Abstract
During revision total shoulder arthroplasty, bone grafting severe glenoid defects without concomitant reinsertion of a glenoid prosthesis may be the only viable reconstructive option. However, the fate of these grafts is unknown. We questioned the durability and subsidence of the graft and the associated clinical outcomes in patients who have this procedure. We retrospectively reviewed 11 patients with severe glenoid deficiencies from aseptic loosening of a glenoid component who underwent conversion of a total shoulder arthroplasty to a humeral head replacement and glenoid bone grafting. Large cavitary defects were grafted with either allograft cancellous chips or bulk structural allograft, depending on the presence or absence of glenoid vault wall defects, without prosthetic glenoid resurfacing. Clinical outcomes (Penn Shoulder Score, maximum 100 points) improved from 23 to 57 at a minimum 2-year followup (mean, 38 months; range, 24–73 months). However, we observed substantial graft subsidence in all patients, with eight of 11 patients having subsidence greater than 5 mm; the magnitude of graft resorption did not correlate with clinical outcome scores. Greater subsidence was seen with structural than cancellous chip allografts. Bone grafting large glenoid defects during revision shoulder arthroplasty can improve clinical outcome scores, but the substantial resorption of the graft material remains a concern.
Level of Evidence: Level III Prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Aseptic loosening of the glenoid component continues to be a frequent indication for revision of a total shoulder arthroplasty (TSA) [1, 7–10, 13, 15, 17–19, 22, 25]. In some instances, the unstable glenoid implant causes large cavitary defects of the cancellous glenoid vault. When combined with defects of the thin cortical vault walls, implantation of a new glenoid component may not be possible. Bone grafting the glenoid defect without new glenoid component implantation combined with hemiarthroplasty is a reconstructive option in these circumstances [1, 17, 18]. However, previous reports have not described the extent of graft subsidence [1], the use of structural allograft for reconstruction of cavitary defects combined with glenoid vault wall defects [17], or the clinical outcomes after glenoid bone grafting [18].
We questioned the extent of resorption of the different allografts used (morsellized chips or bulk structural graft) and whether that resorption related to the functional outcomes for patients in whom large glenoid insufficiencies precluded implantation of a new glenoid component at the time of revision shoulder arthroplasty.
Materials and Methods
We retrospectively reviewed 16 patients (16 shoulders) who underwent revision of a TSA for aseptic glenoid component loosening with conversion to a humeral head replacement and a reconstructed glenoid with allogenic bone graft between May 2001 and May 2005 (Table 1). Inadequate glenoid bone stock precluded implantation of a new prosthetic glenoid component in all patients. The indication for the index shoulder arthroplasty was osteoarthritis in each patient. In all patients, the rotator cuff was reported to be intact at the time of the index surgery. Between the index and bone grafting revision procedures, one patient (Patient 6) underwent pectoralis major transfer for an irreparable subscapularis tear. One patient with preexisting Erb’s palsy (Patient 7) underwent latissimus dorsi muscle transfer before the index arthroplasty. At the time of revision surgery, the rotator cuff was structurally intact in eight of 11 (73%) patients. One patient (Patient 5) had a 2-cm supraspinatus tear, which was reparable at the time of revision surgery. Another patient (Patient 11) had a partially torn subscapularis tendon, which was reparable at the time of revision surgery. The remaining patient (Patient 1) had an irreparable subscapularis tear for which we performed pectoralis major transfer at the time of the revision surgery. All patients had good deltoid muscle function. Glenoid loosening was the result of aseptic osteolysis in all patients. One patient (Patient 3) underwent previous revision arthroplasty for reimplantation of a loose glenoid component 7 years before the bone grafting revision. One patient was lost to followup and four patients died for reasons unrelated to the surgery. The remaining 11 patients (mean age, 69 years; range, 58–83 years; eight men) were available for minimum 2-year (mean, 38 months; range, 24–73 months) clinical and radiographic followups. The original glenoid implant included keeled all-polyethylene glenoid components in five patients and metal-backed glenoid components in six. Information regarding the size and manufacturer of the original humeral component was not routinely available from the retrospective data.
Table 1.
Outcomes of glenoid bone grafting in revision shoulder arthroplasty
| Patient number | Age (years) | Gender | Length of followup (months) | Status of rotator cuff at revision surgery | Humeral components revised | Glenoid rim and wall defects* | Type of allograft used | Graft subsidence (mm) | Total Penn Shoulder Score (total possible = 100) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Followup | |||||||||
| 1 | 73 | M | 24 | Deficient subscapularis (pectoralis major transfer) | Yes (loosening) | 1-cm anterior/inferior wall defect; intact rim | Morsellized cancellous | < 5 | 15 | 40 |
| 2 | 63 | F | 62 | Intact | Yes (loosening) | 1-cm anteroposterior wall defect; intact rim | Morsellized cancellous | 5–10 | 10 | 44 |
| 3 | 63 | M | 53 | Intact | Yes (malpositioning) | Joint line 1.5 cm medial to coracoid base; posterior-inferior rim deficient; 5-mm perforation in posterior-superior wall | Morsellized cancellous | 5–10 | 31 | 50 |
| 4 | 64 | M | 73 | Intact | No | Joint line 1.5 cm medial to coracoid base; absent anterior rim; 1.5-cm defect in posterior wall | Structural | 5–10 | 22 | 82 |
| 5 | 75 | F | 27 | Repaired 2-cm supraspinatus tear | No | Absent anterior wall; 6-mm posterior wall perforation | Structural | 5–10 | 23 | 23 |
| 6 | 58 | M | 38 | Previous pectoralis major transfer for deficient subscapularis | Yes (malpositioning) | 1-cm anterior wall perforation; intact rim | Morsellized cancellous | 5–10 | 35 | 89 |
| 7 | 58 | M | 38 | Previous latissimus dorsi transfer for Erb’s palsy | No | No perforations; intact rim | Morsellized cancellous | < 5 | 36 | 55 |
| 8 | 71 | M | 32 | Intact | Yes (loosening) | 5-mm posterior wall defect; rim intact | Morsellized cancellous | < 5 | 23 | 94 |
| 9 | 83 | F | 24 | Intact | No | 75% glenoid absent; only anterior-superior quadrant remaining; absent rim | Structural | > 10 | 13 | 21 |
| 10 | 64 | M | 24 | intact | Yes (loosening) | Walls intact; deficient rim | Structural (without screws) | 5–10 | 22 | 52 |
| 11 | 82 | M | 24 | Repaired partial tear subscapularis | Yes (loosening) | > 1-cm anterior-superior wall defect; 75% posterior wall absent; 60% loss of rim | Structural | > 10 | 18 | 80 |
* The glenoid vault cancellous bone was absent in each patient; M = male; F = female.
Preoperative radiographic evaluation included anteroposterior, true anteroposterior, and axillary views of the shoulder. We also performed computed tomographic arthrograms in four of the 11 patients when the presence or absence of glenoid loosening was unclear on initial plain radiographs. Evaluation for concomitant infection included intraoperative tissue biopsy and frozen sections in nine of 11 patients. One patient underwent preoperative shoulder aspiration. At the time of surgery, glenoid bone deficiency was evaluated according to the classification described by Antuna et al. [1]; defects are classified as central, peripheral, and combined with further subdivision into mild, moderate, and severe.
The surgical procedure consisted of removal of the loose glenoid component and thorough débridement of all of the devitalized tissues and detritus from the glenoid perimeter and from within the remaining glenoid vault cavity. All procedures were performed by the senior surgeon (JPI). When the remaining insufficient glenoid bone stock prohibited prosthetic glenoid resurfacing, we reconstructed the glenoid vault with allogenic bone graft using packed morsellized cancellous bone (4- to 10-mm chips) or a bulk femoral head structural allograft contoured to fit the defect. We made the decision to use allograft chips or the structural graft intraoperatively. In patients in whom the glenoid rim and walls were deficient and the entire interior of the glenoid vault was lost, these defects would not structurally contain morsellized bone graft material and we used a structural graft. With these uncontained defects, we contoured an allograft femoral head with a high-speed burr to the desired shape and typically secured it in the defect with one or two screws. Two of 11 patients had glenoid vault rim and wall defects of 5 mm to 10 mm and seven of 11 patients had a defect greater than 10 mm. The vault walls were intact in the remaining two patients. The glenoid vault was devoid of cancellous bone in all patients after glenoid component removal and débridement (Fig. 1). Two patients had severe centralized defects and nine had severe combined (glenoid vault and wall) defects. Morsellized allograft cancellous bone, rongeured as necessary to obtain approximately 4- to 7-mm chips, was impacted into the empty vault in six patients and we used a contoured bulk allograft femoral head in five patients (of which four were transfixed with screws). In one patient, the femoral allograft was contoured in such a way that it allowed a secure fit into the vault when impacted in place, obviating the need for further screw fixation (Table 1). In addition to removal of the glenoid component, seven of 11 patients underwent concomitant revision of the original humeral component (using an anatomically sized prosthesis, Global Advantage; DePuy, Warsaw, IN) for loosening (five) or malpositioning (two).
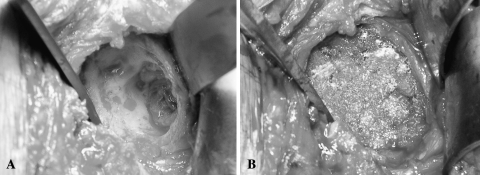
Fig. 1A–B.
(A) An intraoperative photograph shows the glenoid vault completely devoid of cancellous bone after removal of a loose prosthetic glenoid component (Patient 6). (B) The contained defect has been packed with morsellized cancellous allograft bone.
Postoperatively, three of 11 patients were immobilized in an abduction orthosis (SCOI Brace; DonJoy, Vista, CA) for the first 2 postoperative weeks. We used brace immobilization in these patients (Patients 5, 6, 11) to help protect the rotator cuff repair or muscle transfer at the time of the revision surgery. Passive stretching exercises were begun after the initial 2-week period of immobilization. Eight of 11 patients were immobilized in a sling during the first 2 weeks, but we allowed them to perform daily passive range of motion exercises. In all patients, active motion and progressive strengthening were initiated at 6 weeks.
To determine the amount of bone graft subsidence, one of us (JJS), blinded to the clinical outcomes, compared the distance between the lateral edge of the acromion and the lateral edge of the glenoid margin on the immediate postoperative and followup true anteroposterior radiographs of the shoulder (Fig. 2). Subsidence was measured as less than 5 mm, between 5 mm and 10 mm, or greater than 10 mm. We selected ranges rather than raw numbers to account for slight variations (eg, a few millimeters) secondary to differing radiographic techniques and patient positionings, which has been used previously [18].
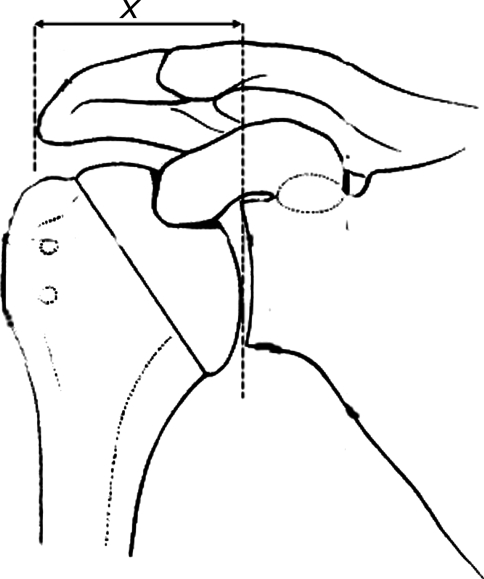
Fig. 2.
To evaluate graft subsidence, the distance (x) was measured from the lateral edge of the acromion to the lateral edge of the glenoid margin on true anteroposterior radiographs of the shoulder.
We determined subjective clinical outcomes with a shoulder-specific patient-generated questionnaire stratifying pain, satisfaction, and function (Penn Shoulder Score), which previously was validated [5, 11, 12, 14]. The pain score is measured out of a possible 30, satisfaction out of a possible 10, and function out of a possible 60. The best possible total score therefore is 100 points.
Results
We observed graft subsidence in all patients (Table 1). Subsidence was as much as 5 mm in three patients, between 5 mm and 10 mm in six patients, and greater than 10 mm in two patients. Mean subsidence for allograft chips and structural allograft was 7 mm and 14 mm, respectively. Greater graft subsidence was seen for structural allograft regardless of the duration of followup. In all four patients in whom transfixation screws were used to secure the structural graft, resorption led to uncovering of the screws and articulation with the prosthetic humeral head. Further medialization led to screw breakage in one of these patients (Patient 5). There was no radiographic evidence of humeral component loosening or malpositioning at the latest followup.
Subjective clinical outcomes improved in all three categories of the Penn Shoulder Score (Table 2). Overall, the average total Penn Shoulder Score improved from 23 to 57. The final Penn Shoulder Score was not correlated to the extent of graft subsidence. There was an insufficient number of subjects without vault wall perforations (two of 11) to make a determination regarding the relationship of bone loss involving the glenoid walls and clinical outcomes.
Table 2.
Change in Penn Shoulder Score
| Penn Shoulder Score | Mean Preoperative Score (range) | Mean Postoperative Score (range) |
|---|---|---|
| Pain (total possible = 30) | 10 (5–13) | 17 (5–30) |
| Satisfaction (total possible = 10) | 2 (0–4) | 7 (3–10) |
| Function (total possible = 60) | 11 (4–21) | 33 (10–55) |
| Total (total possible = 100) | 23 (10–36) | 57 (21–94) |
Two patients underwent additional surgery after the bone grafting. One patient (Patient 1) had revision surgery to a reverse TSA 10 months after the glenoid bone grafting. He had a poorly functioning pectoralis major transfer and an irreparable supraspinatus tear at the time of the glenoid bone grafting. Removal of the loose glenoid component at the time of the first revision left insufficient glenoid volume to support the reverse glenoid component. The glenoid defect was grafted with cancellous chips resulting in 3 mm of subsidence and graft incorporation at the time of the revision to a reverse-type arthroplasty. Another patient (Patient 5) underwent removal of the humeral prosthesis (resection arthroplasty) for sepsis, which developed 26 months after the glenoid bone grafting. At the time of the resection arthroplasty, the anterior and superior rotator cuff was deficient. The structural allograft had incorporated but was eroded medial to the fixation screws. Although the infection cleared clinically, the patient chose not to pursue additional reconstructive surgery and had pain-free, waist-level only shoulder function at the latest followup.
Discussion
In situations of severe glenoid bone defects in failed TSA, bone grafting the defect without prosthetic glenoid reimplantation may be the only viable surgical option, a solution that has received only sparse attention in the literature [1, 17, 18]. The purpose of the current study was to report on the fate of the bone graft and the associated clinical outcomes of patients undergoing revision shoulder arthroplasty with extensive glenoid bone grafting for severe defects.
This study has limitations inherent to its retrospective nature, including a relatively small sample size with some patient variation and the potential for data missing from the patients’ charts. For example, information regarding the size and manufacturer of the original humeral implant was not routinely available in the clinical chart or operative report. Additionally, five of 11 patients had undergone previous rotator cuff repair, muscle transfer for an irreparable rotator cuff tear, or underwent rotator cuff repair at the time of the grafting procedure, which represents further variation in the study group. The potential for continued rotator cuff dysfunction affecting outcomes is possible. However, large numbers of such patients are difficult to assemble secondary to the infrequent nature of this clinical scenario. Recommendations specific to a radiographic finding of screws contacting the prosthetic head could not be drawn from the limited sample size. Given the graft resorption, longer followup is required to determine if the clinical scores will deteriorate with time. Additionally, measurement of graft subsidence was based on radiographs obtained without fluoroscopic control. Fluoroscopic control can minimize variations in measurements as a result of disparities in radiographic technique, beam angle, or patient positioning [21, 24]. This deficiency may have added variation to our subsidence measurements. Like in other series, there was a high rate of humeral implant revision at the time of the bone grafting. This may have contributed to the overall improvement in clinical outcome measures.
Improved clinical outcomes have been reported when the glenoid could be revised with a new resurfacing implant [1, 2, 17, 18]. For this reason, it is our practice to revise the glenoid with a new prosthesis when possible. However, in some severe cases of glenoid erosion such as those reported in the current study, glenoid prosthetic resurfacing is not possible secondary to the large volume of graft required.
Phipatanakul and Norris reviewed 24 patients for whom the glenoid defect was treated with allograft cancellous bone graft [18]. At a mean followup of nearly 3 years, 18 patients had satisfactory pain relief with an additional four patients obtaining good pain relief after conversion to TSA. They reported substantial graft subsidence in 10 of 20 patients for whom comparative radiographs were available. However, they believed the graft had incorporated sufficiently to allow subsequent prosthetic glenoid reimplantation. Neyton et al. reported their experience treating glenoid deficiencies using a corticocancellous iliac crest autogenous graft in nine patients [17]. By placing the cortical surface of the graft in contact with the prosthetic humeral head, they believed they achieved a solid foundation for humeral articulation while allowing ingrowth into the cancellous portion of the autograft. At a minimum of 2 years, medialization of the graft averaged 4.1 mm with two of nine patients having medialization of 10 mm or more. Functional improvements were modest with Constant-Murley scores [3, 4] improving from 46.3 to 49.9. Using the criteria of Neer et al. [16], the functional outcome was unsatisfactory in four of nine patients. An excellent result is considered if the patient has no or slight pain and at least 45° external rotation and 140° active elevation and is satisfied with the procedure. The result is satisfactory if the patient has no, slight, or moderate pain only with vigorous activities and at least 20° of external rotation and 90° of active elevation and is satisfied with the procedure. When not described by either of these scenarios, the result is considered unsatisfactory. The available literature offers no consensus regarding the use of allograft versus autograft bone for these circumstances.
Based on glenoid defects in 48 patients at the time of revision arthroplasty, Antuna et al. [1] classified defects into central, peripheral, and combined with further subdivision into mild, moderate, and severe. They emphasized the difficulties in treating defects involving the glenoid vault and walls. In these extreme cases, however, bone grafting is often the only means by which subsequent glenoid reimplantation can be achieved. Sixty-six percent of the patients in their series with glenoid defects treated with impacted cancellous allograft bone had satisfactory pain relief. They did not report data for graft resorption, however.
Unlike the previous reports, the combined clinical and radiographic data of the current study suggest subjective clinical outcome scores can improve considerably despite substantial graft subsidence. Furthermore, bulk structural allograft, not used in the previous studies, was associated with greater subsidence. By way of comparison, analysis of our Shoulder Surgery Registry shows 52 patients in whom revision TSA was performed, in which another glenoid component was implanted, had an average improvement in total Penn Shoulder Score from 21 to 58 at a minimum 2-year followup.
Simple removal of the loose glenoid component without bone grafting or reimplantation of a new component offers an alternate surgical solution. It is unclear from the current data if glenoid component removal alone would have provided comparable clinical outcomes. However, despite improvements in pain relief, limited functional results and continued medialization of the native glenoid may preclude future glenoid implantation and provide no better opportunity to implant another component later [17, 19].
The use of bulk structural allograft to fill osteolytic defects has had durable use in other orthopaedic applications [6, 20, 23, 26]. However, in many patients, the forces on the graft material may be distributed more favorably by supportive implants (eg, acetabular cage or stemmed distal femoral component). Our decision to use a bulk allograft was based on the findings of severe cortical deficiencies of the native glenoid. In five patients, two-thirds of the glenoid cortical shell was absent. Impacting cancellous chips into these uncontained defects would not have been possible. Because the bulk allograft material articulated directly with the humeral prosthesis without the added support of a glenoid cortical rim, there was little to prevent greater degrees of migration because the graft collapsed during the resorptive phase resulting in substantial subsidence. In other words, the increased subsidence seen in patients using structural allografts is likely the result of the lack of a supportive, native glenoid cortical rim and not the type of allograft material used. The fact that we observed no correlation in subsidence and clinical outcomes is likely attributable to the fact that in all patients, after the graft and prosthesis settled into their new position, the graft partially incorporated and stabilized (Fig. 3).
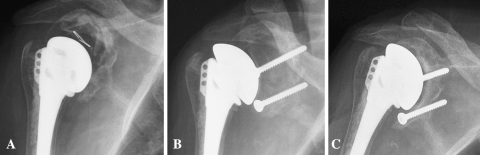
Fig. 3A–C.
(A) A loose and dislocated glenoid component has resulted in massive glenoid bone loss in this failed total shoulder arthroplasty (Patient 9). (B) An initial postoperative radiograph shows the structural allograft has been fixed into the glenoid defect. (C) A 2-year postoperative radiograph shows substantial subsidence of the graft and uncovering of the screws.
In some instances of severe glenoid bone deficiency encountered during revision shoulder arthroplasty, reimplantation of a new glenoid component may not be possible using the current glenoid components that rely on some portion of the subchondral bone to support the backside of the implant. Functional improvement can be achieved at a minimum 2-year followup with bone grafting of the glenoid defect. However, bone grafting of large glenoid defects with allograft bone results in high rates of graft subsidence, particularly when there is absence of a supportive glenoid rim and walls resulting in the use of large structural allografts, which remains a distinct concern. Methods of reconstructing a severely deficient glenoid vault that offer greater durability and minimize subsequent subsidence provide a focus for additional investigations.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was not required by our Institutional Review Board after review and approval of the protocol.
References
- 1.Antuna SA, Sperling JW, Cofield RH, Rowland CM. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg. 2001;10:217–224. [DOI] [PubMed]
- 2.Cheung EV, Sperling JW, Cofield RH. Reimplantation of a glenoid component following component removal and allogenic bone-grafting. J Bone Joint Surg Am. 2007;89:1777–1783. [DOI] [PubMed]
- 3.Constant CR. An evaluation of the Constant-Murley shoulder assessment. J Bone Joint Surg Br. 1997;79:695–696. [PubMed]
- 4.Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160–164. [PubMed]
- 5.Cook KF, Gartsman GM, Roddey TS, Olson SL. The measurement level and trait-specific reliability of 4 scales of shoulder functioning: an empiric investigation. Arch Phys Med Rehabil. 2001;82:1558–1565. [DOI] [PubMed]
- 6.Engh GA, Herzwurm PJ, Parks NL. Treatment of major defects of bone with bulk allografts and stemmed components during total knee arthroplasty. J Bone Joint Surg Am. 1997;79:1030–1039. [DOI] [PubMed]
- 7.Farron A, Terrier A, Buchler P. Risks of loosening of a prosthetic glenoid implanted in retroversion. J Shoulder Elbow Surg. 2006;15:521–526. [DOI] [PubMed]
- 8.Gartsman GM, Roddey TS, Hammerman SM. Shoulder arthroplasty with or without resurfacing of the glenoid in patients who have osteoarthritis. J Bone Joint Surg Am. 2000;82:26–34. [DOI] [PubMed]
- 9.Hawkins RJ, Greis PE, Bonutti PM. Treatment of symptomatic glenoid loosening following unconstrained shoulder arthroplasty. Orthopedics. 1999;22:229–234. [DOI] [PubMed]
- 10.Hill JM, Norris TR. Long-term results of total shoulder arthroplasty following bone-grafting of the glenoid. J Bone Joint Surg Am. 2001;83:877–883. [DOI] [PubMed]
- 11.Iannotti JP, Williams GR Jr. Disorders of the Shoulder: Diagnosis and Management. Philadelphia, PA: Lippincott Williams & Wilkins; 1999.
- 12.Leggin BG, Michener LA, Shaffer MA, Brenneman SK, Iannotti JP, Williams GR Jr. The Penn shoulder score: reliability and validity. J Orthop Sports Phys Ther. 2006;36:138–151. [DOI] [PubMed]
- 13.Martin SD, Zurakowski D, Thornhill TS. Uncemented glenoid component in total shoulder arthroplasty: survivorship and outcomes. J Bone Joint Surg Am. 2005;87:1284–1292. [DOI] [PubMed]
- 14.Michener LA, Leggin BG. A review of self-report scales for the assessment of functional limitation and disability of the shoulder. J Hand Ther. 2001;14:68–76. [DOI] [PubMed]
- 15.Neer CS 2nd, Morrison DS. Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg Am. 1988;70:1154–1162. [PubMed]
- 16.Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64:319–337. [PubMed]
- 17.Neyton L, Walch G, Nove-Josserand L, Edwards TB. Glenoid corticocancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J Shoulder Elbow Surg. 2006;15:173–179. [DOI] [PubMed]
- 18.Phipatanakul WP, Norris TR. Treatment of glenoid loosening and bone loss due to osteolysis with glenoid bone grafting. J Shoulder Elbow Surg. 2006;15:84–87. [DOI] [PubMed]
- 19.Rodosky MW, Weinstein DM, Pollock RG. On the rarity of glenoid component failure. J Shoulder Elbow Surg. 1995;4:3–4. [DOI]
- 20.Shinar AA, Harris WH. Bulk structural autogenous grafts and allografts for reconstruction of the acetabulum in total hip arthroplasty: sixteen-year-average follow-up. J Bone Joint Surg Am. 1997;79:159–168. [DOI] [PubMed]
- 21.Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg Am. 2007;89:588–600. [DOI] [PubMed]
- 22.Sperling JW, Cofield RH, Rowland CM. Neer hemiarthroplasty and Neer total shoulder arthroplasty in patients fifty years old or less: long-term results. J Bone Joint Surg Am. 1998;80:464–473. [DOI] [PubMed]
- 23.Sporer SM, O’Rourke M, Chong P, Paprosky WG. The use of structural distal femoral allografts for acetabular reconstruction: average ten-year follow-up. J Bone Joint Surg Am. 2005;87:760–765. [DOI] [PubMed]
- 24.Vyskocil P, Gerber C, Bamert P. Radiolucent lines and component stability in knee arthroplasty: standard versus fluoroscopically-assisted radiographs. J Bone Joint Surg Br. 1999;81:24–26. [DOI] [PubMed]
- 25.Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78:603–616. [DOI] [PubMed]
- 26.Zmolek JC, Dorr LD. Revision total hip arthroplasty: the use of solid allograft. J Arthroplasty. 1993;8:361–370. [DOI] [PubMed]