Abstract
Heat shock proteins (HSPs) are often upregulated following oxidative and other forms of stress. Based on reports of excessive apoptosis in torn supraspinatus tendon and mechanically loaded tendon cells, we hypothesized heat shock proteins may be present in rodent and human models of tendinopathy due to their central role in caspase dependent apoptotic cell signaling. We used a running rat supraspinatus tendinopathy overuse model with custom microarrays to investigate the process at a genetic level. Additionally torn supraspinatus tendon and matched intact subscapularis tendon samples were collected from patients undergoing arthroscopic shoulder surgery. Control samples of subscapularis tendon were collected from 10 patients undergoing arthroscopic stabilization surgery and evaluated using semiquantative RT-PCR and immunohistochemistry. We identified substantial upregulation of heat shock proteins and apoptotic genes in the rodent model. We further confirmed increased levels of heat shock protein and apoptotic regulatory genes in human supraspinatus and subscapularis tendon. This finding suggests heat shock proteins play a role in the cascade of stress-activated programmed cell death and degeneration in tendinopathy and may provide a novel target in preventing tendinopathies.
Introduction
Rotator cuff tears are an important cause of pain and dysfunction in the adult shoulder [20]. They are common and increase with age. Results from surgical repair of full thickness tears are favorable; however, postoperative imaging often reveals high retear rates. The retears usually occur at the tendon-bone interface and suggest the suture has pulled through the tendon before biological healing has occurred at the tendon-bone interface [3, 5]. The torn margin of the rotator cuff has been targeted as a potential source of healing with studies implicating cytokines [7], matrix metalloprotineases [15], oxygen free radicals [27] and growth factors [4] in the pathophysiology of rotator cuff tears.
Apoptosis or programmed cell death is mediated by the activation of caspases (cysteine-containing aspartate proteases, a group of proteolytic enzymes) and is involved in the stress-induced cascade of tendinopathy [35]. Following oxidative and other forms of stress, one family of stress proteins that is often upregulated are heat shock proteins (HSPs). HSPs play a protective role as molecular chaperones in cells by facilitating the folding, intracellular transport, assembly, and disassembly of other proteins [8]. In addition, HSPs protect cells from oxidative damage both in vivo and in vitro and protect cells from either necrosis or apoptosis [23]. They are potent activators of the innate immune system capable of inducing proinflammatory cytokine induction [29] and their circulating levels are increased in a number of pathological conditions including atherosclerosis [33], cerebrovascular disease [31], and rheumatoid arthritis [9].
Based on our previous observations of increased apoptosis in tendinopathy we hypothesized that due to their central role in caspase dependent apoptotic cell signaling, heat shock proteins may be present in rodent and human models of tendinopathy.
Materials and Methods
Twenty-four male, 30-week-old Sprague-Dawley rats weighing 342–388 g each were randomly divided equally into exercise and control groups. The exercise group underwent a daily treadmill running regimen to model tendon overuse resulting in degeneration as previously described. Rats initially underwent daily training increasing in duration for 2 wk to accustom them to the exercise and surroundings. Subsequent to this, rats were subjected to exercise that consisted of running on a 10° decline at 17 m/min for 1 hour per day, 5 days per week. This regimen equates to ~7,500 strides/day. After 4 weeks of running, rats were euthanized by CO2 inhalation and both supraspinatus tendons were collected. The control group consisted of 12 nonexercised rats. All procedures and protocols were approved by the Animal Care and Ethics Committee of the University of New South Wales under ACEC No. 99/101.
Seventeen supraspinatus tendon samples were collected from patients with rotator cuff tears undergoing shoulder surgery (Table 1). The mean age of the rotator cuff ruptured patients was 57 years (range, 39–75 years). The rotator cuff repair surgery was performed via the standard arthroscopic technique. The size of the tear was recorded at the time of the operation. Samples of the subscapularis tendon were also collected from the same patients. They were only included if there was no evidence of subscapularis tendinopathy on a preoperative MRI scan or macroscopic damage to the subscapularis tendon at the time of arthroscopy.
Table 1.
Patient details
| Patient number | Age (years) | Duration of symptoms (months) | History of trauma | Pain intensity score† | Pain frequency score‡ | Supraspinatus strength (N) | Impingement sign | Size of rotator cuff tear (cm2) |
|---|---|---|---|---|---|---|---|---|
| 1 | 58 | 5 | Y | 8 | 9 | 15 | Y | 2 |
| 2 | 46 | 3 | Y | 9 | 8 | 17 | Y | 2 |
| 3 | 50 | 5 | N | 7 | 6 | 21 | Y | 2 |
| 4 | 64 | 8 | Y | 11 | 10 | 48 | Y | 4.5 |
| 5 | 55 | 7 | Y | 7 | 9 | 38 | Y | 3 |
| 6 | 52 | 2 | Y | 7 | 8 | 20 | Y | 3 |
| 7 | 70 | 6 | Y | 11 | 10 | 26 | Y | 6 |
| 8 | 75 | 3 | Y | 8 | 7 | 29 | Y | 1 |
| 9 | 62 | 4 | Y | 6 | 6 | 38 | Y | 1 |
| 10 | 51 | 3 | Y | 12 | 12 | 13 | Y | 2 |
| 11 | 72 | 6 | N | 6 | 8 | 30 | Y | 3 |
| 12 | 60 | 4 | Y | 8 | 7 | 10 | Y | 5 |
| 13 | 39 | 3 | Y | 11 | 10 | 4 | Y | 1 |
| 14 | 40 | 5 | Y | 6 | 9 | 17 | Y | 3 |
| 15 | 51 | 4 | Y | 6 | 10 | 16 | Y | 4 |
| 16 | 64 | 7 | N | 8 | 10 | 27 | Y | 6 |
| 17 | 62 | 4 | Y | 5 | 8 | 0 | Y | 6 |
†Pain intensity at night, at rest, and with activities was scored as: 0 = no pain, 1 = mild pain, 2 = moderate pain, 3 = severe pain, and 4 = very severe pain, and the three scores combined (maximum, 12).
‡The frequency of severe pain, pain at night, and pain with activities was scored as 0 = never, 1 = monthly, 2 = weekly, 3 = daily, and 4 = constantly, and the three scores combined (maximum, 12).
As an independent control 10 samples of subscapularis tendon were collected from patients undergoing arthroscopic surgery for shoulder stabilization without rotator cuff tears. The absence of rotator cuff tears was confirmed by arthroscopic examination. The mean age of the control group was 35 years (range, 20–41 years).
The subscapularis tendon was harvested arthroscopically from the superior border of the tendon 1 cm lateral to the glenoid labrum. The supraspinatus tendon was harvested from within 1.5 cm of the edge of the tear prior to surgical repair. The harvest and use of human tissue was approved by St. George Private Hospital/Health Care of Australia and The St. George Hospital/South Eastern Sydney Area Health Service. All specimens were immediately placed in RNAlater solution (Ambion, Inc., Austin, TX) and stored at −20°C until RNA extraction. For immunohistochemical staining the tissue samples were immediately fixed in 10% (v/v) formalin for 4 to 6 hours and then embedded in paraffin.
Custom microarrays consisting of 5760 rat oligonucleotide features in duplicate were provided by the Ramaciotti Centre of the University of New South Wales (Sydney, Australia). Fifteen to 20 μg of total RNA was used to synthesize cDNA in which Cy3 or Cy5 fluorescent dyes (Amersham Pharmacia Biotech, North Ryde, New South Wales, Australia) were incorporated using an indirect labeling method. Microarrays were scanned using an Axon GenePix 4000B microarray scanner (Molecular Devices, Union City, CA) and the resulting image analyzed using GenePix prosoftware version 3.0. All genes were represented by at least two independent targets on each microarray (ie, at least six independent targets across three microarrays) and the signal from each target was used to calculate an average signal for each gene. A gene was considered upregulated or downregulated if it had at least a 1.5-fold difference from controls.
Total RNA was isolated from tendon tissue using Trizol reagent (Life Technologies, Grand Island, NY) as per the manufacturer’s instructions. The RNA yield and integrity was evaluated by spectrophotometry and agarose gel electrophoresis, respectively. The OD 260/280 ratio was maintained between 1.7 and 2.1, confirming the quality of the RNA was satisfactory.
RNA extracted from tendon tissue was reverse transcripted to generate the first strand of complimentary DNA (cDNA) and subsequently amplified using PCR to produce amplified targeted segments of cDNA using ImProm-II Reverse Transcription System (Promega, Madison, WI). All the RT steps were performed in a GeneAmp 2400 thermal cycler (Applied Biosystems, Foster City, CA). One to 5 μg of total RNA was mixed with 0.5 μg of oligo dT primer to make a 5-μL solution and denatured at 70°C for 5 minutes. The reaction mixture was then cooled to 4°C for at least 5 minutes. Subsequently, a 15-μL reverse transcription reaction mixture consisting of 1 μL Improm-II Reverse Transcriptase, 0.5 mM of dNTPs, 6 mM MgCl2, 4 μL Improm-II 5× reaction buffer, 20 U Recombinant RNasin ribonuclease inhibitor and nuclease-free water was added to the target RNA and primer combination. The reaction mix was heated to 70°C for 5 minutes to allow annealing of oligo dT primers to the RNA. The mixture was then incubated at 37°C for 1 hour in order to create the cDNA. For each tested RNA sample, a control reaction was run with MMLV reverse transcriptase omitted from the mixture to exclude false positive results from genomic DNA contamination.
One microliter (100 ng) of each cDNA sample from the above extractions was amplified by PCR (GeneAmp System 2400) in a total volume of 20 μL. Platinum Super Mix [22 U/mL complexed recombinant Taq Polymerase with platinum Taq antibody, 22 mH Tris-HCl (pH 8.4), 55 mM KCl, 1.65 mM MgCl2, 220 μM dGTP, 220 μM dATP, 220 μM dTTP, 200 μM cCTP, stabilizers; Invitrogen Life Technologies, Carlsbad, CA] and used together with 0.2 μM each of the specific oligonucleotide primers using published sequences (Table 2). The thermal cycling program consisted of an initial denaturation step of 95°C for 1 minute and 15 seconds, followed by 35 cycles of 95°C for 30 seconds, a 30-second annealing step at varying temperatures, and a 30-second extension step at 73°C. Reaction products (12 μL) were analyzed by electrophoresis through 3% agarose gels stained with ethidium bromide and visualized by transillumination under ultraviolet light at a wavelength of 302 nM. Amplification of the mRNA of β-actin was used as an internal control in all cases.
Table 2.
Human-specific primer sequences and annealing temperatures for PCR reactions
| Product size (bp) | Primer sequence | Annealing temperature (°C) | |
|---|---|---|---|
| Caspase 3 | 398 | Forward TCG GTC TGG TAC AGA TGT CG | 58 |
| Reverse CAT ACA AGA AGT CGG CCT CC | |||
| Caspase 8 | 375 | Forward ACA GTG AAG TCT GGC CTC C | 55 |
| Reverse GCA GGT TCA TGT CAT CAT CC | |||
| CFLIP | 185 | Forward AAG AGG TAA GCT GTC TGT CGG | 52 |
| Reverse TCC TTG CTT ATC TTG CCT CG | |||
| HSP 27 | 330 | Forward ACG AGC ATG GCT ACA TCT CC | 57 |
| Reverse CTT TAC TTG GCG GCA GTC TC | |||
| HSP 70 | 213 | Forward CGA CCT GAA CAA GAG CAT CA | 58 |
| Reverse AAG ATC TGC GTC TGC TTG GT |
Paraffin sections were dewaxed with xylene and graded ethanol. Sections were stained with hematoxylin and eosin and toluidine blue. The presence or absence of edema and degeneration was recorded, together with the degree of fibroblast cellularity, amyloid deposition, and chondroid metaplasia. Tendinopathy was assessed on a basic histological scale derived from Khan et al. [12] (Grade 4 = marked degeneration, 3 = advanced degeneration, 2 = mild/moderate degeneration, 1 = normal tendon) by two independent assessors(AQW, FB) who were blinded to the results.
For immunohistochemical techniques antigen retrieval was achieved using Dako target retrieval solution (Dako, Carpinteria, CA) as per the manufacturer’s instructions. Endogenous peroxidase activity was scavenged with 3% (v/v) H2O2, and nonspecific antibody binding blocked with 10% (w/v) milk in TBS buffer for 15 minutes. Tissue slides were incubated with primary antibody (HSP 27 and 70, caspases 3 and 8, and cellular FLICE-inhibitory protein [cFLIP]) diluted 1:100 in 1% (w/v) BSA/TBS at room temperature for 60 minutes. After two washes, slides were incubated with Dako LINK solution consisting of biotinylated anti-mouse and anti-rabbit Ig. The slides were washed and incubated with streptavidin-peroxidase, followed by extensive washing and exposure to Dako liquid DAB for 5 minutes. Finally the sections were counterstained with hematoxylin. Positive and negative control specimens were included, in addition to the surgical specimens for each individual antibody staining technique.
Results are reported as mean values ± SEM. Comparisons between groups were made with two-way paired Student t tests, Mann-Whitney U tests and Kruskal-Wallis One Way Analysis of Variance on Ranks, using Sigma Stat, version 3.1 (Systat Software Inc, Richmond CA). A power analysis was performed with the beta error set at 0.2 (power = 0.8). Based on the results of the power analysis, it was determined that each group required 11 tissue samples to detect a difference of 20% between each of the groups with regard to the gene expression.
Results
Of the 5760 independent targets on each of the microarrays, the majority had less than a twofold change in expression in the experimental samples versus the controls and thus were considered not differentially expressed. Overall, 91 genes were found to be significantly upregulated, and 37 significantly downregulated. The differential expression of apoptotic related genes represented 6% (5 genes) of the significantly upregulated genes and 8% (3 genes) of significantly downregulated genes. Upregulation (p < 0.01) of HSP 27 (×3.4) and 70 (×2.5), cFLIP (×2.2) receptor and caspase 8 (×3.1) while downregulation (p < 0.05) of Poly(ADP-ribose) polymerase (×4.8), Type-2 angiotensin II receptor (×2.6) and Hypoxia inducible factor 1 (×2.2) occurred in degenerative rat supraspinatus tendon subjected to daily treadmill running for 4 weeks.
Also differentially expressed were cellular communication genes, including members of the glutamate signaling machinery, representing 13% (12 genes) of the genes found to be significantly upregulated and 8% (3 genes) of significantly downregulated genes. Several growth, differentiation, and developmental genes were also differentially regulated in the degenerated tendons, representing 14% (13 genes) of upregulated and 11% (10 genes) of downregulated genes.
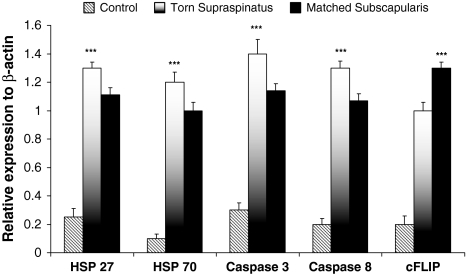
The apoptotic genes cFLIP, caspases 3 and 8, and HSPs 27 and 70 were detected in all samples of torn supraspinatus tendon (Fig. 1). The expression levels of caspases 3 and 8 and HSPs 27 and 70 was higher (p < 0.001) in the torn edges of supraspinatus when compared to matched subscapularis tendon. cFLIP mRNA expression was elevated (p < 0.05) in subscapularis tendon compared to torn supraspinatus samples (Fig. 1). The expression levels of cFLIP, caspases 3 and 8, and HSPs 27 and 70 was elevated (p < 0.001) in torn supraspinatus and matched subscapularis when compared to normal control subscapularis. We observed no correlation between tear size (r = 0.2, p = 0.4), patient age (r = 0.1, p = 0.3), pain, and apoptotic gene expression. These apoptotic genes were confirmed at the protein level where cFLIP, caspases 3 and 8, and HSPs 27 and 70 were confirmed in all samples of torn supraspinatus tendon (Fig. 2). Increased (p < 0.001) immunoactivity of caspases 3 and 8 and HSPs 27 and 70 were found in torn supraspinatus compared to matched and normal subscapularis. The proteins were localized to tendon cells. Negative controls using human epidermis showed no expression of any apoptotic genes. Positive controls of human tonsil tissue confirmed the presence of heat shock proteins 27 and 70 and caspases 3 and 8. cFLIP was identified in human liver tissue as a positive control.
Fig. 1.
The bar graph illustrates the relative expression of apoptosis genes in human tendon samples. The data are displayed as the mean ± SEM, n = 17 for supraspinatus and matched subscapularis, n = 10 for control group (*p < 0.01; **p < 0.001).
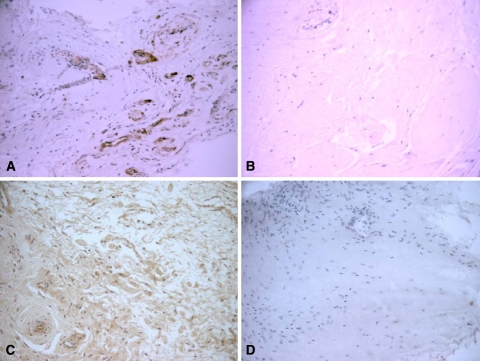
Fig. 2A–D.
The immunohistochemistry of heat shock protein (HSP) 27 is shown in (A) torn human supraspinatus tendon and (B) matched subscapularis tendon. HSP 70 immunohistochemistry in (C) torn human supraspinatus tendon and (D) matched subscapularis tendon is shown. (Haematoxylin and eosin stain, original magnification ×200).
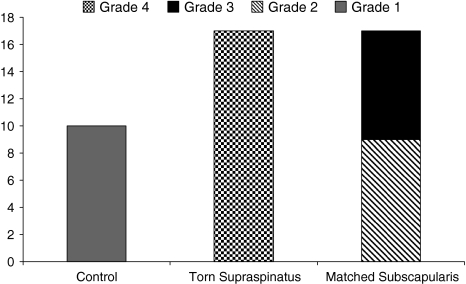
Of additional interest, hematoxylin and eosin staining of matched subscapularis samples showed overall appearances of advanced degenerative change consistent with tendinopathy with seven samples showing grade 3 changes and nine exhibiting grade 2 changes despite no evidence of tendinopathy on preoperative MRI and macroscopically at the time of arthroscopy (Fig. 3). All torn supraspinatus tendon were in keeping with those of advanced mucoid change and chondroid metaplasia as expected in association with marked degenerative change consistent with tendinopathy (Grade 4). We observed no correlation between tear size (r = 0.1, p = 0.6), patient age (r = 0.2, p = 0.5), pain, and the histological grade of tendinopathy.
Fig. 3.
The histological grades of tendinopathy in torn supraspinatus (n = 17), matched subscapularis (n = 17), and control subscapularis (n = 10) are shown. Grade 1 represents normal fibrotendinous tissue; Grade 2 represents mild-moderate degeneration with small increased prominence and roundness of the connective tissue and separation of the collagen fibers; Grade 3 represents advanced degeneration with mucoid ground substance and advanced fibrinoid degeneration; and Grade 4 represents severe degeneration with mucoid ground substance, advanced fibrinoid degeneration, and frank chondroid metaplasia.
Discussion
Our rationale for this study was to further investigate the role played by apoptotic genes in tendinopathy. We used a rat model of supraspinatus tendinopathy to identify novel apoptotic gene expression in degenerative tendon by microarray based on our previous observations of increased apoptosis in tendinopathy. These genes were subsequently identified in degenerative human supraspinatus tendon samples and confirmed at the protein level. Our data are the first to confirm the presence of heat shock protein in both animal and human models of tendinopathy.
We note several limitations. First, age-related changes within the tendon samples could contribute to the overall degenerative picture and gene expression seen in the matched subscapularis tendons. However, the lack of degenerative changes on MRI and arthroscopic examinations may suggest that the differences are truly at the cellular level as suggested by our work. Second, subscapularis tendon is functionally and organizationally distinct from supraspinatus and thus responds to mechanical loading in a different manner, which may alter its apoptotic gene profile. It is reassuring, however, that we found the same genes expressed in matched subscapularis tissue indicating gene expression may be uniform within joints subjected to tendon degeneration. Third, these genes were evaluated at a single time point and thus the pattern of expression we detected may not be uniform for the whole duration of the tendon overuse exercise. Finally, the tendon samples were not stained for fibroblast markers that could have identified precisely which cells were expressing the apoptotic proteins.
Apoptotic cell death is orchestrated by the activation of caspases, a family of cysteine proteases for aspartic acid residues, which cleave specific intracellular substrates to produce the characteristic features of this form of cell death [32]. It is essential for the normal development of multicellular organisms and is involved in cell turnover in healthy adult tissues. Glucocorticoids, growth factor withdrawal, reactive oxygen species (ROS), and cytokine production [29] are some of the signals that can induce apoptosis. Previous studies have highlighted the role of apoptosis in tendon cell degeneration with excessive apoptosis noted in the torn edges of rotator cuff tendons [35], patellar tendinosis [14], and in a mechanically stained tibialis anterior rodent model [25].
A mechanism that protects cells against exercise and oxidative stress is the induction of heat shock proteins. HSP 27 and the inducible HSP 70 are two main representatives of the HSP family modulated by exercise-induced oxidative stress. Induction of HSPs in response to stress serves to protect against the initial insult, augment recovery, and produce a state of resistance to subsequent stress in the cell. This protective role of HSPs can be attributed to several functional properties, including prevention of protein aggregation and promotion of protein disaggregation by catalyzing the refolding of damaged of denatured proteins [6, 21, 22]. Their expression in response to stress also has an important function in protection against apoptosis and in regulation of apoptotic cell signaling [16, 24].
In both our rat and human models of tendinopathy increased levels of HSP 27 and 70 were detected. Overexpression of HSP 27 is essential in preventing cells from undergoing apoptosis, a switch that may be redox-regulated [17]. HSP 27 inhibits specifically the cytochrome C and ATP-triggered activity of caspase 9 on the apoptotic pathway. Furthermore, HSP 27 indirectly interferes with cell death because of its ability to modulate intracellular glutathione [13], a parameter that is also regulated by exercise. Cytochrome C also triggers the oligomerization of Apaf-1, which in turn recruits pro-caspase 9 and pro-caspase 3 into the apoptosome (the caspase activation multiprotein complex). HSP 70 interacts with Apaf-1 thereby preventing its interaction with the caspases preventing apoptosis. HSP 70 also protects cells from heat stress [19], from the cytotoxic effects of TNFα [10], and from nitric oxide [2].
Previous work has shown that oxidative stress-induced apoptosis in human tendon fibroblasts is mediated via the release of the cytochrome C pathway and activation of caspase 3 [34]. Overexpression of nitric oxide synthases is also involved in the induction of stress-related tendinopathy [27]. The increased levels of HSP 27 and 70 in these models of tendinopathy suggest that these molecules are interfering with the caspase-driven apoptotic pathways in an attempt to prevent tendon cell apoptosis. Even in undamaged “control” subscapularis tendon the gene expression of HSPs was greater than that of normal control tendon.
The increased expression levels of other key mediators of apoptosis, caspases 3 and 8 and cFLIP, also suggest an ongoing apoptotic process in both torn and matched subscapularis tendons. cFLIP is a cytoplasmic protein that has sequence homology with caspase 8 and is capable of inhibiting apoptosis by blocking the recruitment and processing of caspase 8 [28]. Based on our observations it would appear that at a cellular level antiapoptotic molecules are attempting to interfere with Fas-L pathways in an attempt to limit the degree of apoptosis.
Pathological studies of rotator cuff tears have demonstrated features of hypoxic degenerative tendinopathy, mucoid degeneration, fragmentation of collagen architecture, and infiltration of fibroblast and vascular tissue [11, 30]. Recently small-sized rotator cuff tears have been shown to have increased fibroblast cellularity and an important inflammatory component while tissue from large and massive tears showed extreme degenerative changes [18]. We found no correlation between tear size, age, patient pain scores, or the duration of symptoms compared to the histological grade of tendinopathy between all samples. Despite no evidence of MRI-determined tendinopathy or gross macroscopic abnormalities at the time of arthroscopy, all matched samples of subscapularis tendons showed evidence of mild/moderate tendinopathy. In a previous investigation subscapularis tendons from patients with full-thickness rotator cuff tears showed approximately 35% of apoptotic cells compared to 20% in subscapularis tendon from patients without a rotator cuff tear [35]. This would appear to agree with our observation that tendinopathy is a feature of intact subscapularis tendon in patients with full-thickness supraspinatus tears. In the future this could provide a useful model for human tendinopathy that at present is unavailable and substituted with animal models [26].
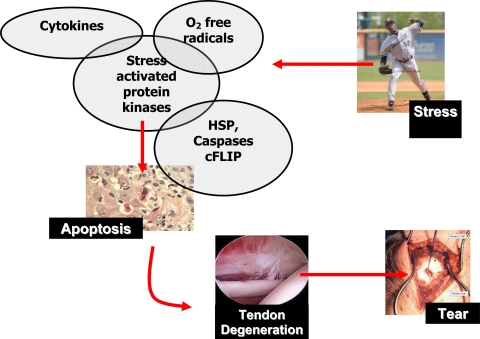
Ultimately there remains a physiological balance regulating the degree of apoptosis. It may be that apoptotic cell death induced by exercise in tissues exposed to stresses is a normal process used to remove partially damaged cells while excessive exercise may cause subsequent mechanical damage leading to necrosis and apoptosis. We therefore hypothesize, based on our and others’ observations, an increase in the amount and duration of load that a tendon cell sees may result in activation of stress-activated protein kinases [1], oxygen free radicals [27], and apoptotic mediators which when persistently activated cause the tendon cells to undergo apoptosis. This results in a weakened collagenous matrix leading to subsequent tendon degeneration and clinical tendon rupture (Fig. 4).
Fig. 4.
The schematic diagram illustrates the manner in which tendinopathies may arise. An increase in the amount and duration of load that a tendon cell experiences may result in activation of protein kinases, cytokines, oxygen free radicals, and apoptotic mediators which, when persistently activated, cause the tendon cells to undergo apoptosis. This results in a weakened collagenous matrix leading to subsequent tendon degeneration and clinical tendon rupture.
The finding of increased levels of heat shock proteins in human and rat models of tendinopathy with the coexpression of other regulators of apoptosis suggests that heat shock proteins play a role in the cascade of degeneration in tendinopathy and may provide a novel target in preventing tendinopathies.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the animal and human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Arnoczky SP, Tian T, Lavagnino M, Gardner K, Schuler P, Morse P. Activation of stress-activated protein kinases (SAPK) in tendon cells following cyclic strain: the effects of strain frequency, strain magnitude, and cytosolic calcium. J Orthop Res. 2002;20:947–952. [DOI] [PubMed]
- 2.Bellmann K, Jaattela M, Wissing D, Burkart V, Kolb H. Heat shock protein hsp70 overexpression confers resistance against nitric oxide. FEBS Lett. 1996;391:185–188. [DOI] [PubMed]
- 3.Boileau P, Brassart N, Watkinson DJ, Carles M, Hatzidakis AM, Krishnan SG. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229–1240. [DOI] [PubMed]
- 4.Fenwick SA, Curry V, Harrall RL, Hazleman BL, Hackney R, Riley GP. Expression of transforming growth factor-beta isoforms and their receptors in chronic tendinosis. J Anat. 2001;199:231–240. [DOI] [PMC free article] [PubMed]
- 5.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219–224. [DOI] [PubMed]
- 6.Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. [DOI] [PubMed]
- 7.Gotoh M, Hamada K, Yamakawa H, Yanagisawa K, Nakamura M, Yamazaki H, Ueyama Y, Tamaoki N, Inoue A, Fukuda H. Interleukin-1-induced subacromial synovitis and shoulder pain in rotator cuff diseases. Rheumatology (Oxford). 2001;40:995–1001. [DOI] [PubMed]
- 8.Hartl FU, Martin J, Neupert W. Protein folding in the cell: the role of molecular chaperones Hsp70 and Hsp60. Annu Rev Biophys Biomol Struct. 1992;21:293–322. [DOI] [PubMed]
- 9.Hayem G, De Bandt M, Palazzo E, Roux S, Combe B, Eliaou JF, Sany J, Kahn MF, Meyer O. Anti-heat shock protein 70 kDa and 90 kDa antibodies in serum of patients with rheumatoid arthritis. Ann Rheum Dis. 1999;58:291–296. [DOI] [PMC free article] [PubMed]
- 10.Jaattela M, Wissing D, Bauer PA, Li GC. Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 1992;11:3507–3512. [DOI] [PMC free article] [PubMed]
- 11.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed]
- 12.Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393–408. [DOI] [PubMed]
- 13.Leeuwenburgh C, Hollander J, Leichtweis S, Griffiths M, Gore M, Ji LL. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J Physiol. 1997;272:R363–369. [DOI] [PubMed]
- 14.Lian O, Scott A, Engebretsen L, Bahr R, Duronio V, Khan K. Excessive apoptosis in patellar tendinopathy in athletes. Am J Sports Med. 2007;35:605–611. [DOI] [PubMed]
- 15.Lo IK, Marchuk LL, Hollinshead R, Hart DA, Frank CB. Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase mRNA levels are specifically altered in torn rotator cuff tendons. Am J Sports Med. 2004;32:1223–1229. [DOI] [PubMed]
- 16.Locke M. The cellular stress response to exercise: role of stress proteins. Exerc Sport Sci Rev. 1997;25:105–136. [DOI] [PubMed]
- 17.Martin-Ventura JL, Nicolas V, Houard X, Blanco-Colio LM, Leclercq A, Egido J, Vranckx R, Michel JB, Meilhac O. Biological significance of decreased HSP27 in human atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:1337–1343. [DOI] [PubMed]
- 18.Matthews TJ, Hand GC, Rees JL, Athanasou NA, Carr AJ. Pathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increases. J Bone Joint Surg Br. 2006;88:489–495. [DOI] [PubMed]
- 19.Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997;17:5317–5327. [DOI] [PMC free article] [PubMed]
- 20.Neviaser RJ. Ruptures of the rotator cuff. Orthop Clin North Am. 1987;18:387–394. [PubMed]
- 21.Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. [DOI] [PubMed]
- 22.Parsell DA, Taulien J, Lindquist S. The role of heat-shock proteins in thermotolerance. Philos Trans R Soc Lond B Biol Sci. 1993;339:279–285; discussion 285–276. [DOI] [PubMed]
- 23.Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jaattela M, Penninger JM, Garrido C, Kroemer G. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3:839–843. [DOI] [PubMed]
- 24.Ruchalski K, Mao H, Li Z, Wang Z, Gillers S, Wang Y, Mosser DD, Gabai V, Schwartz JH, Borkan SC. Distinct hsp70 domains mediate apoptosis-inducing factor release and nuclear accumulation. J Biol Chem. 2006;281:7873–7880. [DOI] [PubMed]
- 25.Scott A, Khan KM, Heer J, Cook JL, Lian O, Duronio V. High strain mechanical loading rapidly induces tendon apoptosis: an ex vivo rat tibialis anterior model. Br J Sports Med. 2005;39:e25. [DOI] [PMC free article] [PubMed]
- 26.Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, Carpenter JE. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. [DOI] [PubMed]
- 27.Szomor ZL, Appleyard RC, Murrell GA. Overexpression of nitric oxide synthases in tendon overuse. J Orthop Res. 2006;24:80–86. [DOI] [PubMed]
- 28.Tran SE, Meinander A, Holmstrom TH, Rivero-Muller A, Heiskanen KM, Linnau EK, Courtney MJ, Mosser DD, Sistonen L, Eriksson JE. Heat stress downregulates FLIP and sensitizes cells to Fas receptor-mediated apoptosis. Cell Death Differ. 2003;10:1137–1147. [DOI] [PubMed]
- 29.Tsan MF, Gao B. Cytokine function of heat shock proteins. Am J Physiol Cell Physiol. 2004;286:C739–744. [DOI] [PubMed]
- 30.Uhthoff HK, Sano H. Pathology of failure of the rotator cuff tendon. Orthop Clin North Am. 1997;28:31–41. [DOI] [PubMed]
- 31.Welch WJ. Heat shock proteins as biomarkers for stroke and trauma. Am J Med. 2001;111:669–670. [DOI] [PubMed]
- 32.Wolf BB, Green DR. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem. 1999;274:20049–20052. [DOI] [PubMed]
- 33.Xu Q, Schett G, Perschinka H, Mayr M, Egger G, Oberhollenzer F, Willeit J, Kiechl S, Wick G. Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation. 2000;102:14–20. [DOI] [PubMed]
- 34.Yuan J, Murrell GA, Trickett A, Wang MX. Involvement of cytochrome c release and caspase-3 activation in the oxidative stress-induced apoptosis in human tendon fibroblasts. Biochim Biophys Acta. 2003;1641:35–41. [DOI] [PubMed]
- 35.Yuan J, Murrell GA, Wei AQ, Wang MX. Apoptosis in rotator cuff tendonopathy. J Orthop Res. 2002;20:1372–1379. [DOI] [PubMed]