Abstract
Monoblock trabecular metal cups are made of a novel porous material intended to enhance ingrowth and improve fixation. We prospectively followed 223 consecutive patients with 245 trabecular metal acetabular cups implanted during primary total hip arthroplasties to determine the overall survivorship of the implant, and any association of survivorship to primary diagnosis and age, and to determine the fate of polar gaps and cysts. Minimum followup was 36 months (mean, 60 months; range, 36–112 months). Patients were assessed with the Harris Hip score and the Oxford questionnaire and radiographically with standardized serial radiographs. At last followup, all cups were radiographically stable with no evidence of migration or progressive radiolucencies. The survivorship with reoperation as the end point was estimated at 98.75% with a 95% confidence interval. Three reoperations occurred during the first 36 months. The Harris hip score increased from 48 to 94 and the Oxford score was 16.4 at the last examination. We observed no difference in terms of survivorship among patients with osteoarthritis, osteonecrosis, or hip dysplasia. Seven of 14 (50%) osteoarthritis cysts and 10 of 33 (33.3%) polar gaps detected on postoperative radiographs decreased or filled, whereas none of the remainder deteriorated with time. Our midterm results suggest this implant may enhance fixation, but long-term followup is needed to confirm our findings.
Level of Evidence: Level IV Therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Cementless fixation, with or without screw augmentation, has evolved during the past few decades as the preferred method for acetabular reconstruction [20]. Favorable early and midterm results of press-fit cementless acetabular component designs have been reported. Latimer and Lachiewicz [24] reported no revision or loosening at 7 years in a series of 136 cups inserted with screws, whereas an overall survivorship rate of 97.7% was reported by Böhm and Bösche [5] at 11 years. Both groups of investigators used the Harris-Galante I implant. Using four different cup designs in 152 patients, Lewallen and Cabanela reported one revision for aseptic loosening and no evidence of osteolysis at a 5-year minimum followup [25]. In a more recent study, Udomkiat et al. reported 99.1% survivorship at 12 years for hemispheric cups implanted without screws [35].
Osteolysis resulting from polyethylene wear has been recognized as a major limitation in long-term survival of modular cementless cups. A deficient locking mechanism and micromotion of the liner in conjunction with screw holes allow backside debris to gain access to the bone-implant interface and accelerate periprosthetic osteolysis and loosening [6, 11, 12, 19, 23]. Younger age and initial diagnosis of osteonecrosis are known to adversely influence the long-term outcome of THA [2, 3, 10, 27, 33].
Initial stability is also fundamental for survivorship of cementless cups. Press-fit techniques provide optimal conditions for bone ingrowth and fixation. Pore size, bone-implant apposition, and material properties all influence bone ingrowth and long-term stability [4, 15].
Improvement on all aspects of acetabular design, including wear-resistant bearing surfaces and innovative approaches to bone ingrowth, theoretically will improve outcomes. One such approach is the monoblock trabecular metal cup made of a novel porous material to enhance ingrowth and eliminate backside wear. Some reports of the TMT cup in primary THA documented no revisions resulting from aseptic loosening or progressive radiolucencies at the interface [16, 22, 26, 29], but the long-term behavior of this cup is unknown. The incidence of gaps in the bone-implant interface is relatively high with this design [16, 22, 26, 34], but it appears they do not influence long-term stability [16, 26]. The use of this elliptic cup designed for rim fit is also of some concern in dysplastic hips with an irregular and shallow acetabulum.
Our primary question was to whether there would be a high survivorship of the TMT cup. Secondarily, we asked whether survivorship of the cup depended on the primary diagnosis and the patient’s age; finally we investigated the fate of the gaps and cysts detected on postoperative radiographs.
Materials and Methods
We prospectively followed 223 consecutive patients with 245 TMT acetabular cups implanted between 1997 and 2004. Data from all patients were collected prospectively for a minimum of 3 years. Two patients died from causes unrelated to their surgery and three were lost to followup. This left 218 patients with 240 hips available for review and with complete clinical and radiographic followups. There were 63 men and 155 women. Their mean age was 56 years (range, 21–80 years; standard deviation, 13.73) at the time of surgery. The primary diagnoses were: primary osteoarthritis (OA) in 127 (52.9%) hips; OA secondary to developmental dysplasia of the hip (DDH) in 61 (25.4%) hips; osteonecrosis (ON) in 37 (15.4%) hips; fractures of the neck of the femur in 12 (5%); rheumatoid arthritis in one; ankylosing spondylitis in one; and Paget’s disease in one. The demographics for patients in the three major groups (OA, DDH and ON) and radiographic classifications for patients with DDH and ON are provided in Tables 1 and 2, respectively. The minimum followup for these groups of patients was 36 months (mean, 60 months; range, 36–112 months). No patients were lost to followup. We obtained prior Institutional Review Board approval and all patients agreed to participate in the study.
Table 1.
Primary diagnosis, age, and followup
| Preoperative diagnosis | Number of hips | Mean age (range) | Standard deviation | Followup (months) |
|---|---|---|---|---|
| Osteoarthritis | 127 | 63.9 (31–80) | 8.5988 | 61 |
| Osteonecrosis | 37 | 36.8 (21–64) | 10.39173 | 60 |
| Developmental dysplasia of the hip | 61 | 50.5 (34–77) | 10.19249 | 61 |
Table 2.
Classifications of developmental dysplasia of the hip* and osteonecrosis†
All patients had a TMT acetabular component with peripheral screw holes (Trabecular Metal™ Technology; Zimmer, Warsaw, IN) through a posterior approach. The TMT cup is elliptic with high surface roughness designed for an interference fit at the periphery of the prepared acetabular cavity. The cup is one piece with the polyethylene liner compression-molded into the porous tantalum to eliminate backside wear. The shell diameter is approximately 2 mm greater at the periphery of the cup than at the dome. Reaming is performed up to the size of the polar diameter of the cup, which is the actual size of the dome. This allows excellent line-to-line coaptation of the dome while press-fit is achieved by the 2-mm greater size of the cup periphery at a range of 20° from the cup equator. All cups were implanted with the press-fit technique. In 27 hips, screws were used to augment initial stability. Polyethylene liners with 10° elevated posterior rims were used in all cases. The outer diameter of the cup ranged from 42 to 60 mm. Femoral heads with a diameter of 22 mm were implanted in 23 hips and 28 mm in the remaining 217. Ninety-two heads were made of cobalt-chromium alloy, whereas the remaining 148 heads were made of alumina ceramic. Cobalt-chromium heads were used for 22-mm heads and when a skirted head was chosen.
A cementless hydroxyapatite-coated femoral stem (Proxilock; Implex, Allendale, NJ) was used in the majority of the patients (214 hips) and a cemented stem (F-115; Implex) was used in 20 hips as a result of poor bone quality or as a result of malformation of the proximal femur in patients with DDH. A cementless distal locking stem (Conelock; Implex) was used in six hips of patients with DDH.
All patients received low-molecular-weight heparin 12 hours postoperatively and for 6 weeks thereafter. One preoperative and three postoperative doses of a second-generation cephalosporin were given for prophylaxis in the majority of these patients.
Patients were evaluated at 6 weeks; 1, 2, 3, and 5 years postoperatively; and yearly thereafter. Clinical assessment was performed by the senior author (KNM) using the Harris hip score [17] at each followup. Patient-reported outcome using the Oxford hip score questionnaire also was obtained from all patients at the last followup. The Oxford questionnaire consists of 12 questions specifically designed to obtain patient-reported outcome score from patients who underwent THA [8].
Standard radiographs included an anteroposterior (AP) view of the pelvis and AP and lateral views of the proximal part of the femur. Two examiners, the senior author (KNM) and a fellowship-trained surgeon (SV) not involved in surgery or patient care, examined the radiographs independently. In two cases in which there was disagreement, a consensus was reached between the examiners. The 6-week postoperative radiograph served as the baseline for identifying polar gaps or cysts, subsequent subsidence, interfacial radiolucencies, osteolysis, and component loosening. Regions in which the surface of the acetabular component was not in contact with bone on the immediate postoperative radiographs were classified as gaps to distinguish them from radiolucent lines that might appear on subsequent radiographs in areas in which no gaps had existed initially or in which gaps had disappeared [33]. Osteoarthritic cysts in the subchondral bone were recorded and progressively evaluated. Periacetabular osteolytic lesions were assessed according to the zones described by DeLee and Charnley [9]. Migration of the cup was assessed using the criteria described by Nunn et al. [30]. We considered cups well fixed if they had no radiolucent lines or migration observed on the radiographs at last followup. Cups with a circumferential radiolucent line of 1 mm or less in width and without migration were considered to have a stable fibrous union. Cups with progressive radiolucent lines and those exhibiting migration were considered loose.
We analyzed any association of diagnosis with reoperation rate using one-way analysis of variance; pairwise comparisons were performed using post hoc t tests with Bonferroni correction to compare the incidence of polar gaps, the size of the cups used, and the mean age at implantation between the three major diagnoses. The level of significance was 5%. We used the Kaplan-Meier method to generate survivorship curves, with 95% confidence intervals, with reoperation as the end point. Curves were generated for the total cohort and for each major diagnosis.
Results
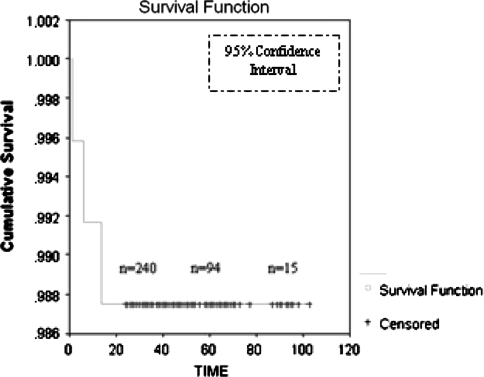
All but one of the 240 hips had a well-fixed, stable cup according to the modified criteria of Engh et al. [11]. There were no progressive radiolucencies or any radiographic evidence of osteolysis or migration around the acetabular components. Increased bone density and thickening of the trabeculae were observed in 58% of the patients. Sixty-nine percent of the densifications were located in Zone I, 12% in Zone II, and 19% in Zone III. All the reoperations occurred during the 36-month followup period. At the end of this period, the survivorship with reoperation for any reason as the end point was estimated at 98.75% with a 95% confidence interval (Fig. 1; Table 3). There were three reoperations in this group. One cup was revised at 4 weeks because of malorientation, one dislocated hip required open reduction and femoral head replacement, and one patient with systemic lupus erythematosus needed extensive surgical débridement. The average Harris hip score increased from 48 points (range, 24–58 points) preoperatively to 94 points (range, 69–97 points) at the final followup. The Oxford questionnaire was completed by185 patients (192 hips) preoperatively and by all patients (239 hips) at the final followup. Preoperatively, the mean score was 46.5 and decreased to 16.4 at the final examination. Two patients had very high postoperative scores (33 and 35 retrospectively) with no clinical or radiographic evidence of hip disease.
Fig. 1.
Kaplan-Meier survivorship curves with reoperation as the end point for the total cohort and as per diagnosis are shown. All the events occurred in the 36-month followup period. To calculate Kaplan-Meier estimates of the survival curve, we evaluated the survival curve at each of the times at which an event occurred. In this period, the survivorship is estimated at 98.75% with a 95% confidence interval.
Table 3.
Number of THA at annual intervals
| Annual interval | Number of THA |
|---|---|
| 3 | 239 |
| 4 | 157 |
| 5 | 94 |
| 6 | 50 |
| 7 | 18 |
| 8 | 15 |
| 9+ | 2 |
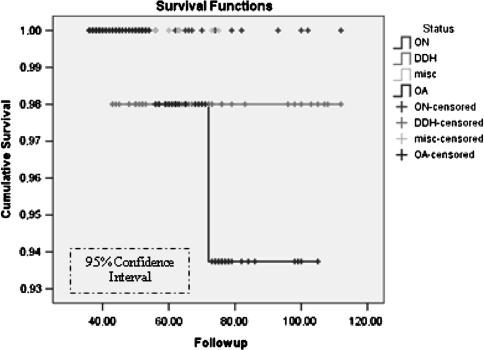
We observed no difference in survivorship among the three major diagnoses (OA, ON, DDH) (Fig. 2). We found no association between diagnosis and reoperation rate, complications, or polar gaps. The size of the cup was smaller (p < 0.05) in patients with DDH when compared with patients with OA and patients with ON. Patients with ON were younger (p < 0.01) than the patients with DDH and the patients with OA at the time of implantation (Table 4). Screws were used in 18 of 61 (29.5%) patients with DDH and only nine of 127 (14.75%) patients with OA.
Fig. 2.
Kaplan-Meier survivorship curves are shown for diagnosis (osteoarthritis [OA], osteonecrosis [ON], and developmental dysplasia of the hip [DDH]) with no considerable differences being seen between the three groups. misc = miscellaneous.
Table 4.
Mean, range, and standard deviation for cup size and age among the groups
| Cup size | Diagnosis | Number of patients | Range | Mean | Standard deviation |
|---|---|---|---|---|---|
| OA | 127 | 46–60 | 51.8493 | 3.09915 | |
| DDH | 61 | 42–56 | 47.7941 | 6.77255 | |
| ON | 37 | 46–58 | 51.6500 | 3.00043 | |
| Age of patients (years) | Diagnosis | Number of patients | Range | Mean | Standard deviation |
| OA | 127 | 31–80 | 63.6096 | 9.46163 | |
| DDH | 61 | 34–77 | 50.4030 | 10.14301 | |
| ON | 37 | 21–64 | 37.6250 | 10.93732 |
Cup size in DDH was significantly smaller (p < 0.05) than in OA group, whereas patients with ON were younger (p < 0.01) than patients with DDH or OA; OA = osteoarthritis; DDH = developmental dysplasia of the hip; ON = osteonecrosis.
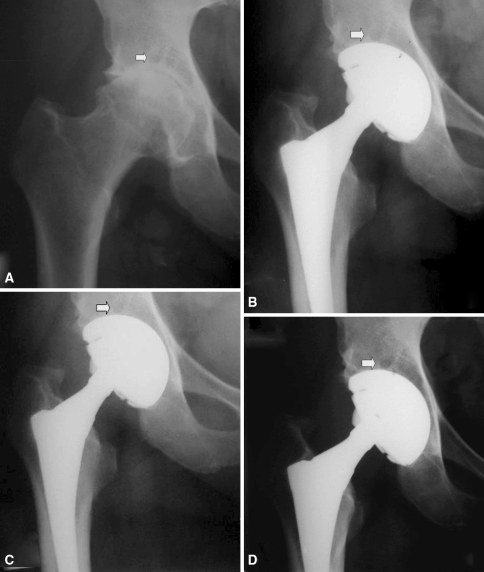
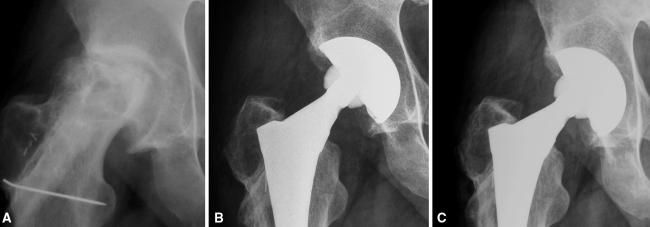
Osteoarthritic cysts and interface gaps were either decreased or filled and they did not affect the stability of the implant. In 14 hips, osteoarthritic cysts in the subchondral bone in Zones I and II were evident on the preoperative radiographs. All of these cysts were left untreated during the baseline procedure. Thickening of the surrounding trabeculae and increased bone density were observed on serial postoperative radiographs. Well-demarcated cysts were evident at the last followup in seven of 14 (50%) of these hips, but they were decreased in size and the cup was clearly well supported by augmented bone trabeculation (Figs. 3, 4). In 33 of 240 hips (13.75%), gaps located in Zone II were seen on the radiographs obtained 6 weeks postoperatively. In 23 (69.7%) cases, they remained unchanged, whereas 10 (30.3%) gaps were decreased (p < 0.01) or filled by the third year (Fig. 5).
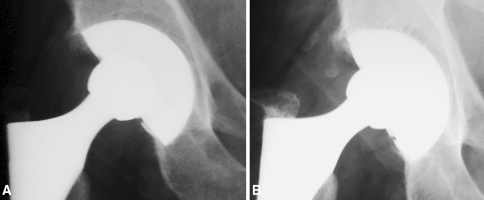
Fig. 3A–D.
(A) Preoperative radiograph and postoperative radiographs at (B) 1, (C) 2, and (D) 5 years of a TMT cup are shown. A well-demarcated stable osteoarthritis cyst (arrow) is evident with no radiographic change over the years.
Fig. 4A–C.
(A) A preoperative anteroposterior radiograph of a patient with osteonecrosis and a failed fibula transfer and anteroposterior radiographs taken at (B) 6 weeks and (C) 3 years postoperatively are shown. Trabeculation radiating from the superior load-bearing part of the cup appears thickened and bone density is increased.
Fig. 5A–B.
(A) A large polar gap is evident on the baseline radiograph. (B) At 5 years, the gap is partially filled, trabecular struts have been formed, and no progressive radiolucencies are seen.
Discussion
The TMT acetabular component incorporates three distinctive innovative characteristics: it is elliptic; it is fitted with a monoblock, nonmodular, compression-molded polyethylene liner; and its metal shell is made of a porous tantalum material that resembles cancellous bone. Because this implant was introduced to our institution soon after it was released for clinical use, this study represents the comprehensive results of its earliest use and longest followup available on implant survivorship and the behavior of gaps and cysts detected on the immediate postoperative radiographs. Furthermore, this is the first report regarding the use of the TMT cup in younger patients and patients undergoing THA because of DDH or ON.
There are several limitations of this study design. We had no control group and the followup was relatively short. Clinical and radiographic outcomes of cementless THA are well documented [1, 5, 35, 36]. As mentioned previously, this study presents the comprehensive results of one of the earliest clinical applications of the TMT cup. A second limitation is that the hips were evaluated radiographically with plain radiographs. Radiographs typically underestimate the true incidence of osteolysis. We chose to include gaps larger than 2 mm to minimize marginal methodologic errors. We believe interpretation of serial radiographs independently from two experienced surgeons reduced this risk. To minimize the possible bias, one of the examiners, a fellowship-trained hip surgeon (VS), not involved in surgery or patient care, evaluated the radiographs independently. No considerable differences were observed between the examiners.
Only three reoperations occurred relatively early and the minimum followup of this cohort is 3 years; therefore, survivorship analysis is indicative only and for the same reasons sample power analysis would not be reliable. Kaplan-Meier curves were generated and included in this report despite these limitations. The cost of the implant is considerably higher than conventional designs and questions have been raised concerning the cost-effectiveness of this implant for use in primary cases. Because there are no data on long-term survivorship of this implant and its performance in comparison with contemporary designs, it is not possible to estimate its cost-effectiveness in association with complication rate, revision incidence and complexity, and total hospital and social costs.
Porous-coated metal-backed cups have had revision rates ranging from 0% to approximately 8% after at least 5 years followup [1, 5, 35]. Most of these revisions are the result of failure of fixation secondary to osteolysis [32, 39]. We had no revisions resulting from loosening and we detected no progressive radiolucencies with the use of the TMT monoblock acetabular component. Our results are similar to the results reported by others [16, 22, 26, 29]. In a multicenter study [16], Gruen et al. reported radiographic results of 414 monoblock acetabular cups with followups ranging from 2 to 5 years. They reported no revisions for loosening and no progressive periacetabular radiolucencies. Macheras et al. [26] reported no revision and no progressive radiolucencies with the TMT cup in 86 primary THAs with an average followup of 7.2 years. Komarasamy et al. [22] and Mulier et al. [29] reported on the same implant at an average of 32 and 46 months followup, respectively. No revisions or radiolucencies and excellent clinical outcomes were reported in both studies. Mulier et al. reported there was cancellous bone densification in Zone I (79%) and Zone III (58%) in 24 hips that had complete radiographic evaluation. We observed similar densification in almost half of our patients (58%). By contrast, stress shielding and decreased bone density have been reported with the use porous-coated implants [37]. The clinical relevance of such a finding and whether it is attributable to the elasticity of the construct or the elliptic shape of the cup are unknown.
The average age of the patients at implantation was 56 years in these series and reflects the high percentage of patients treated for secondary OA either because of DDH or ON. Younger age increases the incidence of osteolysis and aseptic loosening in patients with OA [10, 28]. Although it is unclear whether it is attributable to bone quality, the underlining diagnosis, implants used, or the age of the patients with ON, results of THA in patients with ON are inferior when compared with results in the general population in some reports [2, 3, 31]. Other studies evaluating more contemporary implant designs have had results comparable with those of the general population [7, 14, 38]. Ours is the first report of patients with ON treated with TMT cups and results are comparable with the results of TMT cups in patients with OA and second-generation cementless cups [7, 14, 38].
Despite the fact the shallow acetabulum and irregular rim could pose difficulties when implanting an elliptic rim fit component, we have used TMT cups in patients with DDH. The initial stability was judged as excellent intraoperatively and no difference was observed in this group with the remaining patients. Our results were comparable to those reported with porous-coated cups in patients with DDH [18, 21].
The prevalence of Zone II gaps is inherent to the peripheral press-fit technique monoblock construct and the high friction of trabecular metal. Gruen et al. [16] reported 84% of gaps were filled in their series. In the same report, a cohort of 83 patients was randomized to receive monoblock elliptic cups made either of tantalum or titanium. Filling in of gaps was less in the titanium group, whereas radiolucencies were more frequent in the same group. They attributed it to the monoblock construct, the elasticity, and the osteoconductivity of the trabecular metal. Macheras et al. [26] also reported a high incidence of polar gaps (25 of 82 cases). Using digital measurement analysis of the radiographs, they reported complete filling of all these gaps at 24 weeks. Komarasamy et al. [22] also reported a high incidence (22%) of polar gaps in their series. All gaps were filled at the last followup. In our series, only 30.3% of the gaps and 50% of OA cysts initially detected on plain radiographs were filled by the end of followup. However, there was no evidence of lysis or enlargement of the gaps or cysts. Until now, persistence of residual gaps did not adversely affect the fate of the component. Increased bone density and formation of augmented trabecular struts around the gaps have been observed in most of these patients (Figs. 3–5).
Implantation of a monoblock elliptic acetabular component might be demanding. Assessment of metal-to-bone contact is not possible because of the lack of screw holes on the dome and the presence of the liner. Indirect methods of assessment and a high incidence of dome gaps have been reported [34]. Lack of modularity does not allow use of offset liners when needed and there are concerns regarding potential revisions because the lack of modularity does not allow liner exchange alone. Although extensive wear of the polyethylene liner has not been reported nor did we observe any in our series, it is still of concern.
Our clinical and radiographic results, and the results of others [16, 22, 26, 29], show satisfactory performance of the monoblock trabecular cup at 5 years. However, longer followup is needed to draw safe conclusions.
Acknowledgments
We thank Sokratis Varitimidis, MD, for radiographic evaluation in this series and Dr V. Bagiatis for statistical analysis of our data.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
References
- 1.Archibeck MJ, Berger RA, Jacobs JJ, Quigley LR, Gitelis S, Rosenberg AG, Galante JO. Second-generation cementless total hip arthroplasty: eight to eleven-year results. J Bone Joint Surg Am. 2001;83:1666–1673. [DOI] [PubMed]
- 2.Beaulé PE, Amstutz HC. Management of Ficat stage III and IV osteonecrosis of the hip. J Am Acad Orthop Surg. 2004;12:96–105. [DOI] [PubMed]
- 3.Beaulé PE, Schmalzried TP, Campbell P, Dorey F, Amstutz HC. Duration of symptoms and outcome of hemiresurfacing for hip osteonecrosis. Clin Orthop Relat Res. 2001;385:104–117. [DOI] [PubMed]
- 4.Bobyn JD, Pilliar RM, Cameron HU, Weatherly GC. The optimum pore size for the fixation of porous-surfaced metal implants by the ingrowth of bone. Clin Orthop Relat Res. 1980;150:263–270. [PubMed]
- 5.Böhm P, Bösche R. Survival analysis of the Harris-Galante I acetabular cup. J Bone Joint Surg Br. 1998;80:396–403. [DOI] [PubMed]
- 6.Bono JV, Sanford L, Toussaint JT. Severe polyethylene wear in total hip arthroplasty: observations from retrieved AML PLUS hip implants with an ACS polyethylene liner. J Arthroplasty. 1994;9:119–125. [DOI] [PubMed]
- 7.D’Antonio JA, Capello WN, Manley MT, Feinberg J. Hydroxyapatite coated implants: total hip arthroplasty in the young patient and patients with avascular necrosis. Clin Orthop Relat Res. 1997;344:124–138. [DOI] [PubMed]
- 8.Dawson J, Fitzpatrick R, Carr A, Murray D. Questionnaire on the perceptions of patients about total hip replacement. J Bone Joint Surg Br. 1996;78:185–190. [PubMed]
- 9.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed]
- 10.Duffy GP, Prpa B, Rowland CM, Berry DJ. Primary uncemented Harris-Galante acetabular components in patients 50 years old or younger: results at 10 to 12 years. Clin Orthop Relat Res. 2004;427:157–161. [DOI] [PubMed]
- 11.Engh CA, Bobyn JD, Glassman AH. Porous coated hip replacement: the factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br. 1987;69:45–55. [DOI] [PubMed]
- 12.Fehring TK, Smith SE, Braun ER, Mobley C, Wang PL, Griffin WL. Motion at the modular acetabular shell and liner interface: a comparative study. Clin Orthop Relat Res. 1999;367:306–314. [DOI] [PubMed]
- 13.Ficat RP. Idiopathic bone necrosis of the femoral head: early diagnosis and treatment. J Bone Joint Surg Br. 1985;67:3–9. [DOI] [PubMed]
- 14.Fye MA, Huo MH, Zatorski LE, Keggi KJ. Total hip arthroplasty performed without cement in patients with femoral head osteonecrosis who are less than 50 years old. J Arthroplasty. 1998;13:876–881. [DOI] [PubMed]
- 15.Galante J, Rostoker W, Lueck R, Ray RD. Sintered fiber metal composites as a basis for attachment of implants to bone. J Bone Joint Surg Am. 1971;53:101–114. [PubMed]
- 16.Gruen TA, Poggie RA, Lewallen DG, Hanssen AD, Lewis RJ, O’Keefe TJ, Stulberg SD, Sutherland CJ. Radiographic evaluation of a monoblock acetabular component: a multicenter study with 2- to 5-year results. J Arthroplasty. 2005;20:369–378. [DOI] [PubMed]
- 17.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty: an end result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed]
- 18.Hartofilakidis G, Stamos K, Karachalios T. Treatment of high dislocation of the hip in adults with total hip arthroplasty: operative technique and long-term clinical results. J Bone Joint Surg Am. 1998;80:510–517. [DOI] [PubMed]
- 19.Huk OL, Bansal M, Betts F, Rimnac CM, Lieberman JR, Huo MH, Salvati EA. Polyethylene and metal debris generated by non-articulating surfaces of modular acetabular components. J Bone Joint Surg Br. 1994;76:568–574. [PubMed]
- 20.Huo MH. What’s new in hip arthroplasty. J Bone Joint Surg Am. 2002;84:1894–1905. [DOI] [PubMed]
- 21.Kim YH, Kim JS. Total hip arthroplasty in adult patients who had developmental dysplasia of the hip. J Arthroplasty. 2005;20:1029–1036. [DOI] [PubMed]
- 22.Komarasamy B, Vadivelu R, Bruce A, Kershaw C, Davison J. Clinical and radiological outcome following total hip arthroplasty with an uncemented trabecular metal monoblock acetabular cup. Acta Orthop Belg. 2006;72:320–325. [PubMed]
- 23.Kurtz SM, Ochoa JA, White CV, Srivastav S, Cournoyer J. Backside nonconformity and locking restraints affect liner/shell load transfer mechanisms and relative motion in modular acetabular components for total hip replacement. J Biomech. 1998;31:431–437. [DOI] [PubMed]
- 24.Latimer HA, Lachiewicz PF. Porous-coated acetabular components with screw fixation: five to ten-year results. J Bone Joint Surg Am. 1996;78:975–981. [DOI] [PubMed]
- 25.Lewallen DG, Cabanela ME. Hybrid primary total hip arthroplasty: a 5- to 9-year followup study. Clin Orthop Relat Res. 1996;333:126–133. [DOI] [PubMed]
- 26.Macheras GA, Papagelopoulos PJ, Kateros K, Kostakos AT, Baltas D, Karachalios TS. Radiological evaluation of the metal-bone interface of a porous tantalum monoblock acetabular component. J Bone Joint Surg Br. 2006;88:304–309. [DOI] [PubMed]
- 27.Mont MA, Maar DC, Krackow KA, Jacobs MA, Jones LC, Hungerford DS. Total hip replacement without cement for non-inflammatory osteoarthrosis in patients who are less than forty-five years old. J Bone Joint Surg Am. 1993;75:740–751. [DOI] [PubMed]
- 28.Morscher EW. Current status of acetabular fixation in primary total hip arthroplasty. Clin Orthop Relat Res. 1992;274:172–193. [PubMed]
- 29.Mulier M, Rys B, Moke L. Hedrocel trabecular metal monoblock acetabular cups: mid-term results. Acta Orthop Belg. 2006;72:326–331. [PubMed]
- 30.Nunn D, Freeman MA, Hill PF, Evans SJ. The measurement of migration of the acetabular component of hip prostheses. J Bone Joint Surg Br. 1989;71:629–631. [DOI] [PubMed]
- 31.Salvati EA, Cornell CN. Long-term follow-up of total hip replacement in patients with avascular necrosis. Instr Course Lect. 1988;37:67–73. [PubMed]
- 32.Schmalzried TP, Jasty M, Harris WH. Periprosthetic bone loss in total hip arthroplasty: polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg Am. 1992;74:849–863. [PubMed]
- 33.Schmalzried TP, Wessinger SJ, Hill GE, Harris WH. The Harris-Galante porous acetabular component press-fit without screw fixation: five-year radiographic analysis of primary cases. J Arthroplasty. 1994;9:235–242. [DOI] [PubMed]
- 34.Sculco TP. The acetabular component: an elliptical monoblock alternative. J Arthroplasty. 2002;17(4 suppl 1):118–120. [DOI] [PubMed]
- 35.Udomkiat P, Dorr LD, Wan Z. Cementless hemispheric porous-coated sockets implanted with press-fit technique without screws: average ten-year follow-up. J Bone Joint Surg Am. 2002;84:1195–2000. [DOI] [PubMed]
- 36.Valle AGD, Zoppi A, Peterson MG, Salvati EA. Clinical and radiographic results associated with a modern, cementless modular cup design in total hip arthroplasty. J Bone Joint Surg Am. 2004;86:1998–2004. [DOI] [PubMed]
- 37.Wright JM, Pellicci PM, Salvati EA, Ghelman B, Roberts MM, Koh JL. Bone density adjacent to press-fit acetabular components: a prospective analysis with quantitative computed tomography. J Bone Joint Surg Am. 2001;83:529–536. [DOI] [PubMed]
- 38.Xenakis TA, Beris AE, Malizos KK, Koukoubis T, Gelalis J, Soucacos PN. Total hip arthroplasty for avascular necrosis and degenerative osteoarthritis of the hip. Clin Orthop Relat Res. 1997;341:62–68. [DOI] [PubMed]
- 39.Zicat B, Engh CA, Gokcen E. Patterns of osteolysis around total hip components inserted with and without cement. J Bone Joint Surg Am. 1995;77:432–439. [DOI] [PubMed]