Abstract
It is well established that glucocorticoid hormones strengthen the consolidation of hippocampus-dependent spatial and contextual memory. The present experiments investigated glucocorticoid effects on the long-term formation of conditioned taste aversion (CTA), an associative learning task that does not depend critically on hippocampal function. Corticosterone (1.0 or 3.0 mg/kg) administered subcutaneously to male Sprague–Dawley rats immediately after the pairing of saccharin consumption with the visceral malaise-inducing agent lithium chloride (LiCl) dose-dependently increased aversion to the saccharin taste on a 96-h retention test trial. In a second experiment, rats received corticosterone either immediately after saccharin consumption or after the LiCl injection, when both stimuli were separated by a 3-h time interval, to investigate whether corticosterone enhances memory of the gustatory or visceral stimulus presentation. Consistent with the finding that the LiCl injection, but not saccharin consumption, increases endogenous corticosterone levels, corticosterone selectively enhanced CTA memory when administered after the LiCl injection. Suppression of this training-induced release of corticosterone with the synthesis-inhibitor metyrapone (35 mg/kg) impaired CTA memory, and was dose-dependently reversed by post-training supplementation of corticosterone. Moreover, direct post-training infusions of corticosterone into the insular cortex or basolateral complex of the amygdala, two brain regions that are critically involved in the acquisition and consolidation of CTA, also enhanced CTA retention, whereas post-training infusions into the dorsal hippocampus were ineffective. These findings provide evidence that glucocorticoid effects on memory consolidation are not limited to hippocampus-dependent spatial/contextual information, but that these hormones also modulate memory consolidation of discrete-cue associative learning via actions in other brain regions.
Extensive evidence indicates that adrenocortical hormones (corticosterone in rodents, cortisol in humans) modulate the consolidation of lasting memories of emotionally arousing experiences (McGaugh and Roozendaal 2002). Particularly, glucocorticoids are known to enhance the consolidation of hippocampus-dependent spatial or contextual learning in rodents (Flood et al. 1978; Oitzl and de Kloet 1992; Roozendaal and McGaugh 1996, 1997a; Roozendaal et al. 1996b; Pugh et al. 1997; Cordero and Sandi 1998) and declarative information in humans (Buchanan and Lovallo 2001; Andreano and Cahill 2006; Kuhlmann and Wolf 2006). These findings are in accord with the evidence that the hippocampus has a high density of adrenal steroid receptors (McEwen et al. 1969; Reul and de Kloet 1985) and that glucocorticoids regulate several aspects of hippocampal neuroplasticity (Foy et al. 1987; Diamond et al. 1992; Pavlides et al. 1993; Xu et al. 1997; Korz and Frey 2003).
Findings from our laboratory further indicate that the basolateral complex of the amygdala (BLA) interacts with the hippocampus in mediating glucocorticoid effects on memory consolidation of spatial or contextual information. Lesions or functional inactivation of the BLA block enhancement of spatial memory induced by post-training systemic or intrahippocampal injections of glucocorticoids (Roozendaal and McGaugh 1996; Roozendaal et al. 1996b, 1999; Quirarte et al. 1997; Roozendaal and McGaugh 1997a), whereas direct infusions of glucocorticoids or glucocorticoid receptor (GR) antagonists administered into the BLA also modulate the consolidation of memory of spatial training (Roozendaal and McGaugh 1997b; Roozendaal et al. 2002).
Recent findings have suggested that glucocorticoids also strengthen the memory of emotionally arousing training experiences that do not depend critically on hippocampal functioning, such as object recognition, Pavlovian auditory-cue fear conditioning, and discrete-cue appetitive conditioning (Zorawski and Killcross 2002; Hui et al. 2004; Okuda et al. 2004; Roozendaal et al. 2006a, c). These findings suggest that glucocorticoids also act in other brain regions to modulate memory of other kinds of information. Conditioned taste aversion (CTA) is a discrete-cue associative learning task in which rats acquire a strong and long-lasting aversion to a novel taste when consumption of that taste is associated shortly afterward with the malaise-inducing drug lithium chloride (LiCl) (Bures et al. 1998). The acquisition and consolidation of CTA depend critically on an intact insular cortex (Bures et al. 1998; Welzl et al. 2001; Bermúdez-Rattoni 2004) and, like other kinds of emotionally arousing training, the consolidation is modulated by BLA activity (Miranda and McGaugh 2004). Findings of previous studies examining the role of hypothalamic-pituitary-adrenocortical hormones in CTA are inconsistent. Although adrenalectomy impairs acquisition of CTA (Peeters and Broekkamp 1994), some evidence suggests that systemic administration of the synthetic glucocorticoid dexamethasone to adrenally intact animals also attenuates CTA (Smotherman 1985). Other studies argued that the effects of glucocorticoids on CTA memory may be mediated indirectly by altering levels of adrenocorticotropin (Smotherman 1985).
The present study examined further the role of glucocorticoids in the formation of CTA memory. A first experiment investigated whether systemic injections of corticosterone administered immediately after contingent pairing of saccharin consumption with a mildly aversive dose of LiCl would facilitate later retention. Unlike most other associative learning tasks in which the unconditioned stimulus has to be presented in close proximity to the conditioning stimulus, an interesting and important feature of CTA is that rats acquire aversion to the taste even when the aversive stimulus is administered several hours later (Garcia et al. 1955; Schafe et al. 1995; Bures et al. 1998). This allows the examination of drug effects on the memory trace of the novel taste separate from that of the gastric malaise. Therefore, we also investigated whether systemic administration of corticosterone enhanced aversion when administered either after the novel saccharin taste or after the LiCl injection. Further, as LiCl is known to increase plasma levels of corticosterone and adrenocorticotropin (Hennessy et al. 1976; Smotherman et al. 1976a, b), we examined whether blockade of the endogenous corticosterone response with the synthesis-inhibitor metyrapone impaired memory formation of CTA. A last series of experiments examined whether glucocorticoids enhance CTA memory via direct actions in the insular cortex, BLA, or hippocampus.
Results
Post-training systemic injections of corticosterone enhance memory of taste aversion learning
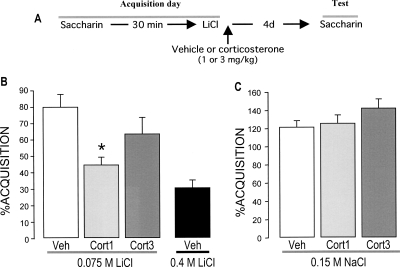
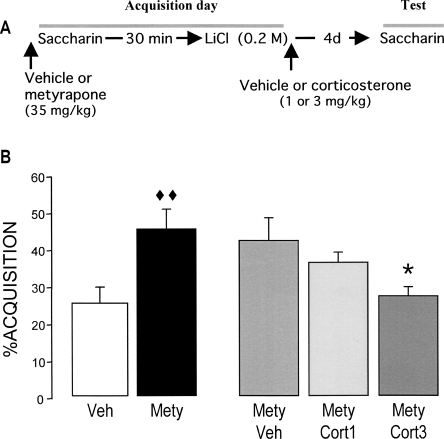
This experiment examined whether corticosterone (1.0 or 3.0 mg/kg, subcutaneously [s.c.]) administered immediately after CTA conditioning with an intraperitoneal injection of a mildly aversive dose of LiCl (0.075 M), 30 min after consuming a novel saccharin drinking solution, would enhance long-term retention of the training experience and, thus, resemble the stronger aversion produced by administration of a much higher molarity (0.4 M) of the aversive stimulant. A diagram of the experimental design is shown in Figure 1A.
Figure 1.
Effects of post-training systemic injections of corticosterone (1.0 or 3.0 mg/kg, s.c.) on taste aversion as assessed on a 96-h retention test trial. (A) Experimental design. (B) Post-training injections of corticosterone enhanced retention of taste aversion. (C) Corticosterone administration did not induce saccharin aversion in rats that were pseudo-conditioned with saline (0.15 M NaCl) rather than LiCl. The aversion index (mean + SEM) is expressed as the percent saccharin consumption on the retention test as compared with that during acquisition. (*) P < 0.05 as compared with the vehicle (Veh) control group (n = 11–13 rats per group). (Cort) Corticosterone.
The average water consumption of the different groups did not differ during baseline (17 ± 2 mL; data not shown). Also, the groups did not differ in saccharin consumption during acquisition, i.e., before drug treatment (data not shown). Figure 1B shows saccharin aversion (expressed as the percentage saccharin consumption during acquisition) on the 96-h retention test trial of rats given immediate post-training injections of either vehicle or corticosterone. Saccharin intake of vehicle-injected rats was only mildly reduced (76.8% compared with acquisition; paired t-test: P < 0.01), indicating a weak aversion. A one-way ANOVA for saccharin aversion on the retention test trial revealed a significant group effect (F(2,33) = 3.70; P < 0.05). Fisher’s post hoc analysis revealed that post-training injection of the lower dose of corticosterone (1.0 mg/kg) enhanced saccharin aversion relative to vehicle-treated rats (P < 0.05). Moreover, saccharin aversion of rats injected with this dose of corticosterone did not differ significantly from that of control rats conditioned with a highly aversive dose of LiCl (0.4 M) (unpaired t-test: P = 0.13). In contrast, the higher dose of corticosterone (3.0 mg/kg) did not significantly affect retention performance compared with the vehicle control group. Corticosterone (1.0 or 3.0 mg/kg) administration did not induce saccharin aversion in rats that were pseudo-conditioned with saline rather than LiCl (F(2,29) = 2.16; P = 0.13), indicating that corticosterone itself did not act as an aversive stimulus (Fig. 1C).
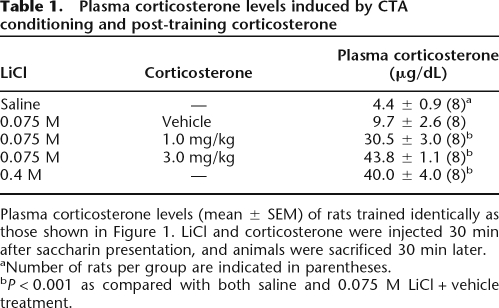
Plasma levels of corticosterone of rats treated identically to those of the behavioral experiment are shown in Table 1. Blood for the corticosterone assay was collected 30 min after the LiCl and corticosterone injections, at the peak of the corticosterone response. A one-way ANOVA revealed a significant group effect (F(4,35) = 46.39; P < 0.001). Post hoc tests indicated that plasma corticosterone levels of vehicle-injected rats conditioned with the low dose of LiCl (0.075 M) were not significantly elevated compared with those of rats that were pseudo-conditioned with saline (P = 0.16). As expected, corticosterone (1.0 or 3.0 mg/kg) injection dose-dependently elevated plasma corticosterone levels 30 min after conditioning (both, P < 0.001 compared with vehicle) and were within the same range as those found in rats conditioned with the highly aversive dose of LiCl (0.4 M) (1 mg/kg vs. LiCl [0.4 M]: P < 0.05; 3 mg/kg vs. LiCl [0.4 M]: P = 0.32).
Table 1.
Plasma corticosterone levels induced by CTA conditioning and post-training corticosterone
Plasma corticosterone levels (mean ± SEM) of rats trained identically as those shown in Figure 1. LiCl and corticosterone were injected 30 min after saccharin presentation, and animals were sacrificed 30 min later.
aNumber of rats per group are indicated in parentheses.
bP < 0.001 as compared with both saline and 0.075 M LiCl + vehicle treatment.
Corticosterone injections do not alter sensory-perceptual effects of the LiCl injection
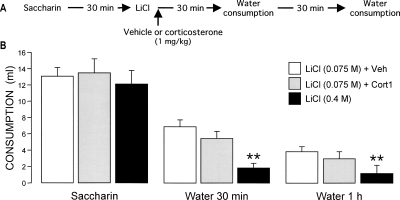
As indicated, extensive evidence indicates that post-training administration of glucocorticoids affects long-term retention via influences on the consolidation of the memory trace (McGaugh and Roozendaal 2002). However, although in the experiment above corticosterone was also administered post-conditioning, the corticosterone administration could have affected long-term memory by influencing sensory-perceptual aspects (i.e., painfulness) of the LiCl stimulus. To investigate this issue, we examined whether corticosterone increased either the severity or duration of the gastric malaise induced by LiCl, as assessed by reduced water consumption during recovery from illness. Water was presented to the rats at 30 min and 1 h after CTA conditioning with either a mildly (0.075 M) or highly aversive dose of LiCl (0.4 M) (Fig. 2A). As is shown in Figure 2B, two-way ANOVA of water consumption indicated significant treatment (F(2,21) = 15.56; P < 0.001) and time effects (F(1,21) = 42.40; P < 0.001) as well as a significant interaction between these two parameters (F(2,21) = 7.23 P < 0.005). Rats administered the high dose of LiCl consumed significantly less water at both 30 min and 1 h after the aversive stimulus presentation than did those injected with the lower dose of LiCl (both, P < 0.01), indicating that the higher dose of LiCl induced a stronger gastric malaise. Importantly, post-training administration of corticosterone, in a dose that enhanced CTA retention (1.0 mg/kg), did not reduce water intake during recovery compared with the water intake of vehicle-treated rats, suggesting that corticosterone does not increase either pain sensation or perception after LiCl treatment.
Figure 2.
Effects of post-training systemic injections of corticosterone (1.0 mg/kg, s.c.) on sensory-perceptual components of the LiCl, as assessed by water consumption during recovery from illness. (A) Experimental design. (B) Corticosterone (Cort) administration did not affect water consumption (mean + SEM) 30 min or 1 h after LiCl (0.075 M), whereas a high dose of LiCl (0.4 M) significantly reduced water intake. (**) P < 0.01 as compared with the LiCl 0.075 M + vehicle (Veh) group (n = 8–9 rats per group).
Corticosterone injection after the aversive stimulus, but not novel taste, enhances conditioned taste aversion learning
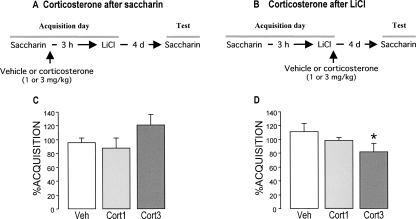
This experiment investigated whether corticosterone would enhance retention of CTA when administered systemically either after the novel taste consumption or after the LiCl injection to examine what aspects of the association are influenced by glucocorticoids. To avoid overlapping drug effects on both stimuli, the delay between saccharin intake and LiCl (0.075 M) injection was increased to 3 h, an interval shown sufficient for corticosterone levels to return to baseline (de Quervain et al. 1998; see Fig. 3A,B for experimental designs).
Figure 3.
Effects of post-training systemic injections of corticosterone (1.0 or 3.0 mg/kg, s.c.) on taste aversion when administered either after the consumption of saccharin or after the LiCl injection as assessed on a 96-h retention test trial. (A,B) Experimental design. (C,D) Corticosterone administration after the LiCl injection, but not after the saccharin consumption, enhanced taste aversion on a 96-h retention test. The aversion index (mean + SEM) is expressed as the percent saccharin consumption on the retention test as compared with that during acquisition. (*) P < 0.05 as compared with the vehicle (Veh) group (n = 12–14 rats per group).
The groups did not differ in baseline water intake and saccharin consumption during acquisition (data not shown). Figure 3C shows saccharin aversion on the retention test trial, 96 h after conditioning and drug treatment, of rats given vehicle or corticosterone immediately after the novel taste presentation. A one-way ANOVA revealed no significant group effect (F(2,35) = 0.94; P = 0.40), suggesting that corticosterone does not enhance memory of the novel taste experience or, alternatively, that an enhanced memory of the taste per se does not result in a greater aversion.
In contrast, a one-way ANOVA of saccharin consumption of rats injected with vehicle or corticosterone immediately after the LiCl treatment showed a significant group effect (F(2,34) = 3.55; P < 0.05). Fisher’s post hoc analyses indicated that the higher dose of corticosterone (3.0 mg/kg) significantly increased saccharin aversion compared with that of vehicle-treated rats (P < 0.05). The lower dose of corticosterone (1.0 mg/kg) did not significantly enhance aversion (Fig. 3D).
Blockade of endogenous corticosterone release during conditioning impairs taste aversion memory
The findings above indicate that corticosterone administered immediately after conditioning with a mildly aversive dose of LiCl enhanced retention of that experience. As the gastric malaise produced by LiCl is known to induce the release of corticosterone (current findings; Smotherman et al. 1976a, b; Smotherman 1985), this increased adrenocortical activity may normally play a role in consolidating the memory of taste aversion. This experiment examined whether blockade of the corticosterone stress response with the 11β-hydroxylase inhibitor metyrapone would impair the strong aversion produced with a high dose of LiCl (0.2 M). Metyrapone (35 mg/kg, s.c.) or vehicle was injected during acquisition 10 min before saccharin consumption (Fig. 4A). Additionally, vehicle or corticosterone (1.0 or 3.0 mg/kg) was administered immediately after the LiCl injection to examine whether glucocorticoid supplementation could attenuate the metyrapone effect.
Figure 4.
Effects of pretraining injection of the 11β-hydroxylase inhibitor metyrapone (35 mg/kg, s.c.) and immediate post-training injections of corticosterone (1.0 or 3.0 mg/kg, s.c.) on taste aversion as assessed on a 96-h retention test trial. (A) Experimental design. (B) Metyrapone (Mety) impaired CTA retention, an effect that was dose-dependently reversed by corticosterone. The aversion index (mean ± SEM) is expressed as the percent saccharin consumption on the retention test as compared with that during acquisition. (◆◆) P < 0.01 as compared with the vehicle (Veh) group; (*) P < 0.05 as compared with the Mety + Veh group (n = 15–16 rats per group).
Metyrapone treatment did not alter saccharin consumption during acquisition (P = 0.82; data not shown). As shown in Figure 4B, a one-way ANOVA of saccharin aversion on the 96-h retention test trial revealed a significant group effect (F(4,74) = 3.15; P < 0.05). Metyrapone treatment significantly reduced saccharin aversion relative to that of rats treated with vehicle (P < 0.01). This metyrapone-induced retention impairment was dose-dependently reversed by post-training supplementation of corticosterone (3.0 mg/kg; P < 0.05).
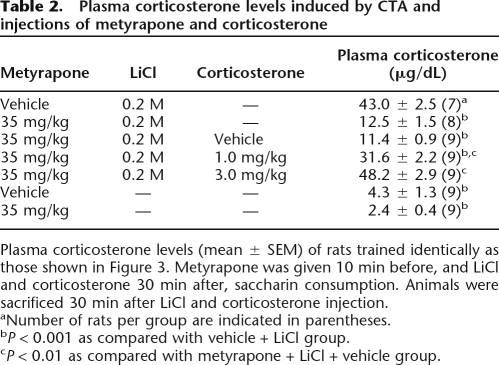
Table 2 shows plasma corticosterone levels of rats sacrificed 30 min after conditioning, following the same CTA procedure and drug treatment regimen used in the behavioral experiment. CTA conditioning with LiCl (0.2 M) significantly increased plasma corticosterone levels compared with those pseudo-conditioned with saline (P < 0.001). Metyrapone significantly attenuated this conditioning-induced increase in plasma corticosterone levels (P < 0.001), and exogenous supplementation of corticosterone (1.0 or 3.0 mg/kg) dose-dependently reversed this metyrapone-induced decrement (both, P < 0.001). Metyrapone alone did not significantly lower plasma corticosterone levels.
Table 2.
Plasma corticosterone levels induced by CTA and injections of metyrapone and corticosterone
Plasma corticosterone levels (mean ± SEM) of rats trained identically as those shown in Figure 3. Metyrapone was given 10 min before, and LiCl and corticosterone 30 min after, saccharin consumption. Animals were sacrificed 30 min after LiCl and corticosterone injection.
aNumber of rats per group are indicated in parentheses.
bP < 0.001 as compared with vehicle + LiCl group.
cP < 0.01 as compared with metyrapone + LiCl + vehicle group.
Post-training infusions of corticosterone into the insular cortex and BLA, but not hippocampus, enhance memory of taste aversion
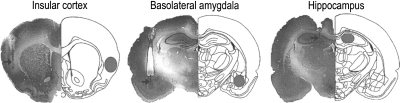
As indicated, the acquisition and consolidation of CTA, as well as that of several other forms of recognition memory, depend critically on the insular cortex (Bures et al. 1998; Welzl et al. 2001; Bermúdez-Rattoni 2004; Bermúdez-Rattoni et al. 2005) and is modulated by BLA activity (Miranda et al. 2003; Miranda and McGaugh 2004). In contrast, the hippocampus appears to be not or only marginally involved in memory formation of CTA (Smotherman et al. 1981). Although the insular cortex expresses a high density of adrenal steroid receptors (Morimoto et al. 1996), it is not known whether glucocorticoids act within this cortical region to enhance memory of CTA. Furthermore, although extensive evidence indicates that glucocorticoids infused post-training into the BLA enhance consolidation of hippocampus-dependent inhibitory avoidance memory and water-maze spatial memory (Roozendaal and McGaugh 1997b; Roozendaal et al. 2002), it is not known whether glucocorticoid administration into the BLA also enhances memory consolidation of CTA. This experiment investigated whether corticosterone administered bilaterally into the insular cortex, BLA, or hippocampus immediately after conditioning with a low dose of LiCl (0.075 M) would enhance retention of the CTA conditioning. Although the infusion volumes administered into the insular cortex (0.5 μL), BLA (0.2 μL), and hippocampus (0.5 μL) differed considering the size of each structure, the concentrations of corticosterone (5 or 10 ng/μL) administered into these brain regions were the same. Figure 5 shows representative infusion sites within these three brain regions.
Figure 5.
Representative photomicrographs and diagrams illustrating placement of cannulae and needle tips in the insular cortex, basolateral amygdala, and hippocampus. (Arrows) Point to the infusion needle tips; (right halves, gray shaded areas) the areas considered as correct infusion needle tip placements.
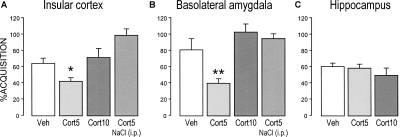
As in the abovementioned experiments, the various treatment groups did not differ in baseline water consumption and saccharin consumption on the acquisition trial (data not shown). As is shown in Figure 6, A and B, one-way ANOVAs for saccharin aversion on the 96-h retention test trial of rats infused with vehicle or corticosterone into either the insular cortex or BLA revealed significant drug effects (insular cortex, F(3,28) = 8.11; P < 0.01; BLA, F(3,35) = 6.15; P < 0.01). In both brain regions, post-training infusions of the lower dose of corticosterone (5 ng/μL) enhanced 96-h retention performance as indicated by a significantly increased saccharin aversion (insular cortex, P < 0.05; BLA, P < 0.01), whereas infusions of the higher dose were ineffective. This lower dose of corticosterone did not induce saccharin aversion by rats that were pseudo-conditioned with saline rather than LiCl. In contrast, one-way ANOVA of saccharin aversion on the 96-h retention test of rats infused with corticosterone (5 or 10 ng/μL) into the hippocampus did not reveal a significant group effect (F(2,26) = 0.60; P = 0.56; Fig. 6C).
Figure 6.
Effects of post-training infusions of corticosterone (5 or 10 ng/μL) administered into the insular cortex (A), BLA (B), or hippocampus (C) on taste aversion as assessed on a 96-h retention test trial. Corticosterone (Cort) infused into either the insular cortex or BLA, but not hippocampus, induced a significant enhancement of taste aversion. Corticosterone infusions into the insular cortex or BLA did not induce aversion when administered to rats that were pseudo-conditioned with saline. The aversion index (mean + SEM) is expressed as the percent saccharin consumption on the retention test as compared with that during acquisition. (*) P < 0.05, (**) P < 0.01 as compared with the corresponding vehicle (Veh) group (n = 8–9 rats per group).
Discussion
An involvement of glucocorticoids in the consolidation of hippocampus-dependent spatial and contextual memory is well established (Flood et al. 1978; Oitzl and de Kloet 1992; Roozendaal and McGaugh 1996, 1997a, b; Pugh et al. 1997; Cordero and Sandi 1998). Such findings are consistent with the evidence that glucocorticoids regulate several aspects of hippocampal plasticity (Foy et al. 1987; Diamond et al. 1992; Pavlides et al. 1993; Xu et al. 1997; Korz and Frey 2003). In part because of such a strong emphasis on hippocampal neuroplasticity and memory, less attention has been given to a possible involvement of glucocorticoids in regulating other kinds of emotionally influenced memory. Emerging evidence, however, indicates that glucocorticoids also enhance memory consolidation of tasks that do not depend, or depend to a lesser extent, on the hippocampus (Zorawski and Killcross 2002; Hui et al. 2004; Okuda et al. 2004; Roozendaal et al. 2006a, b; Medina et al. 2007). Further, there is evidence that glucocorticoids regulate neuroplasticity in other brain regions (e.g., the BLA and prefrontal cortex) (Karst et al. 2002; Maroun and Richter-Levin 2003; Duvarci and Paré 2007). The present findings indicate that corticosterone is involved in the consolidation of memory for CTA and that this modulating influence occurs via direct actions in both the insular cortex and BLA.
In the present experiments, corticosterone administered to rats immediately after CTA training enhanced long-term retention of the taste aversion memory, as indicated by an increased avoidance of the saccharin drinking solution on the retention test. As corticosterone did not enhance retention performance of rats that were pseudo-conditioned with saline instead of LiCl, corticosterone itself did not act as an aversive stimulus (Kent et al. 2002). These findings seem to conflict with early evidence that the synthetic glucocorticoid dexamethasone administered shortly before LiCl injection attenuated CTA (Smotherman 1985). It seems possible that, as with the high dose of corticosterone in the present study, the dose of dexamethasone used in the earlier study may have been too high. Although the mechanism underlying such an inverted-U dose-response relationship on memory consolidation is not known, it is a general phenomenon found with both systemic and local drug administration on a wide range of learning tasks. Further, the efficacy (and even the direction) of corticosterone effects on memory consolidation also depends on the robustness and aversiveness of the training experience (Sandi et al. 1997). Such findings may explain, at least in part, why a different dose of corticosterone enhanced memory consolidation when administered after a paired presentation of saccharin and LiCl than when both stimuli were separated by a 3-h delay. Furthermore, as modulation of memory consolidation depends on a complex, and only partially known, interplay between many hormones and neurotransmitters (McGaugh 2000), it is impossible to quantitatively compare memory effects of corticosterone following systemic administration with those of corticosterone evoked endogenously by highly aversive training in terms of plasma levels.
The use of post-training administration of corticosterone normally provides direct support for the view that the drug affected memory consolidation and that the retention performance was, thus, not confounded by possible effects on attentional, motivational, or sensory-perceptual mechanisms at the time of conditioning or test (McGaugh 1966). However, complicating this issue in CTA, the unconditioned stimulus of visceral malaise persists for 1–2 h after the LiCl injection, depending on the molarity of the LiCl solution, and thus is present after the corticosterone administration. Therefore, we investigated whether glucocorticoids affected sensory-perceptual effects of the visceral malaise (Kent et al. 2002) by examining water consumption of rats during illness at different time intervals shortly after LiCl injection. As rats injected with corticosterone or vehicle after conditioning with a low dose of LiCl consumed comparable amounts of water, but drank significantly more than did rats conditioned with a high dose of LiCl, these findings suggest that corticosterone did not influence CTA retention by modulating the painfulness of LiCl-induced visceral signals. Rather, they are consistent with the view that corticosterone, as found with all other learning tasks investigated to date, enhanced retention of CTA by facilitating time-dependent processes underlying the consolidation of the memory of the training.
As systemic injections of corticosterone enhanced CTA retention only when administered immediately after the LiCl injection, and not after the saccharin consumption, these findings indicate either that corticosterone did not enhance memory of the taste or that such an enhanced memory of the taste stimulus per se is insufficient to increase avoidance. Several studies have indicated that rats initially hesitate consuming a novel taste or food (i.e., neophobia), but increase intake of this food when there are no negative consequences (Bermúdez-Rattoni 2004). It has been reported that a “safe-signal” memory of the novel taste becomes stronger when the delay between the taste presentation and LiCl stimulus increases. Our finding that saccharin intake of rats administered the high dose of corticosterone immediately after saccharin consumption was slightly increased suggests that corticosterone may have enhanced memory of the taste but associated this memory with the initial safe signal. Such enhancement of taste memory is consistent with the finding of increased saccharin consumption in pseudo-conditioned rats given corticosterone (Kent et al. 2002).
Also consistent with previous findings (Smotherman et al. 1976a; Smotherman 1985), CTA conditioning induced a large and dose-dependent elevation in endogenous plasma corticosterone levels. Such findings suggest that increased adrenocortical function may normally play a role in the long-term formation of taste aversion memory. In support of this implication, the corticosterone synthesis-inhibitor metyrapone administered shortly before acquisition reduced the training-induced increase in corticosterone levels as well as impaired the retention of CTA. As post-training injections of corticosterone dose-dependently reversed the metyrapone-induced retention impairment, the memory-impairing effect of metyrapone was most likely directly related to the suppression of adrenocortical function (Roozendaal et al. 1996a). These findings are consistent with evidence that adrenalectomized mice exhibited an impaired acquisition of CTA that was attenuated by a single administration of either corticosterone or dexamethasone 5 min after LiCl injection (Peeters and Broekkamp 1994).
Although several studies have indicated that glucocorticoids influence memory consolidation in a wide variety of learning tasks, prior studies using local infusions have not examined whether glucocorticoids may modulate memory consolidation by acting in brain regions known to support learning and memory assessed in these different tasks. As indicated, CTA depends on a neural circuitry involving the insular cortex, also termed gustatory or taste cortex (Bermúdez-Rattoni 2004). Lesions or functional inactivation of the insular cortex block both the acquisition and retention of CTA (Nerad et al. 1996; Ramirez-Amaya et al. 1998; Cubero et al. 1999; Roman et al. 2006). The insular cortex is primarily involved in the association of the gustatory and visceral stimuli (Bermúdez-Rattoni 2004). The present findings indicate that glucocorticoids act in this brain region to influence CTA memory formation. Although most studies of the involvement of the insular cortex in memory have investigated taste aversion and taste recognition, there is some evidence that the insular cortex is also involved in memory that is not based on taste, including object recognition and inhibitory avoidance (Bermúdez-Rattoni and McGaugh 1991; Bermúdez-Rattoni et al. 1991, 1997, 2005). Such findings fit well with recent evidence of two brain imaging studies suggesting an involvement of the insular cortex in human face and tactile recognition (Paller et al. 2003; Reed et al. 2004). As glucocorticoids are known to also influence the consolidation of other forms of recognition memory (Okuda et al. 2004; Roozendaal et al. 2006c), the insular cortex (and possibly perirhinal cortex) may be a general target for glucocorticoids in influencing recognition memory consolidation. In support of this view, preliminary findings indicate that infusions of the specific GR agonist RU 28362 administered into the insular cortex immediately after object recognition training enhanced memory consolidation of this training (T. Bredy, B. Roozendaal, and J.L. McGaugh, unpubl.).
The finding that corticosterone administered into the hippocampus did not influence retention of CTA is consistent with several findings indicating that CTA memory does not depend critically on an intact hippocampus (Smotherman et al. 1981). However, it should be noted that several researchers have used CTA procedures (e.g., discrimination between different tastes using multiple bottle tests or aversion produced in a particular context) that are hippocampus-dependent (Best and Orr 1973; Krane et al. 1976).
The finding that corticosterone administered into the BLA after CTA conditioning enhanced retention is consistent with extensive evidence that the BLA is also critically involved in CTA (Miranda et al. 2002, 2003). Functional inactivation studies have suggested that the BLA is particularly involved in processing of the aversive visceral signals, i.e., gastric malaise, and not that of the more emotionally neutral taste signal (Gallo et al. 1992). Furthermore, evidence indicates that the BLA interacts with the insular cortex in memory formation of CTA. For example, asymmetrical unilateral infusions of the neurotoxin tetrodotoxin administered into the insular cortex and BLA completely blocked acquisition of CTA, whereas unilateral inactivation of these two brain regions in the same hemisphere was without effect (Bielavska and Roldan 1996). Moreover, unilateral infusions of the cyclic AMP analog 8-bromo-cAMP infused into the insular cortex enhanced CTA, but this effect was blocked by ipsilateral infusions of the β-adrenoceptor antagonist propranolol into the BLA (Miranda and McGaugh 2004). Such an interaction of the BLA with the insular cortex is consistent with extensive evidence implicating the BLA in regulating memory consolidation of other emotionally arousing learning tasks via direct interactions with a wide variety of other brain regions (McGaugh 2002). For example, a GR agonist or antagonist infused into the BLA modulates memory consolidation of inhibitory avoidance, contextual fear conditioning, and water-maze spatial leaning (Roozendaal and McGaugh 1997b; Donley et al. 2005). Glucocorticoid effects on the consolidation of memory for emotionally arousing experiences are intimately linked to noradrenergic activation in the BLA. A β-adrenoceptor antagonist infused into the BLA block memory enhancement induced by glucocorticoids administered systemically or into specific brain regions on inhibitory avoidance, auditory-cue fear conditioning, and object recognition tasks (Quirarte et al. 1997; Roozendaal et al. 2002, 2006a, c). Recent findings of animal and human studies investigating the effects of adrenocortical hormones on memory consolidation strongly suggest that glucocorticoids selectively enhance memory of emotionally arousing experiences (Buchanan and Lovallo 2001; Okuda et al. 2004; Abercrombie et al. 2006; Kuhlmann and Wolf 2006; van Stegeren et al. 2007) because of a critical dependence on training-induced noradrenergic activation within the BLA (Roozendaal et al. 2006b, c).
In conclusion, these data confirm the evidence from many different types of experiments that adrenal stress hormones, released during or after emotionally arousing experiences, play a critical role in consolidating lasting memories. The present findings provide strong evidence for the view that glucocorticoid effects on memory consolidation are not restricted to facilitating memory of context-dependent training but are involved in consolidating memory of many different kinds of emotionally arousing learning experiences.
Materials and Methods
Subjects
Male adult Sprague–Dawley rats (300–350 g) were kept individually in a temperature-controlled (22°C) colony room and maintained on a standard 12-h light:12-h dark cycle (07:00–19:00 h lights on) with ad libitum access to food and water. They were kept in these conditions for at least 5 d until commencement of the experiments. Training and testing were performed during the light phase of the cycle (between 10:00 and 13:00 h), at the rat nadir of the diurnal rhythm of corticosterone. All experimental procedures were in compliance with the NIH Guide for Care and Use of Laboratory Animals and Rules in Health Matters (Ministry of Health, Mexico) and approved by the University of California, Irvine’s Institutional Animal Care and Use Committee as well as the Animal Ethics Committee of Instituto de Neurobiología, Universidad Nacional Autónoma de México.
Taste aversion conditioning and test procedures
The rats were initially deprived of water for 24 h and then habituated to drink water from a graduated bottle for 20 min/d for 5 d. After a stable water consumption baseline was reached, the animals were randomly divided into several treatment groups and acquisition of CTA was performed. For conditioning, the rats were allowed to freely drink a 0.1% saccharin solution from a graduated bottle for 20 min in their home cages, and the volume drunk was recorded. Thirty min or 3 h after completion of saccharin intake, the animals were injected intraperitoneally (i.p.) with LiCl (3 mL), in either a low dose (0.075 M in distilled water) that produces only mild CTA or higher doses (0.2 or 0.4 M in distilled water) known to induce robust CTA (Miranda et al. 2002). For the next 3 d, water baselines were recorded. Ninety-six hours after conditioning, a 0.1% saccharin solution was presented for 20 min to test for retention of the acquired taste aversion (i.e., single bottle test). An aversion index was calculated as the percentage saccharin intake on the retention test as compared with that ingested during the acquisition trial.
Drugs and systemic injection procedures
For systemic injections, corticosterone (1.0 or 3.0 mg/kg; Sigma) was administered subcutaneously in a volume of 2.0 mL/kg immediately after either saccharin consumption or LiCl injection. Corticosterone was first dissolved in 100% ethanol and then diluted in 0.9% saline to reach its appropriate concentration. The final concentration of ethanol was 5%. The vehicle solution contained 5% ethanol in saline only. These doses were selected on the basis of previous experiments (de Quervain et al. 1998).
For adrenocortical suppression, the 11β-hydroxylase inhibitor metyrapone (2-methyl-1,2-di-3-pyridyl-1-propanone; 35 mg/kg; Sigma) was injected in a volume of 2.0 mL/kg on the acquisition trial 10 min before saccharin consumption. The drug was first dissolved in polyethylene glycol and subsequently diluted with 0.9% saline to reach the appropriate concentration. The final concentration of polyethylene glycol was 40%. The vehicle control contained the 40% polyethylene glycol in saline only. All rats were handled for 1 min on the 3 d prior to acquisition and were held gently during the injections.
Surgery for cannula implantation
The rats were anesthetized with sodium pentobarbital (50 mg/kg body weight, i.p.) and given atropine sulfate (0.4 mg/kg, i.p.) to maintain respiration. The skull was positioned in a stereotaxic frame and two 23-gauge stainless-steel guide cannulae were implanted bilaterally, aimed 2.0 mm above the insular cortex (12-mm long; coordinates: anteroposterior [AP], +1.2 mm relative to Bregma; mediolateral [ML], ±5.5 mm from midline; dorsoventral [DV], 3.0 mm below dura), the BLA (15-mm long; AP, −2.8 mm; ML, ±5.0 mm; DV, −5.5 mm), or the dorsal hippocampus (11-mm long AP, −3.4 mm; ML, ±1.5 mm; DV, −1.4 mm). The coordinates were based on the atlas of Paxinos and Watson (2005). The cannulae were fixed to the skull with dental acrylic cement and two surgical screws. Stylets were inserted into the guide cannulae to prevent clogging. The rats were allowed to recover for a minimum of 7 d before initiation of training and were handled three times for 1 min each during this recovery period to accustom them to the infusion procedure.
Drugs and infusion procedures
Corticosterone (5 or 10 ng/μL; Sigma) was first dissolved in 100% ethanol and subsequently diluted in saline to reach the appropriate concentration. The final concentration of ethanol was 2%. Drug doses were chosen on the basis of our previous findings (Medina et al. 2007). Bilateral infusions of drug, or an equivalent volume of vehicle, were made by using 30-gauge injection needles connected to a 10-μL Hamilton microsyringe by polyethylene (PE-20) tubing, driven by an automated microinfusion pump (Carnegie Medicine). The injection needles protruded 2.0 mm beyond the tip of the cannula, and an injection volume of 0.2 μL per hemisphere for the BLA or 0.5 μL per side for the insular cortex and hippocampus was delivered over 1 min. The injection needles were retained within the cannulae for an additional minute following drug infusion to maximize diffusion and prevent dragging along the cannula track. The infusion volume for the BLA was based on findings that this volume of an excitotoxin administered at identical injection sites induces selective lesions of the BLA (Roozendaal and McGaugh 1996). Furthermore, drug infusions of this volume into either the BLA or adjacent central nucleus of the amygdala induce differential effects on memory consolidation (Parent and McGaugh 1994; Quirarte et al. 1997; Roozendaal and McGaugh 1997b; Bahar et al. 2003). The infusion volume for the insular cortex was based on previous findings indicating that infusion of this volume into the insular cortex, but not into the parietal cortex located above the insular cortex, modulates memory consolidation (Bermúdez-Rattoni et al. 2005).
Corticosterone assay
Thirty min after conditioning and drug administration, some animals were injected with an overdose of sodium pentobarbital and decapitated within 90 sec of the injection. Trunk blood was collected in heparinized (500 units/mL) tubes and stored on ice. After centrifugation at 4500g for 10 min, the supernatant was stored at −80°C until assay. Corticosterone plasma concentrations were determined by a commercially available enzyme immunoassay kit, using a 96-well microtiter plate coated with polyclonal antibody raised against corticosterone (Alpco). The absorbance levels were measured with a photometric microplate reader (Thermo Labsystems) at 450 nm. The sensitivity was 0.023 μg/dL, and the coefficients of variation within and between assays were <10%.
Histology
After completion of the behavioral experiments, cannulated rats were overdosed with sodium pentobarbital, and intracardially perfused with 0.9% saline. After decapitation, the brains were removed and stored in a 4% paraformaldehyde solution for 24 h at 4°C. The brains were then immersed in a 30% glucose solution at 4°C. Coronal slices (50 μm thick) were mounted on gelatin-coated glass slides and stained with cresyl violet. The sections were examined under a light microscope and determination of the location of injection needle tips in the target areas was made according to the standardized atlas plates of Paxinos and Watson (2005) by an observer blind to drug treatment condition. When the injection needle tip was not visible in the histological material, the location was extrapolated 2.0 mm from the guide cannula tip. A total of 38 rats with injection needle placements outside the target area or with extensive tissue damage at the injection needle tips was excluded from analysis.
Statistics
The aversion index (percentage of saccharin consumption during retention compared with acquisition) was analyzed using one-way ANOVAs for groups followed by pairwise Fisher post hoc tests, when appropriate. Sensory-perceptual data were analyzed with a two-way ANOVA with the factor time as a repeated measure. For all comparisons, a probability level of <0.05 was accepted as statistical significance. The number of rats per group is indicated in the figure legends.
Acknowledgments
Research was supported by United States Public Health Service Grant MH12526 from the National Institute of Mental Health (J.L.M.), National Science Foundation Grant IOB-0618211 (B.R.), PAPIIT IN201308, CONACyT C54524, 46161 M (M.I.M.), and 46754 Q (M.I.M. and G.L.Q). We thank Dr. Andrea C. Medina for preparation of the histology figure and Ana Yris Guzman, Oscar González, Julian Reyes-López, Angel Méndez, Norma Serafín, Mireya Romero, and Martín García Servin for technical assistance.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.964708.
References
- Abercrombie H.C., Speck N.S., Monticelli R.A. Endogenous cortisol elevations are related to memory facilitation only in individuals who are emotionally aroused. Psychoneuroendocrinology. 2006;31:187–196. doi: 10.1016/j.psyneuen.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Andreano J.M., Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychol. Sci. 2006;17:466–470. doi: 10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Bahar A., Samuel A., Hazvi S., Dudai Y. The amygdalar circuit that acquires taste aversion memory differs from the circuit that extinguishes it. Eur. J. Neurosci. 2003;17:1527–1530. doi: 10.1046/j.1460-9568.2003.02551.x. [DOI] [PubMed] [Google Scholar]
- Bermúdez-Rattoni F. Molecular mechanisms of taste-recognition memory. Nat. Rev. Neurosci. 2004;5:209–217. doi: 10.1038/nrn1344. [DOI] [PubMed] [Google Scholar]
- Bermúdez-Rattoni F., McGaugh J.L. Insular cortex and amygdala lesions differentially affect acquisition on inhibitory avoidance and conditioned taste aversion. Brain Res. 1991;549:165–170. doi: 10.1016/0006-8993(91)90616-4. [DOI] [PubMed] [Google Scholar]
- Bermúdez-Rattoni F., Introini-Collison I.B., McGaugh J.L. Reversible inactivation of the insular cortex by tetrodotoxin produces retrograde and anterograde amnesia for inhibitory avoidance and spatial learning. Proc. Natl. Acad. Sci. 1991;88:5379–5382. doi: 10.1073/pnas.88.12.5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermúdez-Rattoni F., Introini-Collison I., Coleman-Mesches K., McGaugh J.L. Insular cortex and amygdala lesions induced after aversive training impair retention: Effects of degree of training. Neurobiol. Learn. Mem. 1997;67:57–63. doi: 10.1006/nlme.1996.3747. [DOI] [PubMed] [Google Scholar]
- Bermúdez-Rattoni F., Okuda S., Roozendaal B., McGaugh J.L. Insular cortex is involved in consolidation of object recognition memory. Learn. Mem. 2005;12:447–449. doi: 10.1101/lm.97605. [DOI] [PubMed] [Google Scholar]
- Best P.J., Orr J. Effects of hippocampal lesions on passive avoidance and taste aversion conditioning. Physiol. Behav. 1973;10:193–196. doi: 10.1016/0031-9384(73)90296-5. [DOI] [PubMed] [Google Scholar]
- Bielavska E., Roldan G. Ipsilateral connections between the gustatory cortex, amygdala and parabrachial nucleus are necessary for acquisition and retrieval of conditioned taste aversion in rats. Behav. Brain Res. 1996;81:25–31. doi: 10.1016/s0166-4328(96)00039-3. [DOI] [PubMed] [Google Scholar]
- Buchanan T.W., Lovallo W.R. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- Bures J., Bermudez-Rattoni F., Yamamoto T. The CTA paradigm. In: Bures J., et al., editors. Conditioned taste aversion, memory of a special kind. Oxford University Press; Oxford: 1998. pp. 1–25. [Google Scholar]
- Cordero M.I., Sandi C. A role for brain glucocorticoid receptors in contextual fear conditioning: Dependence upon training intensity. Brain Res. 1998;786:11–17. doi: 10.1016/s0006-8993(97)01420-0. [DOI] [PubMed] [Google Scholar]
- Cubero I., Thiele T.E., Bernstein I.L. Insular cortex lesions and taste aversion learning: Effects of conditioning method and timing of lesion. Brain Res. 1999;839:323–330. doi: 10.1016/s0006-8993(99)01745-x. [DOI] [PubMed] [Google Scholar]
- de Quervain D.J.-F., Roozendaal B., McGaugh J.L. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Diamond D.M., Bennett M.C., Fleshner M., Rose G.M. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Donley M.P., Schulkin J., Rosen J.B. Glucocorticoid receptor antagonism in the basolateral amygdala and ventral hippocampus interferes with long-term memory of contextual fear. Behav. Brain Res. 2005;164:197–205. doi: 10.1016/j.bbr.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Duvarci S., Paré D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J. Neurosci. 2007;27:4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood J.F., Vidal D., Bennett E.L., Orme A.E., Vasquez S., Jarvik M.E. Memory facilitating and anti-amnesic effects of corticosteroids. Pharmacol. Biochem. Behav. 1978;8:81–87. doi: 10.1016/0091-3057(78)90127-2. [DOI] [PubMed] [Google Scholar]
- Foy M.R., Stanton M.E., Levine S., Thompson R.F. Behavioral stress impairs long-term potentiation in rodent hippocampus. Behav. Neural Biol. 1987;48:138–149. doi: 10.1016/s0163-1047(87)90664-9. [DOI] [PubMed] [Google Scholar]
- Gallo M., Roldan G., Bures J. Differential involvement of gustatory insular cortex and amygdala in the acquisition and retrieval of conditioned taste aversion in rats. Behav. Brain Res. 1992;52:91–97. doi: 10.1016/s0166-4328(05)80328-6. [DOI] [PubMed] [Google Scholar]
- Garcia J., Kimeldorf D.J., Koelling R.A. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–158. [PubMed] [Google Scholar]
- Hennessy J.W., Smotherman W.P., Levine S. Conditioned taste aversion and the pituitary-adrenal system. Behav. Biol. 1976;16:413–424. doi: 10.1016/s0091-6773(76)91571-6. [DOI] [PubMed] [Google Scholar]
- Hui G.K., Figueroa I.R., Poytress B.S., Roozendaal B., McGaugh J.L., Weinberger N.M. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiol. Learn. Mem. 2004;81:67–74. doi: 10.1016/j.nlm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Karst H., Nair S., Velzing E., Rumpff-van Essen L., Slagter E., Shinnick-Gallagher P., Joëls M. Glucocorticoids alter calcium conductance and calcium channel subunit expression in basolateral amygdala neurons. Eur. J. Neurosci. 2002;16:1083–1089. doi: 10.1046/j.1460-9568.2002.02172.x. [DOI] [PubMed] [Google Scholar]
- Kent W.D., Cross-Mellor S.K., Kavaliers M., Ossenkopp K.P. Acute effects of corticosterone on LiCl-induced rapid gustatory conditioning in rats: A microstructural analysis of licking patterns. Behav. Brain Res. 2002;136:143–150. doi: 10.1016/s0166-4328(02)00105-5. [DOI] [PubMed] [Google Scholar]
- Korz V., Frey J.U. Stress-related modulation of hippocampal long-term potentiation in rats: Involvement of adrenal steroid receptors. J. Neurosci. 2003;23:7281–7287. doi: 10.1523/JNEUROSCI.23-19-07281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krane R.V., Sinnamon H.M., Thomas G.J. Conditioned taste aversions and neophobia in rats with hippocampal lesions. J. Comp. Physiol. Psychol. 1976;90:680–693. doi: 10.1037/h0077236. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S., Wolf O.T. Arousal and cortisol interact in modulating memory consolidation in healthy young men. Behav. Neurosci. 2006;120:217–223. doi: 10.1037/0735-7044.120.1.217. [DOI] [PubMed] [Google Scholar]
- Maroun M., Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J. Neurosci. 2003;23:4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Weiss J.M., Schwartz L.S. Uptake of corticosterone by rat brain and its concentration by certain limbic structures. Brain Res. 1969;16:227–241. doi: 10.1016/0006-8993(69)90096-1. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L. Time-dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L. Memory: A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L. Memory consolidation and the amygdala: A systems perspective. Trends Neurosci. 2002;25:456–461. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L., Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr. Opin. Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- Medina A.C., Charles J.R., Espinoza-Gonzalez V., Sanchez-Resendis O., Prado-Alcala R.A., Roozendaal B., Quirarte G.L. Glucocorticoid administration into the dorsal striatum facilitates memory consolidation of inhibitory avoidance training but not of the context or footshock components. Learn. Mem. 2007;14:673–677. doi: 10.1101/lm.654407. [DOI] [PubMed] [Google Scholar]
- Miranda M.I., McGaugh J.L. Enhancement of inhibitory avoidance and conditioned taste aversion memory with insular cortex infusions of 8-Br-cAMP: Involvement of the basolateral amygdala. Learn. Mem. 2004;11:312–317. doi: 10.1101/lm.72804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M.I., Ferreira G., Ramirez-Lugo L., Bermúdez-Rattoni F. Glutamatergic activity in the amygdala signals visceral input during taste memory formation. Proc. Natl. Acad. Sci. 2002;99:11417–11422. doi: 10.1073/pnas.182200499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda M.I., LaLumiere R.T., Buen T.V., Bermúdez-Rattoni F., McGaugh J.L. Blockade of noradrenergic receptors in the basolateral amygdala impairs taste memory. Eur. J. Neurosci. 2003;18:2605–2610. doi: 10.1046/j.1460-9568.2003.03008.x. [DOI] [PubMed] [Google Scholar]
- Morimoto M., Morita N., Ozawa H., Yokoyama K., Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: An immunohistochemical and in situ hybridization study. Neurosci. Res. 1996;26:235–269. doi: 10.1016/s0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- Nerad L., Ramirez-Amaya V., Ormsby C.E., Bermúdez-Rattoni F. Differential effects of anterior and posterior insular cortex lesions on the acquisition of conditioned taste aversion and spatial learning. Neurobiol. Learn. Mem. 1996;66:44–50. doi: 10.1006/nlme.1996.0042. [DOI] [PubMed] [Google Scholar]
- Oitzl M.S., de Kloet E.R. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav. Neurosci. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- Okuda S., Roozendaal B., McGaugh J.L. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc. Natl. Acad. Sci. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller K.A., Ranganath C., Gonsalves B., LaBar K.S., Parrish T.B., Gitelman D.R., Mesulam M.M., Reber P.J. Neural correlates of person recognition. Learn. Mem. 2003;10:253–260. doi: 10.1101/lm.57403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent M.B., McGaugh J.L. Post-training infusion of lidocaine into the amygdala basolateral complex impairs retention of inhibitory avoidance training. Brain Res. 1994;661:97–103. doi: 10.1016/0006-8993(94)91186-x. [DOI] [PubMed] [Google Scholar]
- Pavlides C., Watanabe Y., McEwen B.S. Effects of glucocorticoids on hippocampal long-term potentiation. Hippocampus. 1993;3:183–192. doi: 10.1002/hipo.450030210. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 2005. [DOI] [PubMed] [Google Scholar]
- Peeters B.W.M.M., Broekkamp C.L.E. Involvement of corticosteroids in the processing of stressful life-events a possible implication for the development of depression. J. Steroid Biochem. Mol. Biol. 1994;49:417–427. doi: 10.1016/0960-0760(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Pugh C.R., Fleshner M., Rudy J.W. Type II glucocorticoid receptor antagonists impair contextual but not auditory-cue fear conditioning in juvenile rats. Neurobiol. Learn. Mem. 1997;67:75–79. doi: 10.1006/nlme.1996.3741. [DOI] [PubMed] [Google Scholar]
- Quirarte G.L., Roozendaal B., McGaugh J.L. Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proc. Natl. Acad. Sci. 1997;94:14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V., Alvarez-Borda B., Bermúdez-Rattoni F. Differential effects of NMDA-induced lesions into the insular cortex and amygdala on the acquisition and evocation of conditioned immunosuppression. Brain Behav. Immun. 1998;12:149–160. doi: 10.1006/brbi.1998.0518. [DOI] [PubMed] [Google Scholar]
- Reed C.L., Shoham S., Halgren E. Neural substrates of tactile object recognition: An fMRI study. Hum. Brain Mapp. 2004;21:236–246. doi: 10.1002/hbm.10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul J.M., de Kloet E.R. Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Roman C., Nebieridze N., Sastre A., Reilly S. Effects of lesions of the bed nucleus of the stria terminalis, lateral hypothalamus, or insular cortex on conditioned taste aversion and conditioned odor aversion. Behav. Neurosci. 2006;120:1257–1267. doi: 10.1037/0735-7044.120.6.1257. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., McGaugh J.L. Amygdaloid nuclei lesions differentially affect glucocorticoid-induced memory enhancement in an inhibitory avoidance task. Neurobiol. Learn. Mem. 1996;65:1–8. doi: 10.1006/nlme.1996.0001. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., McGaugh J.L. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. Eur. J. Neurosci. 1997a;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., McGaugh J.L. Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiol. Learn. Mem. 1997b;67:176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., Bohus B., McGaugh J.L. Dose-dependent suppression of adrenocortical activity with metyrapone: Effects on emotion and memory. Psychoneuroendocrinology. 1996a;21:681–693. doi: 10.1016/s0306-4530(96)00028-5. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., Portillo-Marquez G., McGaugh J.L. Basolateral amygdala lesions block glucocorticoid-induced modulation of memory for spatial learning. Behav. Neurosci. 1996b;110:1074–1083. doi: 10.1037//0735-7044.110.5.1074. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., Nguyen B.T., Power A.E., McGaugh J.L. Basolateral amygdala noradrenergic influence on the memory-enhancing effect of glucocorticoid receptor activation in the hippocampus. Proc. Natl. Acad. Sci. 1999;96:11642–11647. doi: 10.1073/pnas.96.20.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B., Quirarte G.L., McGaugh J.L. Glucocorticoids interact with the basolateral amygdala β-adrenoceptor–cAMP/PKA system in influencing memory consolidation. Eur. J. Neurosci. 2002;15:553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., Hui G.K., Hui I.R., Berlau D.J., McGaugh J.L., Weinberger N.M. Basolateral amygdala noradrenergic activity mediates corticosterone-induced enhancement of auditory fear conditioning. Neurobiol. Learn. Mem. 2006a;86:249–255. doi: 10.1016/j.nlm.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., Okuda S., de Quervain D.J.-F., McGaugh J.L. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006b;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., Okuda S., Van der Zee E.A., McGaugh J.L. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc. Natl. Acad. Sci. 2006c;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C., Loscertales M., Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur. J. Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Schafe G.E., Sollars S.I., Bernstein I.L. The CS–US interval and taste aversion learning: A brief look. Behav. Neurosci. 1995;109:799–802. [PubMed] [Google Scholar]
- Smotherman W.P. Glucocorticoid and other hormonal substrates of conditioned taste aversion. Ann. N. Y. Acad. Sci. 1985;443:126–144. doi: 10.1111/j.1749-6632.1985.tb27068.x. [DOI] [PubMed] [Google Scholar]
- Smotherman W.P., Hennessy J.W., Levine S. Plasma corticosterone levels as an index of the strength of illness induced taste aversions. Physiol. Behav. 1976a;17:903–908. doi: 10.1016/0031-9384(76)90006-8. [DOI] [PubMed] [Google Scholar]
- Smotherman W.P., Hennessy J.W., Levine S. Plasma corticosterone levels during recovery from LiCl produced taste aversions. Behav. Biol. 1976b;16:401–412. doi: 10.1016/s0091-6773(76)91555-8. [DOI] [PubMed] [Google Scholar]
- Smotherman W.P., Kolp L.A., Coyle S., Levine S. Hippocampal lesion effects on conditioned taste aversion and pituitary-adrenal activity in rats. Behav. Brain Res. 1981;2:33–48. doi: 10.1016/0166-4328(81)90037-1. [DOI] [PubMed] [Google Scholar]
- van Stegeren A.H., Wolf O.T., Everaerd W., Scheltens P., Barkhof F., Rombouts S.A.R.B. Endogenous cortisol level interacts with noradrenergic activation in the human amygdala. Neurobiol. Learn. Mem. 2007;87:57–66. doi: 10.1016/j.nlm.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Welzl H., D’Adamo P., Lipp H.P. Conditioned taste aversion as a learning and memory paradigm. Behav. Brain Res. 2001;125:205–213. doi: 10.1016/s0166-4328(01)00302-3. [DOI] [PubMed] [Google Scholar]
- Xu L., Anwyl R., Rowan M.J. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- Zorawski M., Killcross S. Post-training glucocorticoid receptor agonist enhances memory in appetitive and aversive pavlovian discrete-cue conditioning paradigms. Neurobiol. Learn. Mem. 2002;78:458–464. doi: 10.1006/nlme.2002.4075. [DOI] [PubMed] [Google Scholar]