of MMP-13 mRNA expression. Additional research is needed to elucidate the precise stimuli and mechanism(s) by which the mechanostat set point of tendon cells is altered.
The precise consequence(s) of an altered set point in tendon cells following loss of homeostatic tendon strain is unclear. However, this phenomenon may have some clinical relevance in helping to provide a basic science explanation for the reported beneficial effects of some rehabilitation regimes that have been recently advocated in the nonoperative treatment of tendinopathy patients [11, 27, 29, 32, 44, 57]. Recent studies have documented the clinical efficacy of eccentric exercise programs in the nonoperative treatment of tendinopathy [11, 27, 29, 32, 44, 57]. Several of these studies [29, 44, 57] have demonstrated substantial clinical improvement in patients with patellar tendinopathy following 12 weeks of eccentric training performed on a 25° decline board when compared to standard eccentric exercise performed on a flat surface. However, the physiological or biomechanical mechanisms responsible for this improvement have not been identified. A recent study has demonstrated the use of a 25° decline during eccentric loading actually increases the load and strain on the patellar tendon when compared to eccentric loading on a flat surface [32]. While it has been suggested that the controlled progressive stresses utilized in these eccentric exercise regimes can act to increase the tensile strength of pathologic tendons [32], the precise cellular mechanism(s) responsible for the beneficial clinical results reported following the application of increased strain on pathologic tendons has not been completely elucidated. Our data suggest that one potential benefit of increased tendon strain in the treatment of tendinopathy may be related to an increase in the activation threshold of the mechanostat set point of cells in pathologic tendons. Higher tendon strains may be needed in tendinopathy patients to inhibit further catabolic gene expression and encourage homeostasis of the extracellular matrix.
Our data suggest stress deprivation alters the mechanical sensitivity of tendon cells. This change in mechanostat set point could be a result of the increase in MMP-13 mRNA expression and the subsequent loss of intimate cell-matrix contact. It will be critical to determine if alterations in the mechanostat set point of tendon cells, secondary to loss of homeostatic tendon strain, are progressive, self-limiting, or reversible. Additional investigations are also needed to determine if alterations in the mechanostat set point of tendon cells following loss of homeostatic strain have similar effects on both catabolic and anabolic gene expression. Finally, the effect of loading frequency on the gene response of stress-deprived cells must be examined. While the in vitro system used in this study precludes examining the ability of tendon cells to repair the localized, catabolic sequelae of stress deprivation, and possibly reverse the altered set point back to its original calibration, it does provide a novel model with which to investigate how the mechanobiological responsiveness of tendon cells may play a role in tendon homeostasis. Understanding the potential mechanisms involved in the ability, or inability, of tendon cells to reestablish their original mechanostat set point following alterations in cell-matrix connections could be a critical factor in determining the potential reversibility and treatment of various tendon abnormalities.
Acknowledgments
We thank Ralph Commons for his assistance in obtaining the electron photomicrographs.
Footnotes
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Alfredson H, Lorentzon M, Bäckman S, Bäckman A, Lerner UH. cDNA arrays and real time qualitative PCR techniques in the investigation of chronic Achilles tendinosis. J Orthop Res. 2003;21:970–975. [DOI] [PubMed]
- 2.Arnoczky SP, Lavagnino M, Egerbacher M. The response of tendon cells to changing loads: implications in the etiopathogenesis of tendinopathy. In: Woo S-L-Y, Renstrom P, Arnoczky SP, eds. Understanding and Prevention of Tendinopathy in the Athlete, Encyclopedia of Sports Medicine. Blackwell Publishing: Oxford, England; 2007:46–59.
- 3.Arnoczky SP, Lavagnino M, Egerbacher M. The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? Int J Exp Path. 2007;88:217–226. [DOI] [PMC free article] [PubMed]
- 4.Arnoczky SP, Lavagnino M, Whallon JJ, Hoonjan A. In situ cell nucleus deformation under tensile load: a morphologic analysis using confocal laser microscopy. J Orthop Res. 2002;20:29–35. [DOI] [PubMed]
- 5.Arnoczky SP, Tian T, Lavagnino M, Gardner K. Ex vivo static tensile loading inhibits MMP-1 expression in rat-tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J Orthop Res. 2004;22:328–333. [DOI] [PubMed]
- 6.Banes AJ, Horesovsky G, Larson C, Tsuzaki M, Judex S, Archambault J, Zernicke R, Herzog W, Kelley S, Miller L. Mechanical load stimulates expression of novel genes in vivo and in vitro in avian flexor tendon cells. Osteoarthritis Cartilage. 1999;7:141–153. [DOI] [PubMed]
- 7.Banes AJ, Tsuzaki M, Hu P, Brigman B, Brown T, Almekinders L, Lawrence WT, Fischer T. Cyclic mechanical load and growth factors stimulate DNA synthesis in avian tendon cells. J Biomech. 1995;28:1505–1513. [DOI] [PubMed]
- 8.Banes AJ, Tsuzaki M, Yamamoto J, Fischer T, Brigman B, Brown T, Miller L. Mechanoreception at the cellular level: the detection, interpretation, and diversity of responses to mechanical signals. Biochem Cell Biol. 1995;73:349–365. [DOI] [PubMed]
- 9.Bonaldo P, Russo V, Bucciotti F, Doliana R, Colombatti A. Structural and functional features of the α3 chain indicate a bridging role for chicken collagen VI in connective tissues. Biochemistry. 1990;29:1245–1254. [DOI] [PubMed]
- 10.Brown RA, Prajapati R, McGrouther DA, Yannus IV, Eastwood M. Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three dimensional substrates. J Cell Physiol. 1998;175:323–332. [DOI] [PubMed]
- 11.Cannell LJ, Taunton JE, Clement DB, Smith C, Khan KM. A randomized clinical trial of the efficacy of drop squats or leg curl exercises to treat clinically diagnosed jumper’s knee in athletes: pilot study. Br J Sports Med. 2001;35:60–64. [DOI] [PMC free article] [PubMed]
- 12.Chen CS, Ingber DE. Tensegrity and mechanoregulation: from skeleton to cytoskeleton. Osteoarthritis Cartilage. 1999;7:81–94. [DOI] [PubMed]
- 13.Chicurel ME, Chen CS, Ingber DE. Cellular control lies in the balance of forces. Curr Opin Cell Biol. 1998;10:232–239. [DOI] [PubMed]
- 14.Ciarelli MJ, Arnoczky SP, Kilfoyle SJ, Makidon PE. Demonstration of intercellular communications (gap junctions) in tendon cells in situ. Trans Orthop Res. 1996;21:372.
- 15.Donahue HJ. Gap junctional intercellular communications in bone: a cellular basis for the mechanostat set point. Calcif Tissue Int. 1998;62:85–88. [DOI] [PubMed]
- 16.Eastwood M, McGrouther DA, Brown RA. Fibroblast responses to mechanical forces. Proc Instn Mech Eng [H]. 1998;212:85–92. [DOI] [PubMed]
- 17.Egerbacher M, Arnoczky SP, Caballero O, Lavagnino M, Gardner KL. Loss of homeostatic tension induces apoptosis in tendon cells: an in vitro study. Clin Orthop Relat Res. 2008;466. DOI: 10.1007/s11999-008-0274-8. [DOI] [PMC free article] [PubMed]
- 18.Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec 1987;219:1–9. [DOI] [PubMed]
- 19.Fu SC, Chan BP, Wang W, Pau HM, Chan KM, Rolf CG. Increased expression of matrix metalloproteinase 1 (MMP-1) in 11 patients with patellar tendinosis. Acta Orthop Scand. 2002;73:658–662. [DOI] [PubMed]
- 20.Grinnell F. Signal transduction pathways activated during fibroblast contraction of collagen matrices. Curr Topic Pathol. 1999;93:61–73. [DOI] [PubMed]
- 21.Hannafin JA, Arnoczky SP, Hoonjan A, Torzilli PA. The effect of immobilization and cyclic tensile loading on the histology and material properties of canine flexor digitorum profundus tendons: an in vitro study. J Orthop Res. 1995;13:907–914. [DOI] [PubMed]
- 22.Ingber DE. Integrins as mechanochemical transducers. Curr Opin Cell Biol. 1991;3:841–848. [DOI] [PubMed]
- 23.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. [DOI] [PubMed]
- 24.Ireland D, Harrall R, Curry V, Holloway G, Hackney R, Hazleman B, Riley G. Changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol. 2001;20:159–169. [DOI] [PubMed]
- 25.Janmey PA. The cytoskeleton and cell signaling component localization and mechanical coupling. Physiol Rev. 1998;78:763–781. [DOI] [PubMed]
- 26.Järvinen M, Józsa L, Kannus P, Järvinen TL, Kvist M, Leadbetter W. Histopathological findings in chronic tendon disorders. Scand J Med Sci Sports. 1997;7:86–95. [DOI] [PubMed]
- 27.Jensen K, DiFabio RP. Evaluation of eccentric exercise in treatment of patellar tendonitis. Phys Ther. 1989;69:211–216. [DOI] [PubMed]
- 28.Jones GC, Corps AN, Penninton CJ, Clark IM, Edwards DR, Bradley MM, Hazelman BL, Riley GP. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerative human Achilles tendon. Arthritis Rheum. 2006;54:832–842. [DOI] [PubMed]
- 29.Jonsson P, Alfredson H. Superior results with eccentric compared to concentric quadriceps training in patients with jumper’s knee: a prospective randomized study. Br J Sports Med. 2005;39:847–850. [DOI] [PMC free article] [PubMed]
- 30.Józsa LG, Reffy A, Kannus P, Demel S, Elek E. Pathological alterations in human tendons. Arch Orthop Trauma Surg. 1990;110:15–21. [DOI] [PubMed]
- 31.Kannus P, Józsa LG. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed]
- 32.Kongsgaard M, Aagaard P, Roikjaer S, Olsen D, Jensen M, Langberg H, Magnusson SP. Decline eccentric squats increase patellar tendon loading compared to standard eccentric squats. Clin Biomech. 2006;21:748–754. [DOI] [PubMed]
- 33.Lavagnino M, Arnoczky SP. In vitro alterations in cytoskeletal tensional homeostasis control gene expression in tendon cells. J Orthop Res. 2005;23:1211–1218. [DOI] [PubMed]
- 34.Lavagnino M, Arnoczky SP, Egerbacher M, Gardner K, Burns ME. Isolated fibrillar damage in tendons stimulates local collagenase mRNA expression and protein synthesis. J Biomech. 2006;39:2355–2362. [DOI] [PubMed]
- 35.Lavagnino M, Arnoczky SP, Frank K, Tian T. Collagen fiber diameter distribution does not reflect changes in the mechanical properties of stress-deprived tendons. J Biomech. 2005;38:69–75. [DOI] [PubMed]
- 36.Lavagnino M, Arnoczky SP, Tian T, Vaupel Z. Effect of amplitude and frequency of cyclic tensile strain on the inhibition of MMP-1 mRNA expression in tendon cells: in vitro study. Connect Tissue Res. 2003;44:181–187. [DOI] [PubMed]
- 37.Leadbetter WB. Cell-matrix response in tendon injury. Clin Sports Med. 1992;11:533–578. [PubMed]
- 38.Lian O, Scott A, Engebretsen L, Bahr R, Duronio V, Khan K. Excessive apoptosis in patellar tendinopathy in athletes. Am J Sports Med. 2007;35:605–611. [DOI] [PubMed]
- 39.Lo IKY, Marchuk L, Hollinshead R. Matrix metalloproteinases and tissue inhibitors of metalloproteinases mRNA are specifically altered in torn rotator cuff tendons. Am J Sports Med. 2004;32:1223–1229. [DOI] [PubMed]
- 40.Magra M, Maffulli N. Matrix metalloproteases: a role in overuse tendinopathies. Br J Sports Med. 2005;39:789–791. [DOI] [PMC free article] [PubMed]
- 41.Majima T, Marchuk LL, Shrive NG, Frank CB, Hart DA. In-vitro cyclic tensile loading of an immobilized and mobilized ligament autografts selectively inhibits mRNA levels for collagenase (MMP-1). J Orthop Sci. 2000;5:503–510. [DOI] [PubMed]
- 42.McNeilly CM, Banes AJ, Benjamin M, Ralphs JR. Tendon cells in vivo form three dimensional network of cell processes linked by gap junctions. J Anat. 1996;189:593–600. [PMC free article] [PubMed]
- 43.Oh J, Zhao C, Amadio PC, An K-N, Zobitz ME, Wold LE. Immunolocalization of collagen types in the subsynovial connective tissue within the carpal tunnel in humans. J Orthop Res. 2005;23:1226–1231. [DOI] [PubMed]
- 44.Purdam CR, Jonsson P, Alfredson H, Lorentzon R, Cook JL, Khan KM. A pilot study of the eccentric decline squat in the management of painful chronic patellar tendinopathy. Br J Sports Med. 2004;38:395–397. [DOI] [PMC free article] [PubMed]
- 45.Riley GP. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology. 2004;43:131–142. [DOI] [PubMed]
- 46.Ritty TM, Roth R, Heuser JE. Tendon cell array isolation reveals a previously unknown fibrillin-2-containing macromolecular assembly. Structure. 2003;11:1179–1188. [DOI] [PubMed]
- 47.Rosales C, O’Brien V, Kornberg L, Juliano R. Signal transduction by cell adhesion receptors. Biochim Biophys Acta. 1995;1242:77–98. [DOI] [PubMed]
- 48.Rubin C, Sun Y-Q, Hadjiargyrou M, McLeod K. Increased expression of matrix metalloproteinase-1 in osteocytes precedes bone resorption as stimulated by disuse: evidence for autoregulation of the cell’s mechanical environment? J Orthop Res. 1999;17:354–361. [DOI] [PubMed]
- 49.Sachs F. Mechanical transduction in biological systems. Crit Rev Biomed Eng. 1988;16:141–169. [PubMed]
- 50.Scott A, Khan KM, Cook JL, Duronio V. Human tendon overuse pathology: histopathologic and biochemical findings. In: Woo S-L-Y, Renstrom P, Arnoczky SP, eds. Understanding and Prevention of Tendinopathy in the Athlete, Encyclopedia of Sports Medicine. Blackwell Publishing: Oxford, England; 2007:69–84.
- 51.Senga K, Kobayashi M, Hattori H, Yasue K, Mizutani H, Ueda M, Hoshino T. Type VI collagen in mouse masseter tendon, from osseous attachment to myotendinous junction. Anat Rec. 1995;243:294–302. [DOI] [PubMed]
- 52.Shirakura K, Ciarelli M, Arnoczky SP, Whallon JH. Deformation induced calcium signaling. Trans Comb Orthop Res. 1995;2:94.
- 53.Sung K-LP, Whittemore DE, Yang L, Amiel D, Akeson WH. Signal pathways and ligament cell adhesiveness. J Orthop Res. 1996;14:729–735. [DOI] [PubMed]
- 54.Tuoheti Y, Itoi E, Pradhan RL, Wakabayashi I, Takahashi S, Minagawa H, Kobayashi M, Okada K, Shimada Y. Apoptosis in the supraspinatus tendon with stage II subacromial impingement. J Shoulder Elbow Surg. 2005;14:535–541. [DOI] [PubMed]
- 55.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. [DOI] [PubMed]
- 56.Watson PA. Function follows form: generation of intracellular signals by cell deformation. FASEB J. 1991;5:2013–2019. [DOI] [PubMed]
- 57.Young MA, Cook JL, Purdam CR, Kiss ZS, Alfredson H. Eccentric decline squat protocol offers superior results at 12 months compared with traditional eccentric protocol for patellar tendinopathy in volleyball players. Br J Sports Med. 2005;39:102–105. [DOI] [PMC free article] [PubMed]
- 58.Waggett AD, Benjamin M, Ralphs JR. Connexin 32 and 43 gap junctions differentially modulate tenocyte response to cyclic mechanical load. Eur J Cell Biol. 2006;85:1145–1154. [DOI] [PubMed]
- 59.Wang N, Ingber DE. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys J. 1994;66:2181–2189. [DOI] [PMC free article] [PubMed]
- 60.Yuan J, Murrell GA, Wei AQ, Wang MX. Apoptosis in rotator cuff tendinopathy. J Orthop Res. 2002;20:1372–1379. [DOI] [PubMed]
- 61.Yuan J, Wang MX, Murrell GA. Cell death and tendinopathy. Clin Sports Med. 2003;22:693–701. [DOI] [PubMed]