Abstract
Nitric oxide is an important messenger molecule in many physiological processes. The addition of NO via NO-flurbiprofen enhances the material properties of healing tendon, however, flurbiprofen has a detrimental effect on healing. We asked if NO delivered by a cyclooxygenase 3 inhibitor (paracetamol/acetaminophen) would enhance healing in a rat Achilles tendon healing model. Rats were injected subcutaneously daily with NO-paracetamol, paracetamol or vehicle from two days before surgery to the day of tissue harvesting. Paracetamol had no effect on tendon healing compared with vehicle alone. NO-paracetamol did not change the failure load, but did decrease the water content, enhance the collagen content, reduce the cross-sectional area and improve the ultimate stress of healing tendon compared with paracetamol and vehicle. The collagen organization of the healing tendon in the NO-paracetamol group, as determined by polarized light microscopy, was enhanced. Our data suggests NO-paracetamol increases the total collagen content and enhances organization while decreasing the cross-sectional area of healing rat Achilles tendon and is consistent with human clinical trials where NO has improved the symptoms and signs of tendinopathy.
Introduction
Nitric oxide (NO) is an important messenger molecule in many physiological processes [1, 4–6], including tendon healing [7, 8, 10, 11, 13, 14, 22]. We previously demonstrated NO synthase activity increases after injury in a rat healing Achilles tendon model [10]. When NO synthase was inhibited, healing tendon failure load decreased by 25% [10]. Addition of NO through nitroflurbiprofen enhanced the material properties of healing tendon [22], whereas the parent compound caused a decrease in failure load of healing tendon [22].
Paracetamol is a widely used antipyretic and analgesic compound. Although it is frequently classified with nonsteroidal inflammatory drugs (NSAIDs), paracetamol differs from other NSAIDs in a number of ways. Unlike other NSAIDs, paracetamol exhibits little or no antiinflammatory activity. Paracetamol does not cause gastrointestinal damage and has no antiplatelet aggregation effect. The mechanism of its analgesic effect was not understood until cyclooxygenase-3 (Cox-3) was recently identified and reported abundantly expressed in the mature brain and the spinal cord [3]. Nitroparacetamol is a novel compound derived from paracetamol and designed to deliver NO along with the analgesic compound [2].
We asked if NO-paracetamol enhanced day 10 healing rat Achilles tendon failure load, with cross-sectional area, water content, ultimate stress, strain collagen content and collagen organization as secondary outcomes measures.
Materials and Methods
We evaluated the effects of NO delivered by NO-paracetamol on tendon healing in a rat Achilles tendon healing model. The primary endpoint was failure load of the healing Achilles tendon construct at day 10. A priori power analysis with an effect size of 1.5, α error probability of 0.05, power 0.8 indicated a sample size of 18 rats in total. Secondary endpoints were tendon cross-sectional area, water content, ultimate stress, strain, collagen content and collagen organization. Rats in every experiment were randomly divided into three groups: vehicle, paracetamol, and a NO-paracetamol group. Outbred male Sprague-Dawley rats, weighing 250 to 300 g (6–8 weeks old), were used for tendon wet/dry weight and collagen content assays (n = 34; vehicle 11, paracetamol 12, NO-paracetamol 12), biomechanical testing (n = 17; vehicle 5, paracetamol 6, NO-paracetamol 6), and histologic assessment (n = 18; vehicle 6, paracetamol 6, NO-paracetamol 6), respectively. We obtained prior approval from the Committee on Animal Research of the University of New South Wales, Sydney.
Rats were housed in beta-chip-lined plastic cages, three animals per cage, with a 12-hour light/dark cycle in a central animal care facility and were fed conventional rat chow (Gordons Specialty Stock Feeds, Yanderra, N.S.W., Australia) and tap water ad libitum. All rats were given health checks and were allowed at least 2 weeks to adapt to their new environment before surgery. A rat healing Achilles tendon model was used as previously described [9]. Anesthesia was achieved with 40 mg/kg pentobarbitone sodium by intraperitoneal injection. Briefly, a 1.0-cm incision was made over the skin of the right Achilles tendon. After the Achilles tendon and plantaris tendon were completely isolated from the surrounding fascia, the Achilles tendon was transected. The tendinous portion of the plantaris tendon was removed to prevent any possible action as an internal splint. The skin was then sutured with 4–0 Ethilon (Piscataway, NJ) monofilament nylon.
Postoperatively we applied no dressing or cast and the animals were unrestricted and fed a normal diet after surgery. The animals were euthanized by fluothane inhalation at day 10 postoperatively for sample collection for biomechanical testing and collagen content assays and at days 5, 10, and 15 postoperatively for histologic assessment.
NO-paracetamol and paracetamol (Tylenol®, McNeil Laboratories, Raritan, NJ) were provided by NicOx (Paris, France). The compounds were dissolved in a vehicle of DMSO and polyethylene glycol (1:1) and diluted with 0.9% normal saline before injection. Rats were injected daily subcutaneously with NO-paracetamol (170 μM or 48 mg/kg per day), paracetamol (170 μM or 25.7 mg/kg per day), or vehicle from 2 days before surgery to the day of tissue harvesting.
Healing Achilles tendon samples were harvested at day 10 postoperatively. After removing all extraneous tissues, the middle segment of the healing tendon (approximately 7 mm) was collected and the original tendons at the two ends were removed by transverse sharp transection. The original tendon was white, while the healing tendon more translucent. Fresh healing tendon specimens were weighed immediately (wet weight), then dried overnight in a freeze-dryer (RVT-400, Savant, Farmingdale, NY) and weighed again (dry weight).
We determined the collagen content of tendons by measuring the concentration of hydroxyproline in each tissue specimen as previously described [16, 20]. Briefly, 5 mg of dried tendon tissue was hydrolyzed with 1 mL of 6 N hydrochloric acid (HCl) by heating at 110°C for 12 hours. Hydrolyzed tissues were transferred to beakers and kept at 60°C until the HCl was completely evaporated, then reconstituted in 20 mL H2O and the pH adjusted to 7.0 with NaOH. Hydroxyproline was dissolved and diluted to the concentration of 80, 40, 20, 10, 5, 2.5, and 0 μg/mL as standard solutions. One hundred microliters of the standard solutions or samples were added to a 96-well plate. We added 50 microliters of chloramine T to initiate hydroxyproline oxidation. After mixing by gently shaking, we incubated the plate for 20 minutes at room temperature. Fifty microliters of perchloric acid (HClO4) was added to each well and incubated for 5 minutes at room temperature to terminate the reaction. To develop the color we added 50 μL of p-dimethylaminobenzaldehyde (p-DMAB; Sigma, St Louis, MO) solution to each well and incubated the plate in a water bath (60°C) for 20 minutes, then cooled it in tap water for 5 minutes to stop further development of color. The absorbance was read at 550 nm using a spectrophotometer (SPECTRAmaxTM 250; Molecular Devices, Sunnyvale, CA). We calculated the hydroxyproline concentration against the standard curve. The total collagen content of each sample was calculated based on the hydroxyproline concentration and the hydrolyzed sample volume. Results were expressed as microgram hydroxyproline per milligram dry tendon tissue. Normal uninjured Achilles tendon samples (n = 4) were included in each assay as a quality control. All assays were performed in quadruplicate.
For mechanical testing we harvested healing rat Achilles tendons with the calcaneus on one end and a portion of muscle on another end, placed them into a container with 0.9% (w/v) saline soaked gauze at day 10, and stored at −20°C until testing. Before mechanical testing, Achilles tendon samples were completely thawed. We measured the cross-sectional areas of the healing tendons with an area micrometer similar to that described previously [9]. After measurement of the cross-sectional area, the samples proceeded to biomechanical testing. Failure load and stiffness of healing tendon were assessed on an Instron hydraulic testing system (Instron Test Equipment Ltd, High Wycombe, Bucks, UK) as previously described [9]. Briefly, the tendon was held on a cryoclamp by gripping muscle and intramuscular tendinous fibers. The cryoclamp was attached to the testing system. Dry ice was used to keep the gripped muscle and intramuscular tendinous tissue frozen. The calcaneus was fixed to another clamp, which was attached to a 450-N load cell (Xtran, Applied Measurement Australia Pty Ltd, Eastwood, New South Wales, Australia). Throughout the testing procedure, the tendon was kept moist and unfrozen by continuous irrigation with 0.9% saline. The in situ resting length of the tendon was measured with a micrometer and each specimen was loaded to failure at a constant distraction rate of 1 mm/sec (strain rate approximately 10%/sec). Force and displacement data were collected at a rate of 1000 samples/second using a dedicated computer and software (National Instruments/LabVIEW 5.1, Austin, TX). On completion of testing, we inspected each specimen for the type of failure, including substance rupture versus avulsion. We also calculated the mechanical properties (stiffness in N/mm, and ultimate stress at failure (MPa) - maximum energy at failure and total energy) on uninjured contralateral tendons [18]. Failure load was expressed as maximum force, measured in Newtons (N), which caused tendon rupture.
To assess organization we harvested healing Achilles tendon samples on postoperative day 10. After removing all extraneous tissues, the healing tendon (approximately 7 mm) was collected with a small portion of the original tendons. The tendons were sandwiched in a plastic container to maintain orientation and immediately fixed in buffered formalin saline (pH 7.4) for 24 hours, then dehydrated, and embedded in paraffin wax. Coronal sections of the healing Achilles tendons were cut in 5-μm sections and stained with hematoxylin and eosin. Four to five tissue sections were examined under a Leica DMLB polarized light microscope (Leica, Postach, Germany). Tendon collagen fibril organization was semiquantified by scoring 1 to 5 as previously described [9]. Briefly, tendon sections were examined under a polarized light microscope and scored in a blinded manner by one observer (G.T.). Score 1 represented “immature” and score 5 represented “mature” tendon organization. The scores were based on the orientation and network structure of collagen fibers, the formation of collagen fiber bundles, and the length and diameter of the bundles: score 1, collagen fibers showed no unique orientation and no birefringence of the collagen fiber bundles formed; score 2, some collagen fiber bundles were formed but the length and diameter of collagen fiber bundles were less than 100 μm and 15 μm, respectively, and the areas of collagen fiber bundles were less than 50% with a loose network structure; score 3, length and diameter of collagen fiber bundles were over 100 μm and 15 μm, respectively, and these bundles formed a tight network structure with the area of collagen fiber bundles less than 50%; score 4, area of collagen fiber bundles over 50%, and the bundles were longitudinally aligned along tendon; and score 5, normal tendon structure—wide, tightly packed, well-organized collagen fiber bundles were longer with homogenous birefringence and occupying more than 95% of the area. We previously reported the interobserver reliability of this assessment with an ICC = 0.92 [22].
All values in the text and figures are expressed as mean ± standard deviation of the number of observations. Analysis of the primary endpoint (failure load of the healing Achilles tendon construct at day 10) was made using unpaired two-tailed Student’s t-tests. Comparisons between injured and uninjured tendon was made with paired Student’s t-tests. With the exception of collagen organization, statistical analysis of all the secondary endpoints were made using un-paired two-tailed Student’s t-tests with the alpha level at 0.01. The alpha level was set at 0.01 in order to account for multiple comparisons. Analysis of collagen organization was made using a nonparametric test (Kruskal-Wallis one way analysis of variance on ranks). Comparisons between experimental groups were performed using unpaired two-tailed Student’s t-tests.
Results
The healing tendon constructs of animals in the vehicle group (40 ± 18 N, mean ± SD) had similar failure loads to those of the other control group: paracetamol (35 ± 12; p = 0.57) (Table 1). Addition of NO-paracetamol (49 ± N) neither enhanced nor reduced (p = 0.15) the failure load of the healing constructs. The failure site in all samples was in the tendinous portion of the healing tendon.
Table 1.
The biomechanical properties of uninjured and healing (injured) Achilles tendons in rats treated with NO-paracetamol, paracetamol, or vehicle at day 10 postoperation
| Failure load (N) | Cross-sectional area (mm2) | Displacement (mm) | Maximum energy | Total energy | Length (mm) | Stiffness (N/mm) | Ultimate stress (MPa) | Strain (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Uninjured | |||||||||
| Vehicle | |||||||||
| Mean | 70 | 2.8 | 3.6 | 134 | 333 | 8.6 | 7.6 | 25 | 42 |
| SD | 24 | 0.1 | 1.5 | 69 | 145 | 1.0 | 2.7 | 9 | 14 |
| n | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 5 | 4 |
| Paracetamol | |||||||||
| Mean | 76 | 2.8 | 3.3 | 141 | 364 | 10.1 | 7.5 | 27 | 33 |
| SD | 15 | 0.1 | 0.9 | 76 | 99 | 0.4 | 1.6 | 5 | 9 |
| n | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Controls | |||||||||
| Mean | 68 | 2.8 | 3.3 | 120 | 346 | 8 | 8 | 24 | 30 |
| SD | 31 | 1.2 | 1.3 | 66 | 208 | 2.9 | 2.8 | 10 | 14 |
| n | 11 | 11 | 11 | 11 | 11 | 10 | 10 | 11 | 10 |
| NO-paracetamol | |||||||||
| Mean | 93 | 3 | 2.9 | 138 | 527 | 9.2 | 10.1 | 32 | 31 |
| SD | 7 | 0 | 0.3 | 18 | 208 | 0.7 | 1.1 | 4 | 4 |
| n | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| T-tests | |||||||||
| Vehicle vs paracetamol | 0.64 | 0.69 | 0.72 | 0.89 | 0.71 | 0.02 | 0.95 | 0.62 | 0.26 |
| Controls vs NO-paracetamol | 0.05 | 0.24 | 0.29 | 1.00 | 0.06 | 0.58 | 0.02 | 0.10 | 0.35 |
| Injured | |||||||||
| Vehicle | |||||||||
| Mean | 40 | 7.9 | 5 | 119 | 172 | 10.2 | 4.5 | 5 | 51 |
| SD | 18 | 0.5 | 2 | 84 | 57 | 0.5 | 1.6 | 2 | 21 |
| n | 5 | 5 | 5 | 5 | 5 | 4 | 4 | 5 | 4 |
| Paracetamol | |||||||||
| Mean | 35 | 7.2 | 4 | 79 | 131 | 11.5 | 3.0 | 5 | 31 |
| SD | 12 | 0.5 | 1 | 37 | 34 | 0.8 | 1.0 | 1 | 10 |
| n | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Controls | |||||||||
| Mean | 37 | 7.5 | 4 | 97 | 150 | 10.0 | 3.6 | 5 | 36 |
| SD | 14 | 0.6 | 2 | 63 | 49 | 3.4 | 1.4 | 2 | 20 |
| n | 11 | 11 | 11 | 11 | 11 | 11 | 10 | 11 | 11 |
| NO-paracetamol | |||||||||
| Mean | 49 | 6.0 | 4 | 116 | 169 | 11.3 | 4.4 | 8 | 37 |
| SD | 16 | 0.6 | 2 | 67 | 52 | 1.0 | 1.7 | 2 | 15 |
| n | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| T-tests | |||||||||
| Vehicle vs paracetamol | 0.57 | 0.04 | 0.41 | 0.33 | 0.18 | 0.02 | 0.11 | 0.81 | 0.08 |
| Controls vs NO-paracetamol | 0.15 | 0.0002 | 0.99 | 0.57 | 0.44 | 0.39 | 0.32 | 0.007 | 0.92 |
SD = standard deviation, n = sample size. Statistical analysis using unpaired two-tailed Student’s t-tests. The alpha level was set at 0.01 in order to account for multiple comparisons. P values that meet these criteria are in bold.
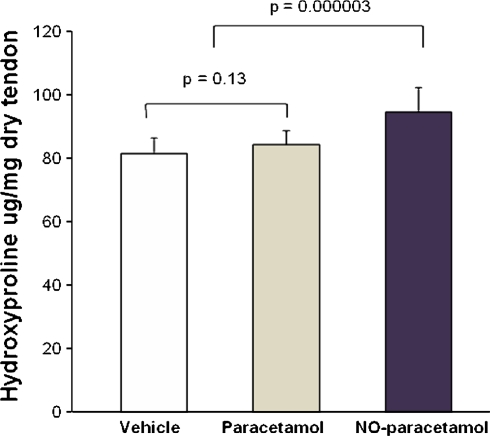
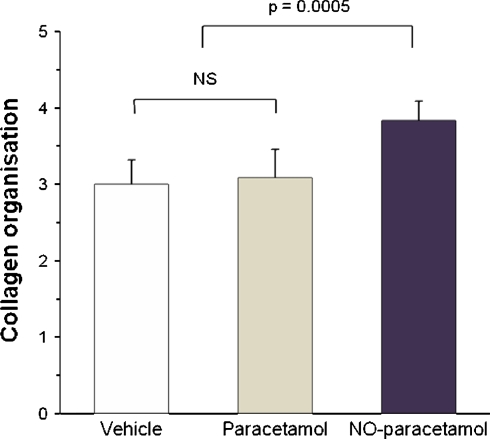
NO-paracetamol affected a number of secondary endpoints of tendon healing, even when adjusted for multiple comparisons (Tables 1, 2). Specifically, the day 10 healing tendons of the NO-paracetamol group had a smaller cross-sectional area (p = 0.0002), less wet weight (p = 0.005), greater dry weight (p = 0.004), less water content (p = 0.0001), a higher collagen content (p = 0.00003), better collagen organization (p = 0.005), and better values for ultimate stress (p = 0.007) than the healing tendons of the combined control group. Paracetamol had no effect on tendon healing (Table 1). As there were no differences between the two control groups (vehicle and paracetamol), the data from those two groups was combined and compared with the NO-paracetamol group.
Table 2.
Water content of healing tendon on day 10 postoperation
| Wet weight (mg) | Dry weight (mg) | Water content (%) | |
|---|---|---|---|
| Uninjured | |||
| Vehicle | |||
| Mean | 41 | 19 | 54% |
| SD | 5 | 2 | 4% |
| n | 11 | 11 | 11 |
| Injured | |||
| Vehicle | |||
| Mean | 50 | 10 | 80% |
| SD | 6 | 2 | 3% |
| n | 11 | 11 | 11 |
| Paracetamol | |||
| Mean | 50 | 10 | 79% |
| SD | 5 | 2 | 4% |
| n | 11 | 11 | 11 |
| Controls | |||
| Mean | 50 | 10 | 80% |
| SD | 5 | 2 | 3% |
| n | 22 | 22 | 22 |
| NO-paracetamol | |||
| Mean | 45 | 12 | 73% |
| SD | 4 | 2 | 5% |
| n | 12 | 12 | 12 |
| T-tests | |||
| Vehicle vs paracetamol | 0.93 | 0.94 | 0.80 |
| Controls vs NO-paracetamol | 0.005 | 0.004 | 0.0001 |
SD = standard deviation, n = sample size. Statistical analysis using unpaired two-tailed Student’s t-tests. The alpha level was set at 0.01 in order to account for multiple comparisons. P values that meet these criteria are in bold.
The organizational structure of healing rat was much better (approximately 30% improvement in scores) in the NO-paracetamol compared with the control groups with better (p < 0.005) aligned collagen bundles (Fig. 1).
Fig. 1.
Nitric oxide improved collagen content. Total collagen content of healing Achilles tendons at day 10 in rats treated with vehicle, paracetamol, or nitric oxide (NO)-paracetamol is shown. Values are mean ± standard deviation, n = 11, 11, and 12 for vehicle, paracetamol, and NO-paracetamol. Statistical analysis was performed using unpaired two-tailed Student’s t-tests.
Uninjured Achilles tendon constructs failed at the tendon-calcaneus interface. Uninjured tendons had better load to failure properties, less cross-sectional area, total energy and ultimate stress than day 10 healing tendon in the same animals (all p < 0.001) (Table 1, Figure 2).
Fig. 2.
Nitric oxide improved collagen organization of healing Achilles tendons at day 10 in rats. The graph shows semiquantification of healing Achilles tendon collagen organization observed under polarized microscope on sections stained with hematoxylin & eosin by a blinded observer. The organization was scored from 1 to 5 based on the orientation and network structure of collagen fibers, the formation of collagen fiber bundles, and the length and diameter of the bundles, where 5 was the most mature. Values are mean ± standard deviation, n = 6 for each group. Statistical analysis was performed using Kruskal-Wallis one way analysis of variance on ranks.
NO-paracetamol had no effect on any of the measured parameters in uninjured tendons: failure load, cross-sectional area, displacement, maximum energy, total energy, length, ultimate stress or strain (Table 1).
Discussion
Nitric oxide is an important messenger molecule in many physiological processes. While the addition of NO via NO-flurbiprofen enhances the material properties of healing tendon, however, flurbiprofen has a detrimental effect on healing. We therefore asked whether NO-paracetamol enhanced select aspects of healing Achilles tendon: primarily failure load, with cross-sectional area, water content, ultimate stress, strain collagen content and collagen organization as secondary outcomes measures.
We note several limitations. We used an adolescent rat model, and the animals received the drug via subcutaneous injection two days prior to tendon division. In humans, tendon ruptures usually occur in middle-aged or elderly individuals, and it is usually not possible to administer a drug prior to rupture. The healing potential is likely enhanced in adolescent rats compared to adults so the results might not be generalizable to the population of interest. We also only measured one time-point. The model, however, is well-established and is sensitive to changes in tendon healing. The study also allowed a comparison with paracetamol with vehicle, as well as with NO-paracetamol. It is possible the analgesic effect of paracetamol may change rat activity and tendon healing. However the results of the paracetamol group were the same as those of the vehicle alone group.
Tendons have a high proportion of collagen fibers, which are closely packed and parallel to the direction of force and have the highest tensile strength of all connective tissue. After tendon injury, the tendon healing process includes cell infiltration and migration, angiogenesis, fibroblast proliferation, collagen synthesis, and collagen fibril reorganization. Our data suggest the addition of NO through NO-paracetamol enhanced rat Achilles tendon healing by improving the amount of collagen in the healing tendon, promoting better collagen reorganization and improving the material properties (including ultimate stress and stiffness) of the healing constructs.
The mechanism by which NO-paracetamol increased collagen content of healing tendon is undetermined; however, studies performed by other authors suggest the exogenous addition of NO donors in low concentration stimulates collagen synthesis in cultured dermal fibroblasts and in dermal fibroblasts in healing wounds [19]. We reported addition of NO to cultured human rotator cuff tendon cells enhances collagen synthesis [21]. Reduced cell proliferation, impaired collagen synthesis, and decreased contractile properties of dermal fibroblast were also observed in inducible NO synthase (iNOS) gene knockout mice [15], whereas transfecting the iNOS gene into rat skin wounds increased collagen accumulation in the healing wounds [17].
We reported similar but less obvious improvements in tendon healing using NO-flurbiprofen in the same animal model [22]. In that experiment, flurbiprofen alone reduced failure load and cross-sectional area. Flurbiprofen also reduced the animals’ body weight gain compared with vehicle and NO-flurbiprofen. NO-flurbiprofen reduced cross-sectional area, had no effect on failure load, and improved ultimate stress compared with vehicle. In this experiment, paracetamol did not impair tendon healing.
We also demonstrated NO-paracetamol considerably reduced the cross-sectional area of healing tendon compared with paracetamol and vehicle. However, the failure load in the NO-paracetamol group was higher than that in paracetamol and vehicle groups. NO-paracetamol increased dried weight and decreased the water content of the healing tendon. It is possible the improvements in collagen content and organization, reduction in cross-sectional area, and ultimate stress in the NO-paracetamol group reflect an improvement in the rate of Achilles tendon healing. The design of these experiments does not allow us to determine if the improved healing is sustained. However, we do have data in humans [12] suggesting patients with Achilles tendonitis treated with a transdermal preparation of NO have improvement of symptoms over the placebo group and the improvement persists for up to 3 years after cessation of treatment. The beneficial effects of systemic NO on rat Achilles tendon healing is also consistent with human randomized clinical trials for tendinopathy of supraspinatus, lateral epicondylitis and Achilles tendonitis, where subjects receiving NO via glyceryltrinitrate patches had improved pain and function compared with subjects receiving placebo patches [11, 13, 14].
While daily injections of NO-paracetamol did not affect failure load of day 10 healing tendons, it did reduce tendon cross-sectional area, water content, improve collagen content and organization and ultimate stress, so that the healing tendons of rats receiving NO-paracetamol were closer to uninjured tendon.
Footnotes
One or more of the authors (GM,GT, MW) have received funding from a grant from the NiCox Corporation and by St George Hospital/South Eastern Sydney Area Health Service.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Bult H, Boeckxstaens GE, Pelkmans PA, Jordaens FH, Van Maercke YM, Herman AG. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990;345:346–347. [DOI] [PubMed]
- 2.Burgaud JL, Riffaud JP, Del Soldato P. Nitric-oxide releasing molecules: a new class of drugs with several major indications. Curr Pharm Des. 2002;8:201–213. [DOI] [PubMed]
- 3.Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci USA. 2002;99:13926–13931. [DOI] [PMC free article] [PubMed]
- 4.Dusting GJ. Nitric oxide in cardiovascular disorders. J Vasc Res. 1995;32:143–161. [DOI] [PubMed]
- 5.Kirkeboen KA, Andreassen AK, Kvernmo HD, Strand OA. Nitric oxide–basal biochemistry and physiological aspects [in Norwegian]. Tidsskr Nor Laegeforen. 1999;119:4056–4060. [PubMed]
- 6.Li H, Forstermann U. Nitric oxide in the pathogenesis of vascular disease. J Pathol. 2000;190:244–254. [DOI] [PubMed]
- 7.Lin J-H, Wang M-X, Wei A, Zhu W, Diwan AD, Murrell GAC. Temporal expression of nitric oxide synthase isoforms in healing Achilles tendon. J Orthop Res. 2001;19:136–142. [DOI] [PubMed]
- 8.Lin J-H, Wang M-X, Wei A, Zhu W, Murrell GAC. The cell specific temporal expression of nitric oxide synthase isoforms during Achilles tendon healing. Inflamm Res. 2001;50:1–8. [DOI] [PubMed]
- 9.Murrell GAC, Lilly EG, Collins A, Seaber AV, Goldner RD, Best TM. Achilles tendon injuries: a comparison of surgical repair versus no repair in a rat model. Foot Ankle. 1993;14:400–406. [DOI] [PubMed]
- 10.Murrell GAC, Szabo C, Hannafin JA, Jang D, Dolan MM, Deng X, Murrell DF, Warren RF. Modulation of tendon healing by nitric oxide. Inflamm Res. 1997;46:19–27. [DOI] [PubMed]
- 11.Paoloni J, Appleyard R, Murrell GAC. A randomized double-blind placebo controlled clinical trial investigating the use of topical nitric oxide application in the treatment of Achilles tendonitis. J Bone and Joint Surg Am. 2004;86:916–922. [DOI] [PubMed]
- 12.Paoloni J, Murrell GAC. Three-year followup study of topical glyceryl trinitrate treatment of chronic noninsertional Achilles tendinopathy. Foot Ankle Int. 2007;28:1064–1068. [DOI] [PubMed]
- 13.Paoloni JA, Appleyard RC, Nelson J, Murrell GAC. Topical nitric oxide application in the treatment of chronic extensor tendinosis at the elbow: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2003;31:915–920. [DOI] [PubMed]
- 14.Paoloni JA, Appleyard RC, Nelson J, Murrell GAC. Topical glyceryl trinitrate application in the treatment of chronic supraspinatus tendinopathy: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med. 2005;33:806–813. [DOI] [PubMed]
- 15.Shi HP, Efron DT, Most D, Barbul A. The role of iNOS in wound healing. Surgery. 2001;130:225–229. [DOI] [PubMed]
- 16.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–273. [DOI] [PubMed]
- 17.Thornton FJ, Schaffer MR, Witte MB, Moldawer LL, MacKay SLD, Abouhamze A, Tannahill CL, Barbul A. Enhanced collagen accumulation following direct transfection of the inducible nitric oxide synthase gene in cutaneous wounds. Biochem Biophys Res Comms. 1998;246:654–659. [DOI] [PubMed]
- 18.Whittle NJR. Nitric oxide in physiology and pathology. Histochem J. 1995;27:727–737. [PubMed]
- 19.Witte MB, Kiyama T, Barbul A. Nitric oxide enhances experimental wound healing in diabetes. Br J Surg. 2002;89:1594–1601. [DOI] [PubMed]
- 20.Woessner JF Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. [DOI] [PubMed]
- 21.Xia W, Szomor Z, Wang Y, Murrell GAC. Nitric oxide enhances collagen synthesis in cultured human tendon cells. J Orthop Res. 2006;24:159–172. [DOI] [PubMed]
- 22.Yuan J, Murrell GAC, Wei AQ, Appleyard RC, Del Soldato P, Wang M-X. Addition of nitric oxide via nitroflurbiprofen enhances the material properties of healing Achilles tendon. Inflamm Res. 2003;52:230–237. [DOI] [PubMed]