Abstract
The reported results of acetabular cage reconstruction for pelvic deficiency are widely variable. Our primary question was: what is the survivorship of cage reconstruction with a primary end point of cage revision and secondary end points of radiographic loosening and any reoperation? Secondary questions were: which factors predict cage failure, and what is the functional outcome (SF-36, WOMAC, Harris hip score) of this reconstructive method? We reviewed 72 cage reconstructions in 68 patients. Minimum followup was 1.2 years (mean, 5.1 years; range, 1.2–10.7 years). Five-year cage revision-free survivorship was 87.8%. Five-year loosening-free and acetabular reoperation-free survivorships were 80.7% and 81.3%, respectively. No single preoperative factor (age, gender, severity of pelvic defect, degree of heterotopic ossification, difference in limb lengths and centers of rotation) or intraoperative factor (type of bone graft, type of cage, changes in limb length and center of rotation) predicted cage failure. Functional outcomes were 28.9 (SF-36 Physical Component), 52.4 (SF-36 Mental Component), 33.7 (WOMAC), and 44.2 (Harris). We judged these outcomes acceptable for this sometimes challenging problem. Future techniques for treating pelvic deficiency will need to be compared with these and other outcomes in the literature.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Severe pelvic deficiency presents a difficult problem in hip arthroplasty. Specifically, the goals of restoring or preserving pelvic bone stock, placing the acetabular component in the correct anatomic position, optimizing joint stability, equalizing leg lengths, and achieving stable fixation are not as readily achieved in such a situation [16]. Different ways of treating this problem have been proposed, including acceptance of a high hip center [28, 30], cementing a cup onto structural bone graft [22], or using a bilobed cup [8], a custom triflanged cup [9, 19], a jumbo cup [11, 40], a trabecular metal cup with augments [20], or a reconstructive cage [5, 24]. Despite the number of proposed solutions, none appear clearly superior over others, and pelvic deficiencies continue to be a perplexing problem.
Cage reconstruction is attractive in that it acts as a plate to bridge the bony defect, protecting underlying bone graft as it incorporates [17]. This may then eventually restore pelvic bone stock allowing later revision, if necessary, to be achieved with even a regular cementless hemispherical cup [15]. One of the disadvantages is a nonporous back surface that does not promote bony ingrowth and making it susceptible to fixation failure with time [3].
Prior reports on the reconstructive cages have shown wide variability of results with cage survival ranging from 69% to 100% at varying times [5, 6, 14, 21, 23, 25, 27, 34, 37, 38, 41], complicating comparison with existing and future methods of reconstruction. This is partly attributable to nonactuarial methods and unclear definitions of end points (cage removal, any acetabular reoperation, or radiographic loosening) in estimating cage survivorship. Furthermore, only one study attempted to look at factors that may be related to cage failure [6]. However, in this study, the authors primarily reported the results of allograft bone for acetabular revision and thus included reconstructions with cage and noncage devices. Similar mixing of cage reconstruction with other methods occurred in other studies [4, 13, 35], thus precluding easy comparison of outcomes.
We therefore first asked: what is the medium-term survivorship of acetabular cage reconstruction using the primary end point of cage revision and secondary end points of radiographic loosening and any acetabular reoperation? Second we asked which preoperative or intraoperative factors, if any, predict eventual cage failure? Finally, we asked: what is the functional outcome of patients who have undergone this reconstructive procedure as measured by the SF-36, WOMAC index, and Harris hip score (HHS)?
Materials and Methods
We identified consecutive patients with acetabular cage reconstruction from our institution’s surgical database. These were then retrospectively reviewed to determine any subsequent reoperations with or without removal of the cage. Followup radiographs likewise were obtained to look for any radiographic evidence of loosening or failure. Finally, subjective outcome measurements were obtained. Institutional Review Board approval was obtained before initiation of the study.
We reviewed 68 patients (72 reconstructions in 71 hips) undergoing cage reconstructions between November 1996 and July 2006. Inclusion criteria were pelvic deficiency and implantation of an acetabular cage device with or without bone grafting. Our definition of a cage device was one that provided circumferential support with superior and inferior fixation, either a flange or a hook. This distinguished a cage from other fixation devices such as roof rings, which were excluded. Three patients had bilateral cage reconstructions and one patient had another cage placed after her first cage failed; these then were treated as separate index surgeries. There were 27 males and 41 females. The average age at the time of cage surgery was 60.8 years (range, 12–89 years). Minimum followup was 1.2 years (mean, 5.1 years; range, 1.2–10.7 years). Among the original cohort of 68 patients, 11 died. All but one of the 57 surviving patients was contacted by telephone and was asked to provide information regarding further surgical procedures and to fill out a validated outcomes questionnaire (see below). Questionnaires were sent to patients by mail and were returned either when the patients came in for their followup appointment or by mail in stamped, self-addressed envelopes. At the initiation of this review, only 23 of the 57 surviving patients had recent radiographs within the past year. The 34 other patients were requested to come in for clinical and radiographic followups. Twenty-three patients either came in as requested or, because of logistic constraints, went to see their local physician instead and had their new radiographs forwarded to us. Therefore, 46 of the 57 (81%) surviving patients had final radiographs available for review.
All surgeries were performed by one of three adult reconstructive staff surgeons. There were 62 revisions and 10 primary reconstructions. Of the former, the major indications for revision were aseptic loosening (38.7%) and protrusio (21.0%) (Table 1). The most common underlying diagnoses in these patients were degenerative joint disease (37.1%), osteonecrosis of the femoral head (17.7%), and developmental hip dysplasia (11.3%) (Table 2). Eight of the 10 primary reconstructions were in conjunction with tumor excision, either metastatic (40%) or primary (40%). The other two patients had radiation-induced pelvic osteonecrosis and severe osteoarthritis (Table 3). In all cases, whether revision or primary, the main indication for cage reconstruction was a pelvic deficiency that was deemed by the surgeon to be best reconstructed with a cage rather than a regular cup or other reconstructive device.
Table 1.
Indications for revision in hip reconstructions revised with an acetabular cage (n = 62)
| Indication for revision | Number of hips |
|---|---|
| Aseptic loosening | 24 (38.7%) |
| Protrusio acetabuli | 13 (21.0%) |
| Liner dislodgement | 7 (11.3%) |
| Periprosthetic fracture | 6 (9.7%) |
| Osteolysis | 5 (8.1%) |
| Infection | 3 (4.8%) |
| Instability | 3 (4.8%) |
| Wear | 1 (1.6%) |
Table 2.
Diagnoses in patients with hip reconstructions revised with an acetabular cage (n = 62)
| Diagnosis | Number of hips |
|---|---|
| DJD | 23 (37.1%) |
| ONFH | 11 (17.7%) |
| SLE | 5 |
| MS | 2 |
| Posttrauma | 2 |
| Renal transplant | 2 |
| DDH | 7 (11.3%) |
| RA | 6 (9.7%) |
| Trauma | 4 (6.5%) |
| Metastasis | 3 (4.8%) |
| Ovarian CA | 1 |
| Renal cell CA | 1 |
| SCCA | 1 |
| SCFE | 2 (3.2%) |
| Primary tumor | 2 (3.2%) |
| Synovial sarcoma | 1 |
| Chondrosarcoma | 1 |
| Paget’s disease | 1 (1.6%) |
| CP spastic hip | 1 (1.6%) |
| Psoriasis | 1 (1.6%) |
| Pelvic ON postradiation | 1 (1.6%) |
DJD = degenerative joint disease; ONFH = osteonecrosis of the femoral head; SLE = systemic lupus erythematosus; MS = multiple sclerosis; DDH = developmental dysplasia of the hip; RA = rheumatoid arthritis; CA = carcinoma; SCCA = squamous cell carcinoma; SCFE = slipped capital femoral epiphysis; CP = cerebral palsy; ON = osteonecrosis.
Table 3.
Diagnoses in primary hip cases reconstructed with an acetabular cage (n = 10)
| Diagnosis | Number of hips |
|---|---|
| Metastasis | 4 (40%) |
| Renal cell CA | 2 |
| Breast CA | 2 |
| Primary tumor | 4 (40%) |
| Osteosarcoma | 2 |
| Ewing’s sarcoma | 1 |
| Multiple exostoses | 1 |
| Pelvic ON postradiation | 1 (10%) |
| Degenerative joint disease | 1 (10%) |
CA = carcinoma; ON = osteonecrosis; DJD = degenerative joint disease.
The different types of cages used were the Gap® (Stryker-Osteonics, Allendale, NJ; n = 4), Gap II® (Stryker-Osteonics; n = 45), Burch-Schneider (Protek, Bern, Switzerland; n = 2), Contour™ (Smith and Nephew Richards, Memphis, TN; n = 11), and Protrusio Cage™ (DePuy Orthopaedics, Warsaw, IN; n = 10). All cages were made of titanium alloy and designed to transfer load from the acetabulum and allow fixation to periacetabular bone. Type of inferior fixation was recorded. Forty-eight of the cages were inferiorly fixed by a hook that was crimped around the inferomedial wall or teardrop; 17 had screws going through an inferior flange; five had intraosseous placement of the flange; and two did not use the inferior flange for fixation. The total number of screws used for cage fixation ranged from two to 14. In each case, the number of screws used was determined by the surgeon’s assessment of whether optimal fixation had already been achieved. The bearing surface consisted of polyethylene cups, either constrained or nonconstrained, cemented into the cage. In 23 patients, structural bone graft (femoral head, distal femur, or proximal tibia) was used with or without supplemental morselized allograft. In 29 patients, morselized allograft bone alone was used. In the remaining 20 patients, only local autograft or no graft was used (Table 4). In addition, 17 of the patients (15 with morselized and two with structural grafts) had supplementation with demineralized bone matrix (Table 4). The average duration of surgery was 259 ± 121 minutes (range, 101–703 minutes). Mean blood loss was 1652 ± 1954 mL (range, 100–12,000 mL). The median hospital stay was 5 days (range, 3–31 days).
Table 4.
Types of bone graft used for cage reconstruction (n = 72)
| With or without DBM | Structural graft | Morselized graft | No graft | Total |
|---|---|---|---|---|
| Without DBM | 21 (29.2%) | 14 (19.4%) | 20 (27.8%) | 55 (76.4%) |
| With DBM | 2 (2.8%) | 15 (20.8%) | 0 | 17 (23.6%) |
| Total | 23 (31.9%) | 29 (40.3%) | 20 (27.8%) | 72 (100%) |
DBM = demineralized bone matrix.
One of the authors (JNS), who was not involved in any of the index surgeries, analyzed preoperative radiographs (pelvis anteroposterior and Judet views) and CT scans, when available, to classify the pelvic defect according to three available classification systems. Using the D’Antonio/AAOS system [10], there were seven segmental or noncontained defects, 12 cavitary or contained, 29 combined, and 19 pelvic discontinuities. In the Paprosky system [22], there were 16 Type II defects (five IIA, eight IIB, and three IIC) and 51 Type III (28 IIIA and 23 IIIB) defects. In the Toronto system [29], there were six Type II, 26 Type III, 16 Type IV, and 19 Type V defects. Five patients did not have preoperative radiographs available for review. Among the 62 revision surgeries, 39 already had Brooker Grade II or higher preexisting heterotopic ossification [7]. Using the method of Ranawat et al. [26], the horizontal and vertical distances between the anatomic hip center and the prosthetic femoral head center were measured. Mean preoperative vertical and horizontal displacement values of the center of rotation were 23.9 mm superior and 4.6 mm lateral, respectively. Mean vertical change in center of rotation before and after surgery was 15.7 mm and horizontal change was 2.5 mm.
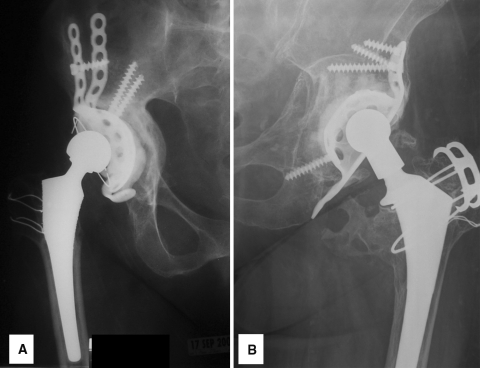
We obtained final radiographs within the past year on 46 of the 57 surviving patients. These were evaluated by one of the authors (JNS) for evidence of loosening using a modification of the criteria defined by Gill et al. [12]: screw or cage fracture, progressive radiolucencies, or cage migration (Fig. 1A). Conversely, a well-fixed cage was defined as one with the cage and screws intact, no progressive radiolucencies, and no migration when compared with previous radiographs (Fig. 1B). Vertical and horizontal migration values of the center of rotation between the immediate postoperative and final followup radiographs were measured. These were divided by the interval in years to come up with vertical and horizontal migration per-year values, the average values of which were 1.4 mm per year and 1.6 mm per year, respectively.
Fig. 1A–B.
Representative final hip radiographs are shown. (A) A loose acetabular cage is evidenced by loss of inferior fixation and resultant migration and radiolucency. (B) A well-fixed acetabular cage has an intact cage and screws and no radiolucency or migration.
Forty-eight of the 57 surviving patients completed the questionnaire at the time of final followup; this consisted of the SF-36 [33, 39], the WOMAC [1, 2], and the HHS [18].
We made Kaplan-Meier survivorship estimates using three hard end points: (1) cage revision-free survivorship, (2) radiographic loosening-free survivorship, and (3) acetabular reoperation-free survivorship. Log rank and Wilcoxon univariate tests and Cox regression multivariate analysis were used to look for correlation of different factors with cage failure, defined as either cage removal or radiographic loosening Factors analyzed were age, gender, degree of pelvic deficiency, preoperative and postoperative differences in limb lengths and centers of rotation, type of cage used, method of inferior cage fixation, total number of screws used, whether bone graft was used, type of bone graft, and presence of heterotopic ossification. Analysis was performed using SAS® Software for Windows Version 9.1.3 (SAS Institute, Cary, NC).
Results
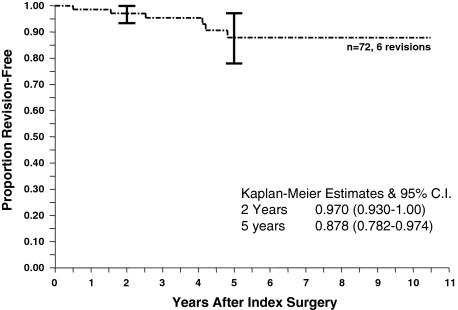
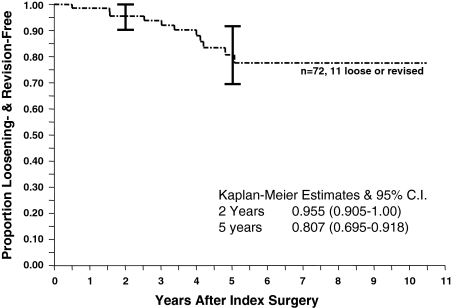
The 5-year revision-free survivorship of acetabular cages was 87.8% ± 9.6% (95% confidence interval [CI]) (Fig. 2). Of the 72 cages implanted, six had been removed at an average 2.9 years after implantation, three for aseptic loosening and three for deep infection. Of the patients without sepsis, one had revision surgery with yet another cage, one had revision surgery to a standard ingrowth cup, and one had a resection arthroplasty. The three patients with infection had insertion of an antibiotic spacer. Two patients have pending second-stage reimplantation, whereas one had reimplantation performed at another institution using a custom-made device. Using the end point of radiographic loosening, survivorship at 5 years was 80.7% ± 11.1% (95% CI) (Fig. 3). Five of the 66 (71.6%) unrevised cages were loose on the final or most recent radiographs. Using an end point of acetabular reoperation for any reason, the 5-year survivorship was 81.3% ± 10.9% (95% CI) (Fig. 4). There were five reoperations (in four hips) solely for acetabular liner exchange; four of these were liner-cage dissociation and one was a recurrent dislocation secondary to a malpositioned liner.
Fig. 2.
The Kaplan-Meier survivorship curve of cage reconstruction with an end point of cage removal shows acceptable 5-year survivorship of 87.8%. CI = confidence interval.
Fig. 3.
The Kaplan-Meier survivorship curve of cage reconstruction with an end point of radiographic loosening or revision shows stepwise progression of loosening up to 6 years and a 5-year survivorship of 80.7%. CI = confidence interval.
Fig. 4.
The Kaplan-Meier survivorship curve of cage reconstruction with an end point of any acetabular reoperation shows a 5-year survivorship of 81.3%. In addition to cage revisions, other reoperations were polyethylene liner revisions for dislodgement or cup malpositioning. CI = confidence interval.
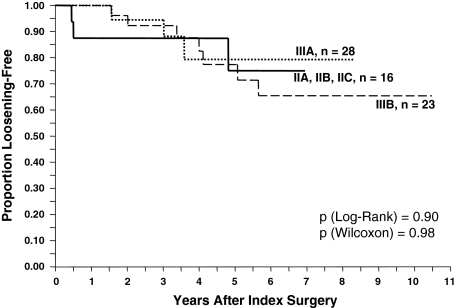
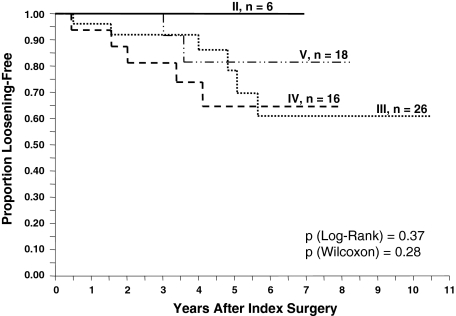
No single preoperative or intraoperative factor correlated with failure (Tables 5, 6). None of the three classification systems (D’Antonio [p = 0.58]; Paprosky [p = 0.78]; Toronto [p = 0.54]) correlated with eventual cage failure (Figs. 5, 6). Even when the classes were grouped together (D’Antonio I/II versus D’Antonio III/IV; Toronto II/III versus Toronto IV/V), we observed no correlation (p = 0.20 and 0.54, respectively) (Table 5). Likewise, none of the different types of bone graft correlated with cage failure (Table 6).
Table 5.
Influence of factors with numeric variables using proportional hazards regression analysis
| Factor | p Value |
|---|---|
| Age | 0.28 |
| Male gender | 0.74 |
| D’Antonio classification [10] | 0.92 |
| D’Antonio I/II versus III/IV | 0.32 |
| Paprosky classification [22] | 0.95 |
| Toronto classification [29] | 0.74 |
| Toronto II/III versus IV/V | 0.60 |
| Total number of screws | 0.66 |
| Postoperative COR-V | 0.67 |
| Postoperative COR-H | 0.14 |
| LLD | 0.55 |
| HO (Brooker 0/I versus II–IV) | 0.37 |
COR-V = vertical distance from anatomic to prosthetic center of rotation; COR-H = horizontal distance from anatomic to prosthetic center of rotation; LLD = leg length discrepancy; HO = heterotopic ossification.
Table 6.
Influence of factors with categorical variables using log rank and Wilcoxon tests
| Factor | Log rank p value | Wilcoxon p value |
|---|---|---|
| Type of inferior fixation (hook versus screw versus intraosseous flange) | 0.68 | 0.60 |
| Cage type (GAP I versus GAP II versus Protrusio Cage versus Contour) | 0.58 | 0.60 |
| DBM versus no DBM | 0.96 | 0.96 |
| Morselized versus structural graft | 0.80 | 0.70 |
DBM = demineralized bone matrix.
Fig. 5.
Kaplan-Meier survivorship curves by type of pelvic deficiency according to the Paprosky classification [22] failed to show a correlation between worsening pelvic deficiency and likelihood of cage failure defined as either cage removal or radiographic loosening.
Fig. 6.
Kaplan-Meier survivorship curves by type of pelvic deficiency according to the Toronto classification [29] failed to show a correlation between worsening pelvic deficiency and likelihood of cage failure defined as either cage removal or radiographic loosening.
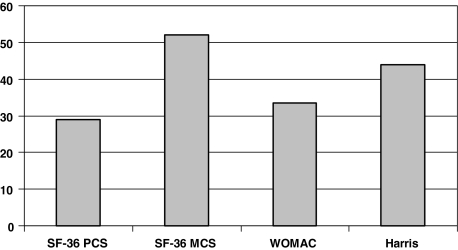
At final followup, the mean SF-36 physical component score for the 47 patients who completed the questionnaire was 28.9 ± 8.5. The mean SF-36 mental component score was 52.4 ± 11.5. Mean WOMAC index was 33.7 ± 19.8 and mean HHS was 44.2 ± 20.4 (Fig. 7).
Fig. 7.
Mean SF-36 physical component score (PCS), SF-36 mental component score (MCS), the WOMAC osteoarthritis index, and Harris hip score for all patients with unrevised acetabular cages still show considerable impairment. This may be partly attributable to effects of medical comorbidities and other systemic illnesses. In the WOMAC, a lower score suggests better function.
Discussion
The reported outcomes of acetabular cage reconstruction have been conflicting. Few reports have used actuarial methods in estimating survivorship, thus making comparison of results difficult. Our first question therefore was: what is the survivorship of acetabular cage reconstruction, with the primary end point of cage revision and secondary end points of radiographic loosening and any acetabular reoperation? Secondary questions were: which preoperative or intraoperative factors, if any, predict eventual cage failure, and what is the functional outcome, as measured by the SF-36, WOMAC index, and HHS, of patients who have undergone cage reconstruction?
This study has several limitations. It was a retrospective analysis of a single cohort with no comparison group. The relatively small number of subjects makes it prone to Type II (beta) error (ie, failing to recognize a true difference or correlation). However, our study is one of the largest series on this method of reconstruction and all operations were performed at one institution. Severe pelvic deficiencies in hip arthroplasty are uncommon, and compiling a large series is challenging. Furthermore, each patient tends to be unique in terms of the anatomy of the deficiency, types of devices previously implanted, and local and systemic patient factors, thus making it difficult to randomize patients to different treatment arms. Not surprisingly, all previous reports have been Level IV studies and it is unlikely a Level I/II study is feasible. Still, our study addresses some of the weaknesses of prior studies. The methodologic flaws inherent in these were corrected by using actuarial methods to determine implant survivorship and Cox proportional hazards regression to analyze the relationship of different factors with cage failure. In addition, there were strict inclusion criteria and a uniform type of reconstruction.
The results of cage reconstruction have been highly variable, ranging from 69% to 100% [5, 6, 14, 21, 23, 25, 27, 34, 37, 41]. Unfortunately, few of these studies used survivorship analysis that would make it possible to compare results at specific times. Moreover, there is huge variability in the selection criteria for cage reconstruction in terms of the underlying pelvic deficiency. For example, in the study of Paprosky et al. in 2006 [21], all 16 of their patients had pelvic discontinuities, the most severe form of deficiency [4]. However, in the report of Rosson and Schatzker [27], only one of 20 patients had a pelvic deficiency. Not surprisingly, the former study had a much higher revision rate (five of 16 cages revised within 4.5 years’ average followup) than the latter (no revision at 5 years’ average followup).
Our result of 87.8% cage revision-free survivorship at 5 years (Fig. 2) falls well within the range of previous reports and helps narrow the estimate of cage results from the wide spectrum previously reported. In addition, we have shown radiographic loosening-free survivorship of 80.7% at 5 years (Fig. 3) and acetabular reoperation-free survivorship of 81.3% at 5 years (Fig. 4). Considering the magnitude of the problem these patients initially presented with and the lack of clearly superior alternatives, we judge these results acceptable. The success of cage reconstruction may be the result of the immediate stability and mechanical load protection of the bone graft, optimizing the conditions for successful incorporation. Once this occurs, there is less stress on the cage, minimizing the likelihood of fatigue fracture and offsetting its lack of biologic fixation. We strongly believe all efforts should be made to maximize the initial stability of the cage construct to provide support to enhance graft incorporation.
A comparison of acetabular cage reconstruction to other techniques for addressing pelvic bone deficiencies reveals no other techniques are clearly superior. Currently, there is enthusiasm for using trabecular metal (TM) cups in severe pelvic deficiencies. The appeal of this method is that it promotes biologic fixation to host bone. The main disadvantage is fitting the TM implant to the bone defect. Either modular augments must be cemented together in a piecemeal fashion or host bone must be cut away to allow placement of a larger TM cup. This compromises future reconstructions that likely will be necessary. Unfortunately, the value of TM cups as compared with cage reconstructions cannot be determined at present because the results of the former at 5 years or longer duration is still unknown [20, 31, 32, 36].
Determining prognostic factors related to cage outcome might provide insight into better patient selection or improved techniques. Only one study other than ours looked at the influence of different factors on cage failure. Bohm and Banzhaf [6] identified three factors that correlated with failure: lack of radiographic incorporation of allograft, graft resorption, and use of particulate (morselized) as opposed to bulk (structural) allograft. However, the first two factors may only become radiographically evident when failure is imminent or has occurred. Therefore, only the third factor may be of use in optimizing results of cage reconstructions by using structural bone graft as opposed to morselized grafts alone. Intuitively, it is logical that in severe noncontained defects, structural support is necessary to ease the load off the cage as the bone graft incorporates, whereas in less severe and contained deficiencies, which may not even require a cage, morselized graft is adequate. However, our analysis did not reproduce their results in that the type of bone graft (structural versus morselized) did not predict cage failure. In fact, in our analysis of different factors, we were unable to identify a single preoperative or intraoperative factor that correlated with failure. We acknowledge this may reflect a beta error secondary to the low total number of failures (six cages removed plus five loose on radiographs). Future reevaluation with additional patients, a longer followup, and meta-analysis with other series may help identify these factors.
Surprisingly, worsening severity of pelvic deficiency as reflected in any of the three available classification systems failed to predict eventual cage failure (Figs. 5, 6).
Specifically looking at the 19 patients with pelvic discontinuities (Toronto Type V), none have undergone subsequent cage removal, and among the 14 surviving patients, two had a loose cage on radiographs. A possible explanation might be that cage reconstruction can treat different types of pelvic deficiencies equally well such that failure rate is not affected by the severity of deficiency.
Our functional outcome score at final followup as reflected in the HHS (mean, 44.2) is noticeably lower when compared with scores from previous studies that used the same measurement tool, with their scores ranging from 66 to 82.8 [27, 35, 37, 41]. There may be two reasons for this. One is many of our patients who live far away only sent back their questionnaires and radiographs by mail and thus did not have the clinical examination component included in their HHS. Another reason might be related to systemic comorbidities, a reflection of our general patient population and referral base. Our study population included a disproportionately high number of individuals with primary and metastatic malignancies, and other systemic illnesses.
Acetabular cage reconstruction for pelvic deficiency yielded actuarial 5-year survivorship of 87.8% ± 9.6% (cage removal), 80.7% ± 11.1% (radiographic loosening), and 81.3% ± 10.9% (any acetabular reoperation). In light of the complexity of the problem of pelvic deficiency, we believe these outcomes are acceptable. Given our statistical methods, we believe these also can serve as appropriate historical controls for comparison against future results of other reconstructive methods.
Acknowledgments
We thank Paul Lender for help on the construction of the database and statistical analysis and Nancy Borgstrom, Barbara Lace-Langdon, and Claire Lewandowski for help in contacting the patients in the study.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed]
- 2.Bellamy N, Goldsmith CH, Buchanan WW, Campbell J, Duku E. Prior score availability: observations using the WOMAC osteoarthritis index. Br J Rheumatol. 1991;30:150–151. [DOI] [PubMed]
- 3.Berry DJ. Antiprotrusio cages for acetabular revision. Clin Orthop Relat Res. 2004;420:106–112. [DOI] [PubMed]
- 4.Berry DJ, Lewallen DG, Hanssen AD, Cabanela ME. Pelvic discontinuity in revision total hip arthroplasty. J Bone Joint Surg Am. 1999;81:1692–1702. [DOI] [PubMed]
- 5.Berry DJ, Müller ME. Revision arthroplasty using an anti-protrusio cage for massive acetabular bone deficiency. J Bone Joint Surg Br. 1992;74:711–715. [DOI] [PubMed]
- 6.Böhm P, Banzhaf S. Acetabular revision with allograft bone: 103 revisions with 3 reconstruction alternatives, followed for 0.3–13 years. Acta Orthop Scand. 1999;70:240–249. [DOI] [PubMed]
- 7.Brooker AF, Bowerman JW, Robinson RA, Riley LH Jr. Ectopic ossification following total hip replacement: incidence and a method of classification. J Bone Joint Surg Am. 1973;55:1629–1632. [PubMed]
- 8.Chen WM, Engh CA Jr, Hopper RH Jr, McAuley JP, Engh CA. Acetabular revision with use of a bilobed component inserted without cement in patients who have acetabular bone-stock deficiency. J Bone Joint Surg Am. 2000;82:197–206. [DOI] [PubMed]
- 9.Christie MJ, Barrington SA, Brinson MF, Ruhling ME, DeBoer DK. Bridging massive acetabular defects with the triflange cup: 2- to 9-year results. Clin Orthop Relat Res. 2001;393:216–227. [DOI] [PubMed]
- 10.D’Antonio JA, Capello WN, Borden LS, Bargar WL, Bierbaum BF, Boettcher WG, Steinberg ME, Stulberg SD, Wedge JH. Classification and management of acetabular abnormalities in total hip arthroplasty. Clin Orthop Relat Res. 1989;243:126–137. [PubMed]
- 11.Dearborn JT, Harris WH. Acetabular revision arthroplasty using so-called jumbo cementless components: an average 7-year follow-up study. J Arthroplasty. 2000;15:8–15. [DOI] [PubMed]
- 12.Gill TJ, Sledge JB, Muller ME. Total hip arthroplasty with use of an acetabular reinforcement ring in patients who have congenital dysplasia of the hip: results at five to fifteen years. J Bone Joint Surg Am. 1998;80:969–979. [DOI] [PubMed]
- 13.Gill TJ, Sledge JB, Muller ME. The management of severe acetabular bone loss using structural allograft and acetabular reinforcement devices. J Arthroplasty. 2000;15:1–7. [DOI] [PubMed]
- 14.Goodman S, Saastamoinen H, Shasha N, Gross A. Complications of ilioischial reconstruction rings in revision total hip arthroplasty. J Arthroplasty. 2004;19:436–446. [DOI] [PubMed]
- 15.Gross AE. Restoration of acetabular bone loss 2005. J Arthroplasty. 2006;21(suppl 1):117–120. [DOI] [PubMed]
- 16.Gross AE, Goodman S. The current role of structural grafts and cages in revision arthroplasty of the hip. Clin Orthop Relat Res. 2004;429:193–200. [DOI] [PubMed]
- 17.Gross AE, Wong P, Saleh KJ. Grafts and cages: managing massive bone loss. Orthopedics. 2000;23:973–974. [DOI] [PubMed]
- 18.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed]
- 19.Joshi AB, Lee J, Christensen C. Results for a custom acetabular component for acetabular deficiency. J Arthroplasty. 2002;17:643–648. [DOI] [PubMed]
- 20.Nehme A, Lewallen DG, Hanssen AD. Modular porous metal augments for treatment of severe acetabular bone loss during revision hip arthroplasty. Clin Orthop Relat Res. 2004;429:201–208. [DOI] [PubMed]
- 21.Paprosky W, Sporer S, O’Rourke MR. The treatment of pelvic discontinuity with acetabular cages. Clin Orthop Relat Res. 2006;453:183–187. [DOI] [PubMed]
- 22.Paprosky WG, Magnus RE. Principles of bone grafting in revision total hip arthroplasty: acetabular technique. Clin Orthop Relat Res. 1994;298:147–155. [PubMed]
- 23.Perka C, Ludwig R. Reconstruction of segmental defects during revision procedures of the acetabulum with the Burch-Schneider anti-protrusio cage. J Arthroplasty. 2001;16:568–574. [DOI] [PubMed]
- 24.Peters CL, Curtain M, Samuelson KM. Acetabular revision with the Burch-Schneider antiprotrusio cage and cancellous allograft bone. J Arthroplasty. 1995;10:307–312. [DOI] [PubMed]
- 25.Pieringer H, Auersperg V, Bohler N. Reconstruction of severe acetabular bone-deficiency: the Burch-Schneider antiprotrusio cage in primary and revision total hip arthroplasty. J Arthroplasty. 2006;21:489–496. [DOI] [PubMed]
- 26.Ranawat CS, Dorr LD, Inglis AE. Total hip arthroplasty in protrusio acetabuli of rheumatoid arthritis. J Bone Joint Surg Am. 1980;62:1059–1065. [PubMed]
- 27.Rosson J, Schatzker J. The use of reinforcement rings to reconstruct deficient acetabula. J Bone Joint Surg Br. 1992;74:716–720. [DOI] [PubMed]
- 28.Russotti GM, Harris WH. Proximal placement of the acetabular component in total hip arthroplasty: a long-term follow-up study. J Bone Joint Surg Am. 1991;73:587–592. [PubMed]
- 29.Saleh KJ, Holtzman J, Gafni A, Saleh L, Davis A, Resig S, Gross AE. Reliability and intraoperative validity of preoperative assessment of standardized plain radiographs in predicting bone loss at revision hip surgery. J Bone Joint Surg Am. 2001;83:1040–1046. [DOI] [PubMed]
- 30.Schutzer SF, Harris WH. High placement of porous-coated acetabular components in complex total hip arthroplasty. J Arthroplasty. 1994;9:359–367. [DOI] [PubMed]
- 31.Sporer SM, Paprosky WG. The use of a trabecular metal acetabular component and trabecular metal augment for severe acetabular defects. J Arthroplasty. 2006;21(suppl 2):83–86. [DOI] [PubMed]
- 32.Sporer SM, Paprosky WG. Acetabular revision using a trabecular metal acetabular component for severe acetabular bone loss associated with a pelvic discontinuity. J Arthroplasty. 2006;21(suppl 2):87–90. [DOI] [PubMed]
- 33.Stewart AL, Hays RD, Ware JE Jr. The MOS short-form general health survey. Reliability and validity in a patient population. Med Care. 1988;26:724–735. [DOI] [PubMed]
- 34.Symeonides P, Petsatodes G, Pournaras J, Kapetanos G, Christodoulou A, Papadopoulos P. Replacement of deficient acetabulum using Burch-Schneider cages: 22 patients followed for 2–10 years. Acta Orthop Scand. 1997;275:30–32. [DOI] [PubMed]
- 35.Udomkiat P, Dorr LD, Won YY, Longjohn D, Wan Z. Technical factors for success with metal ring acetabular reconstruction. J Arthroplasty. 2001;16:961–969. [DOI] [PubMed]
- 36.Unger AS, Lewis RJ, Gruen T. Evaluation of a porous tantalum uncemented acetabular cup in revision total hip arthroplasty: clinical and radiological results of 60 hips. J Arthroplasty. 2005;20:1002–1009. [DOI] [PubMed]
- 37.van Koeveringe AJ, Ochsner PE. Revision cup arthroplasty using Burch-Schneider anti-protrusio cage. Int Orthop. 2002;26:291–295. [DOI] [PMC free article] [PubMed]
- 38.Wachtl SW, Jung M, Jakob RP, Gautier E. The Burch-Schneider antiprotrusio cage in acetabular revision surgery: a mean follow-up of 12 years. J Arthroplasty. 2000;15:959–963. [DOI] [PubMed]
- 39.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [DOI] [PubMed]
- 40.Whaley AL, Berry DJ, Harmsen WS. Extra-large uncemented hemispherical acetabular components for revision total hip arthroplasty. J Bone Joint Surg Am. 2001;83:1352–1357. [DOI] [PubMed]
- 41.Winter E, Piert M, Volkmann R, Maurer F, Eingartner C, Weise K, Weller S. Allogeneic cancellous bone graft and a Burch-Schneider ring for acetabular reconstruction in revision hip arthroplasty. J Bone Joint Surg Am. 2001;83:862–867. [DOI] [PubMed]