Abstract
Down syndrome (DS) is a genetic disorder arising from the presence of a third copy of the human chromosome 21 (Hsa21). Recently, O’Doherty and colleagues in an earlier study generated a new genetic mouse model of DS (Tc1) that carries an almost complete Hsa21. Since DS is the most common genetic cause of mental retardation, we have undertaken a detailed analysis of cognitive function and synaptic plasticity in Tc1 mice. Here we show that Tc1 mice have impaired spatial working memory (WM) but spared long-term spatial reference memory (RM) in the Morris watermaze. Similarly, Tc1 mice are selectively impaired in short-term memory (STM) but have intact long-term memory (LTM) in the novel object recognition task. The pattern of impaired STM and normal LTM is paralleled by a corresponding phenotype in long-term potentiation (LTP). Freely-moving Tc1 mice exhibit reduced LTP 1 h after induction but normal maintenance over days in the dentate gyrus of the hippocampal formation. Biochemical analysis revealed a reduction in membrane surface expression of the AMPAR (α-amino-3-hydroxy-5-methyl-4-propionic acid receptor) subunit GluR1 in the hippocampus of Tc1 mice, suggesting a potential mechanism for the impairment in early LTP. Our observations also provide further evidence that STM and LTM for hippocampus-dependent tasks are subserved by parallel processing streams.
Down syndrome (DS) results from trisomy of human chromosome 21 (Hsa21) (Lejeune et al. 1959) and is the most common genetically defined cause of mental retardation. Cognitive impairment in individuals with DS is characterized by developmental delay and deficits in language and memory (for reviews, see Chapman and Hesketh 2000; Silverman 2007). Although Hsa21 has been sequenced (Hattori et al. 2000), the functions of many of the Hsa21 genes are unknown, and their contributions to mental retardation remain to be clarified.
The Tc1 mouse is the most complete animal model for DS currently available. Tc1 mice are trisomic for ∼92% of Hsa21 (O’Doherty et al. 2005). They not only exhibit many aspects of human DS but also recapitulate several of the DS features present in other mouse models (Reeves 2006). Tc1 mice show alterations in cerebellar neuronal number, heart development, and mandible size. In addition, they have impaired short-term recognition memory and display reduced long-term potentiation (LTP) in the dentate gyrus of the hippocampus of the anesthetized animal (O’Doherty et al. 2005). The hippocampus is critically important for episodic memory processing in humans (for review, see Squire 1992) and various forms of spatial and nonspatial learning in rodents (Morris et al. 1982; Phillips and LeDoux 1992; Kim et al. 1995). The hippocampus is among the most severely and consistently affected cortical structures in DS (Aylward et al. 1999; Pinter et al. 2001; Pennington et al. 2003).
In order to provide insights into the neurobiology of DS, we have undertaken a more detailed analysis of synaptic plasticity and cognition in Tc1 mice. In 1966, McGaugh (1966) proposed three distinct systems for encoding memory: Immediate memory, lasting seconds to a few minutes (usually now called working memory [WM]) (Izquierdo et al. 1999); short-term memory (STM), which develops in a few seconds or minutes and lasts for several hours; and long-term memory (LTM), which consolidates slowly and lasts at least 24 h. WM is defined as an online, nonarchival system (Goldman-Rakic 1996). STM and LTM are, in contrast, systems whose primary roles are to preserve memories for use offline. To investigate WM, STM, and LTM in Tc1 mice, animals were tested in the Morris watermaze and the novel object recognition task. A key feature that qualifies LTP as a potential memory mechanism is its persistence over time (Hebb 1949; Bliss and Collingridge 1993; Abraham et al. 2002). Studies of the duration of LTP have shown that it has the potential to support memories ranging from the short-term to the very long-term. With this in mind we induced LTP in awake, freely-moving Tc1 and wild-type (WT) mice. The freely-moving animal is the preferred choice because it allows a longitudinal assessment of the persistence of LTP over time scales comparable with those necessary for the acquisition and storage of all components of memory. Finally, to further analyze a potential mechanism involved in impaired LTP, we compared AMPAR (α-amino-3-hydroxy-5-methyl-4-propionic acid receptor) expression at the synaptic membrane in the hippocampus of Tc1 and WT mice.
Results
Tc1 mice show normal spatial reference memory (RM) in both the standard and reversal versions of Morris watermaze
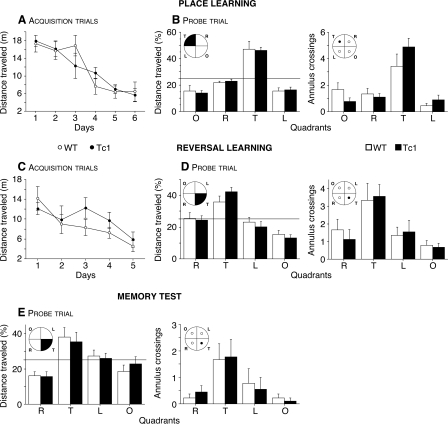
Tc1 mice were tested for spatial learning and memory performance in the Morris watermaze. Figure 1A shows that acquisition curves were similar to Tc1 and WT mice. Tc1 mice and WT littermates swam a similar distance to find the hidden escape platform (F(1,96) = 0.01, P = 0.93). The distance traveled decreased during training in the two genotypes, indicating that learning had occurred (F(5,96) = 14.91, P < 0.0001). Furthermore, during the probe trial on the seventh day, when the platform was removed, all mice showed a strong preference for the target (T) quadrant (Fig. 1B, left, F(3,64) = 46.81, P < 0.0001) and for the place where the platform had been located (Fig. 1B, right, F(3,64) = 19.77, P < 0.0001), demonstrating that Tc1 mice utilized a spatial strategy to find the platform.
Figure 1.
Performance of WT (open circles, n = 9) and Tc1 (filled circles, n = 9) mice during acquisition trials of place (A) and reversal (C) learning in the Morris watermaze. The results are expressed as distance traveled (m) and show similar performance for Tc1 and WT mice in both versions of the task. Probe trial of place learning (B), reversal learning (D), and memory test (20 d later) (E). Mice were scored for the percentage of distance traveled in the four quadrants: opposite (O), target (T), left (L), and right (R), and for the number of annulus crossings. The targeted quadrant and platform are indicated in black. The horizontal line indicates the distance traveled using a random search strategy. No difference was observed between Tc1 (black bars) and WT (white bars) mice in the different probe trials, indicating a normal spatial memory in Tc1 mice. Values represent means ± SEM.
We then examined learning flexibility in a reversal test, in which the hidden platform was moved to a new location in the pool. As in the standard version, Tc1 mice showed no impairment in reversal learning. Indeed, both groups improved with training (Fig. 1C, F(4,80) = 4.93, P < 0.01), traveling a similar distance to find the platform (F(1,80) = 1.287, P = 0.26) and exhibiting a spatial strategy during the probe trial, favoring the target quadrant (Fig. 1D, left, F(3,64) = 24.88, P < 0.0001) and the place where the platform had been located (Fig. 1D, right, F(3,64) = 7.78, P < 0.001). Therefore, Tc1 mice are able to adapt their behavior following a change of the platform position.
Then, to test the LTM of the Tc1 mice, we did a probe trial 20 d after the last probe trial. Tc1 mice displayed intact spatial LTM. Indeed, all mice showed a strong preference for the target quadrant (Fig. 1E, left, F(3,64) = 10.59, P < 0.0001) and for the target annulus (Fig. 1E, right, F(3,64) = 5.53, P < 0.01). Our behavioral data showing that Tc1 mice have spared performance in spatial RM and in reversal learning, strongly suggest that they have no deficit either in behavioral flexibility or in acquisition of new spatial memories. In addition, the results of the probe trial carried out 20 d after the reversal learning indicate a recency effect in memory recall (they are able to retain specifically the information they learned during the second version of the maze), suggesting again that they have the capability to develop and retain new spatial memories.
Tc1 mice have significantly impaired spatial WM in the Morris watermaze
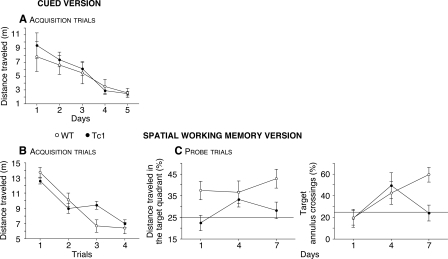
To test spatial WM in Tc1 mice, a new group of animals was trained in the Morris watermaze. First, in order to familiarize the animals with the procedure (i.e., to swim, to locate, and to climb onto the escape platform), mice were trained in the cued version of the Morris watermaze using a platform onto which a visual cue was affixed. In this version, no difference was observed between genotypes. The distance traveled to escape decreased significantly with time for all the mice (Fig. 2A, F(4,95) = 8.40, P < 0.0001). This result demonstrates that Tc1 mice have no impairment in this form of associative learning.
Figure 2.
Performance of WT (open circles, n = 10) and Tc1 (filled circles, n = 12) mice during acquisition trials of the cued (A) and spatial WM (B) version of the Morris watermaze. The results are expressed as distance traveled (m) and show intact associative learning, but impaired spatial WM acquisition in Tc1 mice. (C) Probe trials of the spatial WM version. Mice are scored for the percentage of distance traveled in the target quadrant and for the number of target annulus crossings expressed as the percentage of the total number of annulus crossings in all four quadrants. The horizontal line indicates the distance traveled and the annulus crossings using a random search strategy. This test confirms the impaired spatial WM in Tc1 mice. Values represent means ± SEM.
Spatial WM was then evaluated in a series of learning-reversal tests in which the location of the hidden platform was changed each day. Four trials were administrated daily. We defined spatial WM as short-term retention of the location of the hidden platform. The distance traveled on each training trial was averaged for the 9 d of training. Although the distance decreased with training for all the mice (Fig. 2B, F(3,76) = 37.69, P < 0.0001), Tc1 mice showed a delay in the acquisition of the task (genotype × trial interaction: F(3,76) = 4.03, P < 0.01). However, by the last trial, all mice exhibited the same level of performance. Furthermore, only WT mice exhibited a spatial strategy as indicated by the probe trials (Fig. 2C, left, F(1,57) = 10.84, P < 0.01). In addition, whereas WT mice improved their performance over time, Tc1 mice performed at close to chance level (25%) even at the end of testing (Fig. 2C, right, genotype × time interaction: F(2,57) = 3.03, P = 0.05). Tc1 mice showed significantly impaired spatial WM and deficiencies in the use of spatial strategies to solve the task. In fact, they adopted a nonspatial strategy to find the platform. The deficit observed in Tc1 mice in the WM version of the Morris watermaze is robust and unlikely to result from sensorimotor or motivational deficits, because these animals showed normal spatial RM acquisition and performance on the same apparatus with the same sensorimotor and motivational demands, and the same spatial cues.
Tc1 mice are impaired in STM but have intact LTM in the novel object recognition task
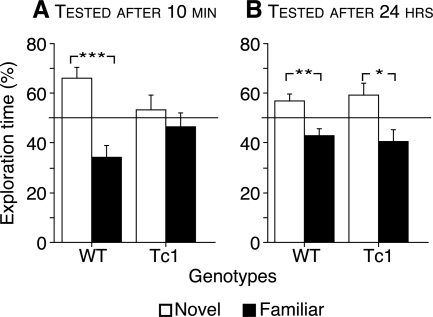
To further assess STM and LTM in Tc1 mice, we trained mice in the novel object recognition task. During the training sessions, mice were allowed to explore three objects. Following a 10-min or 24-h delay, one of the familiar objects was replaced with a novel object, and the time spent exploring the different objects was measured. No significant difference in total exploration levels was observed between genotypes during the training and the test sessions. For both genotypes, no spontaneous preference for an object was observed during the training phase. At the 10-min delay, whereas WT mice spent significantly more time exploring the novel object than familiar objects, Tc1 mice failed to show significantly greater exploration of the novel object (Fig. 3A, genotype × object interaction: F(1,28) = 5.24, P < 0.05). However, 24 h after the training session, all the mice spent significantly more time exploring the novel object than the familiar objects (Fig. 3B, F(1,34) = 16.90, P < 0.001), indicating that both genotypes recognized the novel object. Thus, while STM in novel object recognition is impaired in Tc1 mice, LTM, surprisingly, is unaffected.
Figure 3.
Discrimination learning at short and long delays in WT and Tc1 mice in the object recognition task. The results are expressed as percentage of time spent exploring the novel (white bars) and familiar (back bars) objects. The horizontal line indicates performances close to the chance level. Tc1 mice show a recognition memory deficit at a 10-min delay (n = 7 WT and n = 9 Tc1) (A), but not at a 24-h delay (n = 9 WT and n = 10 Tc1) (B). Values represent means ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001.
Reduced early LTP but normal late LTP expressed over days in the dentate gyrus of freely-moving Tc1 mice
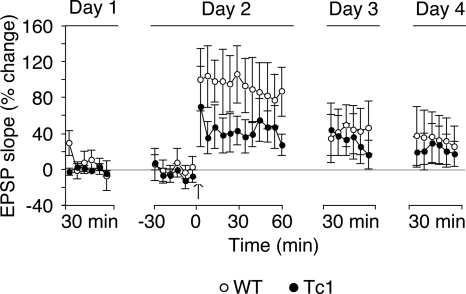
Since synaptic plasticity is widely believed to be the neural substrate of memory (for review, see Martin and Morris 2002), we next examined whether the combination of impaired STM and normal LTM in Tc1 mice is paralleled by a corresponding pattern in LTP. We therefore investigated hippocampal LTP at medial perforant path (MPP)–granule cell synapses in the dentate gyrus of freely-moving mice, allowing us to follow late LTP over several days. One hour after tetanic stimulation of the MPP, Tc1 mice exhibited significantly reduced LTP compared with that of WT littermates (Fig. 4, F(1,180) = 19.28, P < 0.0001). However, both 24 and 48 h after the tetanus, LTP was of similar magnitude in both genotypes (genotype effect: at 24 h, F(1,90) = 0.56, P = 0.46, and at 48 h, F(1,90) = 0.63, P = 0.43; day effect: day 1 vs. day 3, F(1,180) = 21.36, P < 0.0001, and day 1 vs. day 4, F(1,180) = 11.43, P < 0.001).
Figure 4.
LTP in the dentate gyrus of awake freely-moving WT and Tc1 mice. The graph plots the time course of LTP induced at MPP-granule cell synapses in freely-moving animals (WT: open circles, n = 8; Tc1: filled circles, n = 9). Baseline responses were recorded for 2 d (30 min per day). A tetanus (six series of six trains of six stimuli at 400 Hz, 200 msec between trains, 20 sec between series) was delivered (arrow) on the second day, and responses were monitored for 1 h, and again for 30 min per day for the next 2 d. Each data point presented is an average of ten successive sampled responses over 5 min. The horizontal line represents percentage of baseline. Tc1 mice showed reduced LTP after the delivery of the tetanus, but normal maintenance at days 3 and 4. Values represent means ± SEM.
Reduced surface membrane expression of the AMPAR subunit GluR1 in Tc1 mice
To process information at very short time intervals, mice require a flexible, rapid-onset neuronal mechanism. A large body of in vitro data indicates that a key component of LTP 30–60 min post-induction is the insertion of AMPAR into the postsynaptic membrane (Malinow and Malenka 2002). We therefore set out to identify whether impaired trafficking of AMPAR in Tc1 mice might offer an explanation for the striking dissociation between the impaired early LTP and intact late LTP in Tc1 mice.
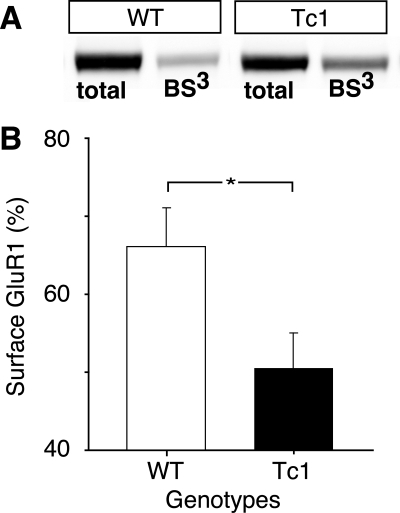
In order to quantify levels of surface expression of glutamate receptors (GluR1 and NR1), we utilized the membrane-impermeable cross-linking reagent BS3 (bis-[sulfosuccinimidyl] suberate), combined with quantitative Western blotting (Grosshans et al. 2002). This enabled us to calculate the percentage of GluR1 and NR1 present at the membrane surface in Tc1 mice compared with control littermates. While there was no significant difference in surface levels of the NR1 subunit of the NMDAR (N-methyl-D-aspartate receptor) between the two groups, there was a clear decrease in the levels of the GluR1 subunit of the AMPAR in Tc1 mice compared with controls (Fig. 5A,B, F(1,11) = 5.51, P < 0.05). This result is consistent with an abnormality of AMPAR insertion. In view of the reduced percentage of AMPAR at the surface in Tc1 mice, we used quantitative Western blotting to look at any potential differences in the total protein levels of molecules known to be important for AMPAR trafficking (for review, see Bredt and Nicoll 2003). However, we found no differences in the levels of either PSD-95 (postsynaptic density protein-95) or the γ8 isoform of stargazin between controls and Tc1 mice (data not shown).
Figure 5.
Surface expression of the AMPAR subunit GluR1 assayed by quantitative Western blot analysis in WT and Tc1 mice. (A) Blot showing the samples of total and internal (BS3) protein levels from hippocampal slices taken from WT and Tc1 mice. (B) Quantification of the percentage of GluR1 present at the membrane surface in WT (white bars, n = 6) and Tc1 (black bars, n = 7) mice. Surface expression was calculated from total minus internal protein levels. Data show a reduced percentage of AMPAR at the surface in Tc1 mice compared with WT mice. Values represent means ± SEM. *P < 0.05.
Discussion
The present study reveals several striking findings. Tc1 mice show spared spatial RM, long-term spatial and recognition memories, and exhibit normal maintenance of LTP over days at perforant path–granule cell synapses in the dentate gyrus. Conversely, Tc1 mice are selectively impaired in spatial WM and short-term recognition memory. These behavioral impairments were paralleled by a deficit in early LTP, which might be explained by a reduced surface expression of AMPAR.
In 2005, O’Doherty et al. identified a short-term recognition memory deficit in Tc1 mice and impaired induction of LTP in the dentate gyrus of urethane-anesthetized Tc1 mice. Our study confirms their results, reflecting the robustness of the phenotypes seen in Tc1 mice in spite of their noninbred genetic background and significant degree of mosaicism (O’Doherty et al. 2005), and significantly extends the characterization of the Tc1 mice by revealing normal long-term maintenance of LTP in awake, freely-moving animals and a dissociation between short- and long-term recognition memory.
The issue of whether STM is an independent memory stream or is just an early phase of LTM remains controversial. Many procedures disrupt LTM without effect on STM, for example, administration of protein synthesis inhibitors (Davis and Squire 1984), or protein kinase inhibitors (Schafe and LeDoux 2000); a similar behavioral pattern is seen in transgenic animals in which a protein kinase A inhibitory peptide is expressed (Abel et al. 1997), or in which immediate early gene expression is disrupted (Jones et al. 2001; Plath et al. 2006). However, these experiments do not distinguish between STM as a phase of memory that is in series with LTM and the opposing hypothesis that the two processes are independent parallel streams. In the latter case, it should be possible to selectively abolish STM without affecting the subsequent expression of LTM of the same task. Observations of this sort have been reported for immediate/WM in humans, (Scoville and Milner 1957; Warrington and Shallice 1969; Sullivan and Sagar 1991), for short-term associative memory in flies (Tully and Gold 1993), and for sensitization in Aplysia (Emptage and Carew 1993). Similarly, a range of pharmacological interventions have been reported to affect STM but not LTM when rats are trained in a passive avoidance task (Izquierdo et al. 1999), and short-term but not long-term recognition memory is impaired when the selective GluK5 kainate receptor antagonist UBP302 is infused into the perirhinal cortex (Barker et al. 2006). Our findings extend these findings, providing evidence that STM and short lasting LTP involve, at least in part, mechanisms that are not necessary for LTM and long-lasting LTP. Analysis of the Tc1 model should therefore help to provide a better understanding of the mechanisms specifically involved in the early phase of LTP and the formation of STM, and how these are distinguished from the cellular mechanisms underlying the late phase of LTP and the long-term storage of memory.
In rodents, the hippocampus is a critical structure for encoding spatial information (O’Keefe and Nadel 1978; Olton and Papas 1979; Morris et al. 1982). It is required for the formation of both spatial WM (Olton and Papas 1979) and spatial RM. Recent studies suggest that spatial WM and spatial RM are subserved by different mechanisms within the hippocampus. For example, impaired spatial WM with spared spatial RM performance has been observed in genetically modified mice with a global deletion of the GluR-A (GluR1) subunit of the AMPAR (Reisel et al. 2002; Schmitt et al. 2003, 2005). Schmitt et al. (2004) suggested that, within the hippocampus, two distinct and dissociable systems are recruited for information processing mechanisms: a GluR1-dependent system that allows the animal to respond rapidly and flexibly on the basis of trial-specific information that needs to be retrieved from memory. This presumably underlies spatial WM performance. The other system is GluR1-independent, allowing the associative strength or reward valence of places or locations in the environment to be increased and/or decreased gradually or incrementally over many trials. The latter could underpin spatial RM acquisition on tasks such as the Morris watermaze (for review, see Bannerman et al. 2006). Here, we report a deficit of rapid-onset LTP coupled with an intact late-onset LTP in Tc1 mice, in conjunction with a reduced expression of the AMPAR subunit GluR1 at the membrane surface. It is possible, therefore, that a reduced surface expression GluR1 in Tc1 mice underlies not only the impaired early LTP but also the dissociation between spatial WM and spatial RM. Changes in GluR1 phosphorylation have been linked to deficits in LTP in the Ts65Dn mouse model of DS (Siarey et al. 2006) but changes in receptor GluR1 surface expression have not previously been reported.
Various DS models in mice have been developed in order to study the consequences of increased gene dosage in DS and to specifically address phenotype/genotype relationships. While Tc1 mice are trisomic for 92% of Hsa21, two other well-studied models contain a partial murine trisomy 16 (MMU16): Ts65Dn (Reeves et al. 1995) and Ts1Cje (Sago et al. 1998) with, respectively, 132 and 85 orthologs of chromosome 21 genes in three copies. All these DS models display DS-like features relating to learning and memory such as abnormal spatial cognition (for review, see Uecker et al. 1993). However, it is still too early to make direct comparisons between these different models, and between these mouse models and DS. More has still to be done to define precisely the nature of their deficits to further understand the relationships between genes and cognitive impairments. Indeed, all these mice are from different genetic backgrounds and the studies have been conducted on animals of different ages under different experimental conditions. In terms of memory, they all have deficits but in various degrees or types of memory. For example, compared with Ts1Cje mice (Sago et al. 1998, 2000), Ts65Dn have more severe impairments of spatial RM in the Morris maze (Escorihuela et al. 1995; Reeves et al. 1995; Holtzman et al. 1996; Sago et al. 2000; Stasko and Costa 2004), potentially due to the larger size of their triplicated region. However, surprisingly, Tc1 mice, which carry the longest segment, show normal performances. Ts1Cje mice have normal recognition memory (Fernandez and Garner 2007), whereas Ts65Dn mice show a deficit in long-term novel object recognition (Fernandez et al. 2007, supplementary information; Fernandez and Garner 2008) and Tc1 have impaired short- but spared long-term recognition memory. Despite these apparent discrepancies between these different mouse models, some general conclusions can be drawn. Indeed, they all exhibit widespread morphological and/or physiological changes in the major subfields of the hippocampus (Siarey et al. 1997; Insausti et al. 1998; Belichenko et al. 2004; Kleschevnikov et al. 2004; Kurt et al. 2004; Costa and Grybko 2005; O’Doherty et al. 2005; Siarey et al. 2005; Lorenzi and Reeves 2006; Belichenko et al. 2007) that are concomitant with significant learning deficits in various behavioral tasks that are putatively hippocampus-dependent. For example, in terms of synaptic plasticity, these three DS models exhibit major alterations in hippocampal LTP (Siarey et al. 1997, 2005; Kleschevnikov et al. 2004; Costa and Grybko 2005; O’Doherty et al. 2005; Belichenko et al. 2007). The presence of these hippocampal phenotypes in DS mouse models is of great interest because a growing body of evidence now suggests that hippocampal dysfunction contributes significantly to the intellectual disabilities that characterize DS (Aylward et al. 1999; Pinter et al. 2001; Pennington et al. 2003). In addition, these different animal models for DS with different degrees of phenotypic impact illustrate the fact that we are trying to model a human condition of multidimensional nature. DS affects the central nervous system in many different ways, producing various degrees of intellectual disability.
While mental retardation remains the most striking and permanent feature of DS, a quantitative and qualitative phenotypic heterogeneity has been observed. The IQ of DS persons ranges from 30–70, depending on the severity of the disability, with an average around 50 (Chapman and Hesketh 2000). Whereas most individuals with DS have normal performance levels for simple tasks (Caycho et al. 1991), cognitive impairments arise with an increased complexity of the task (Oliver et al. 2005) and seem mainly to reflect persistent use of old strategies (Wishart 1993) and/or attention deficits (Brown et al. 2003; Maatta et al. 2006). Since the discovery of the chromosomal anomaly causing DS and the involvement of increased gene dose in changes in DS brain structure and function, one major goal of DS research has been to characterize the number and nature of genes involved in determining the pathology. This is an essential step to understanding the molecular basis of the disease and the development of appropriate therapeutics. Comparison of the phenotype and genotype of individuals with partial trisomy 21 has helped to localize genes whose dosage imbalance contributes to DS features. However, the number of these individuals is limited, and the presentation of DS, even with full trisomy 21, is highly variable, limiting the resolution of the phenotype map. All memory types have been reported impaired in DS persons (Bower and Hayes 1994; Carlesimo et al. 1997; Chapman and Hesketh 2000, 2001; Jarrold and Baddeley 2001; Lanfranchi et al. 2004; Brock and Jarrold 2005; Vicari et al. 2006; Jarrold et al. 2007; Silverman 2007), including various forms of LTM, STM, as well as WM. The Tc1 mouse model will give insights into this challenging gene–phenotype correlational analysis and in the identification of genetic mechanisms underlying memory processes. We argue that the reduced surface expression of the AMPAR subunit GluR1 might provide a molecular mechanism for cognitive and physiological alterations reported in Tc1 mice. Recent studies demonstrate that amyloid peptides, generated from the amyloid precursor protein (APP), can drive loss of surface glutamate receptors (Almeida et al. 2005; Snyder et al. 2005; Hsieh et al. 2006; Dewachter et al. 2007). The APP gene is located on Hsa21 (Patterson et al. 1988) and is overexpressed in Tc1 mice (O’Doherty et al. 2005). We hypothesize that overexpression of the APP gene leads to synaptic dysfunction by reducing surface expression of GluR1 at synapses. This, in turn, might induce the spatial WM deficit observed in Tc1 mice. Taken together these data indicate that the APP gene is a candidate for the cognitive and LTP deficits observed in Tc1 mice. However, we have not so far been able to confirm that the observed over-expression of the APP gene (O’Doherty et al. 2005) leads to an increase in human APP protein (data not shown). Further experiments will be needed to confirm this hypothesis, including rescue of the phenotype by crossing mice that are heterozygotic with respect to APP with Tc1 mice to produce an animal expressing APP with the normal double copy. Individuals with DS develop neuropathological features similar to Alzheimer’s disease (AD) early in life. β-Amyloid peptide is widely believed to underlie the pathophysiology of AD. These observations suggest that Tc1 mice may provide important information regarding the biological mechanisms responsible for DS and AD.
Using a selective GluK5 (or GluR5) kainate receptor antagonist, Barker et al. (2006) demonstrated that memory acquisition underlying recognition memory is kainate receptor dependent at short delays but not at long delays. The GLUR5 gene maps to Hsa21 (Gregor et al. 1993). Overexpression of GluR5 could alter the subunit composition and properties of heteromeric GluR-associated ion channels and have a detrimental effect on the short-term recognition memory in Tc1 mice. Thus, GLUR5 represents a possible candidate gene for the striking dissociation between STM and LTM seen in Tc1 mice and may have a role in mental retardation seen in DS. Again, further experiments will be needed to confirm this hypothesis, for example by using a breeding strategy to rescue the dosage of GLUR5.
A more daunting challenge than isolating genes responsible for single gene disorders is the identification of genes involved in complex traits. DS is a complex condition and the ultimate phenotype of the syndrome represents the epistatic and synergistic effects of many genes interacting together. Disentangling additive and interactive effects is the most challenging aspect to the genetic analysis of DS. Our mouse model represents a powerful genetic tool with the potential to help unravel the role of epistasis (modifier genes), pleiotropism, and environmental effects that are common to the genetic architecture of complex traits. Finally, the results presented here suggest that a significant part of the phenotype generated by the genetic disorder in DS can be related to the consequences of hippocampal dysfunction.
Materials and Methods
Animals
O’Doherty et al. (2005) generated the transchromosomic mouse line Tc1 by using irradiation microcell-mediated chromosome transfer (XMMCT). This trans-species aneuploid mouse line stably transmits a freely segregating almost complete copy of Hsa21 in a C57BL/6J × 129S8(F2) genetic background. Tc1 and their WT littermates are obtained from the mating of C57BL/6J×129S8(F1) Tc1 females with C57BL/6J×129S8(F1) males. The genotype of the mice was determined by polymerase chain reaction analysis as previously described (O’Doherty et al. 2005). Animals were weaned at 3 wk and were then housed by gender and litter under standard conditions, with food and water available ad libitum.
Experiments were conducted on adult male mice during the light phase of a 12-h light/dark schedule (with lights on at 0730 h) by experimenters who were blind to genotypes. All experiments were performed in compliance with UK Home Office regulations. All behavioral studies were conducted on independent groups of naive animals and were replicated several times. Furthermore, to avoid any confounding effect in the Morris watermaze, fully balanced experimental designs were used. For example, when mice were tested in the cued version first and then in the spatial version, another independent group of animals was then tested in the spatial version first to verify that the same results were obtained irrespective of experimental order.
Morris watermaze
The watermaze consists of a circular pool (150-cm diameter, 60-cm height) filled to a depth of 40 cm with water maintained at 20°C–22°C and made opaque using a white aqueous emulsion (Acusol OP 301 opacifier). The escape platform, made of rough plastic, was submerged 1 cm below the water surface. A video tracking system (HVS Image) was used to monitor activity.
Hidden-platform version
During the training phase of the standard place learning version of the Morris watermaze, mice learned the fixed position of a small hidden platform (6-cm diameter), using prominent distal extramaze cues arranged in the room around the pool. Each trial started with the mice facing the interior wall of the pool and ended when they climbed onto the platform or after a maximum searching time of 90 sec. The starting position was changed pseudo-randomly between trials. Animals that did not find the platform were gently guided and placed on it for 20 sec. The animals received one habituation trial on the first day and then two trials per day for five consecutive days. The mice were left undisturbed in their home cage for the 90-min intertrial interval. On the seventh day, mice were given the 60-sec probe test in which the platform had been removed. The distance traveled in each quadrant (target, opposite, left, and right) and the number of times the animal crossed each of the four possible platform sites (annulus) were automatically calculated from the video tracking system. After the first probe trial, all mice were given a reversal test in which the hidden platform was moved to a new position. The mice were trained for 5 d following the same training procedure and then tested for the second probe trial on the sixth day. To test mice for LTM, animals were left undisturbed for 20 d before being given a third probe trial.
Cued-platform version
During the cued version of the watermaze, an independent group of naive mice were trained to find a wider platform (9-cm diameter), made visible to the mice by a small dark ball (4.5-cm diameter) on a post 11 cm above the platform. The training procedure was identical to that of the spatial version except that (1) the trials on the sixth day were omitted, (2) both the platform’s position and the animal’s starting position were pseudo-randomized for each trial, and (3) the pool was surrounded by a white curtain to mask distal extramaze cues.
Spatial WM version
A group of mice, previously trained in the cued-platform version of the Morris watermaze, was assessed in the spatial WM version. Our protocol was adapted from the method of Janus (2004). In this test, each mouse was given four consecutive 60-sec training trials (intertrial interval: 20–25 sec) every day for 9 d. The location of the hidden escape platform (9-cm diameter) was fixed for all four trials each day but was changed pseudo-randomly between days. The starting position was changed pseudo-randomly among trials. Animals that did not find the platform were gently guided and placed on it for 20 sec. Upon finding the platform, the mice were allowed a 20-sec post-trial period on the platform. After the fourth training trial of each day, a fifth trial was given in which the mouse was allowed to search for the platform for 30 sec. On three of the 9 d (first, fourth, and seventh), the platform was removed for this fifth trial, allowing an assessment of spatial memory to be made (probe trial). On the other 6 d, the platform was present on the fifth trial to reduce the possibility of mice associating this trial with the absence of the platform.
Novel object recognition
Mice were tested for learning and memory deficits in the novel object recognition task using the method described by O’Doherty et al. (2005), with three objects in a fixed position to minimize the potential confound of intrinsic object preference. The apparatus consisted of a dark circular arena (65-cm diameter, 70-cm height). Mice were given a habituation session in this arena for 10 min on a single day. Training commenced the following day with two 10-min trials separated by 10 min. In each of the trials, mice were placed at the center of the arena and left to explore three differently shaped and colored objects (made of Lego) placed in fixed positions. Objects were cleaned with hot water after each trial. Memory of these objects was then tested 10 min or 24 h later. Mice were placed back into the same arena, but one of the objects was replaced by a novel object of a different shape and color to any of the training objects. For each mouse, the objects are randomly assigned as either familiar or novel, thus eliminating any effect due to spontaneous preference for an object. Time spent exploring each object was scored. Normally, rodents tend to explore a novel object in preference to a familiar object. A discrimination ratio was calculated by dividing the time spent exploring the novel object by the time spent exploring the novel object plus the mean time exploring the familiar objects.
Electrophysiology in awake, freely-moving mice
Extracellular field recording was used to study LTP at MPP-granule cell synapses in the dentate gyrus of freely-moving mice. LTP experiments were performed on behaviorally tested animals after a minimum interval of 2 mo following the last behavioral test. Surgery was performed under pentobarbital anesthesia (6 μg/g, i.p., Pentoject, Pentobarbitone Sodium Ph.Eur. Animalcare Ltd) and carprofen analgesia (5 μg/g, s.c., Rimadyl, Pfizer). Bipolar electrodes were made from 50-μm diameter formvar-insulated nickel-chrome wire (Advent Research Materials) running through beveled fine-gauge steel cannulae (Plastics1). Stimulating and recording electrodes were placed according to stereotactic coordinates. The stimulating electrode was positioned in the MPP, 3 mm lateral to lambda and at a depth of ∼1.5 mm from brain surface. The recording electrode was lowered into the hilus of the ipsilateral dentate gyrus, 2 mm posterior to bregma, 1.6 mm lateral to the midline, and at a depth of ∼1.5 mm. Electrode depths were adjusted to maximize the amplitude of evoked field responses. Electrodes were fixed in place with an initial application of Super-Bond C&B dental cement (Morita Europe), followed by several layers of Kemdent (Associated Dental Products Ltd). After surgery, animals were allowed to recover fully for at least 8 d and then were habituated to the recording chamber and the handler over 2 d. After recovery and habituation, cables were connected to the headcap, and low frequency baseline stimuli (monophasic pulse, 60-μsec pulse-width, 0.033 Hz) were delivered for 30 min per day to evoke a population field potential. The early component of the evoked response was sampled as a measure of MPP-granule cell excitatory post-synaptic potentials (EPSP). These recordings were sampled from day to day until stable baselines were obtained. To study LTP, the baseline stimulus intensity was selected to evoke a population spike of ∼1 mV in amplitude. Following 2 d of stable baseline, a tetanus of pulses was delivered to the perforant path. The tetanus consisted of six series of six trains of six stimuli at 400 Hz, 200 msec between trains, 20 sec between series. During the tetanus, pulse-width was doubled. Responses were measured for 60 min after tetanus and again over 30 min, 24 h, and 48 h after the tetanus. LTP was indexed as a percentage change in the field EPSP slope relative to the baseline.
Biochemical measurements of surface expressed receptors
Hippocampal slice preparation
Brains were removed and placed in ice-cold artificial cerebrospinal fluid (ACSF: 120 mM NaCl, 3 mM KCl, 1.2 mM NaH2PO4, 23 mM NaHCO3, 11 mM D-glucose, 2 mM MgSO4, 2 mM CaCl) bubbled with 95%O2/5%CO2. Hippocampi were dissected out and 400-μm slices prepared on a McIlwain tissue chopper (Mickle Laboratory Engineering Co.). Alternate slices taken from Tc1 animals and their control littermates were placed in either ice-cold ACSF, or ice-cold ACSF containing the cross-linking agent BS3 (1 mg/mL, Pierce) for 45 min on ice (to prevent further receptor trafficking) with 95%O2/5%CO2 blown into the chamber. The BS3 treatment results in all surface proteins being extensively cross-linked. To quench BS3, slices were washed three times in cold ACSF containing 20 mM Tris (pH 7.6). Following dounce homogenization in lysis buffer (1%NP-40, 20 mM Hepes at pH 7.4, 100 mM NaCl, 10 mM Na orthophosphate, Halt protease inhibitor cocktail [Pierce, 1:100], and phosphatase inhibitor cocktail set II [Calbiochem, Merck KgaA, 1:100]), protein concentrations were determined using the BCA protein assay (Pierce) and surface expression assayed by quantitative Western blot analysis.
Western blot analysis
For each blot, equivalent amounts of total protein were loaded into each well. Equivalent samples underwent SDS-PAGE gel electrophoresis and gels were transferred onto Immobilon-FL membranes (Millipore) using Xcell II Blot module (Invitrogen). Only internal proteins appear at the appropriate molecular weight band, as under cross-linking conditions surface proteins run at very high molecular weight. Membranes were stained simultaneously with pairs of primary antibodies and then secondary antibodies conjugated to infrared fluorescent dyes IR700 and IR800 (Rockland). Detection and quantification was done using the Odyssey infrared scanning imaging system (LI-COR Biosciences). All values were normalized to levels of the control protein actin (C-11 antibody, Santa Cruz Biotechnology) using multiplexed analysis of dual color blotting with simultaneous detection. Anti-GluR1 antibody was obtained from Chemicon (Millipore), anti-NR1 from BD Pharmingen (BD), anti-PSD-95 from Affinity Bioreagents, and anti-TARP γ8 was a kind gift of Chris Thompson (Durham University, Durham, UK). For surface expression studies, total and internal (cross-linked) samples were always run in adjacent lanes. Surface expression was calculated from total minus internal protein levels.
Statistical analysis
Repeated- ANOVA was performed to assess the interaction between genotypes (between factor) and time (within factor). For two groups comparisons, Student’s t-test was used. Statistical significance was set at a P value ≤0.05. Data are given as mean ± SEM.
Acknowledgments
We thank M. Nosten-Bertrand for helpful comments on the manuscript. We thank C. Brazil and L. Fern for animal care. E.M. was supported by a fellowship from the Fondation Fyssen.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.969608.
References
- Abel T., Nguyen P.V., Barad M., Deuel T.A., Kandel E.R., Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Abraham W.C., Logan B., Greenwood J.M., Dragunow M. Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus. J. Neurosci. 2002;22:9626–9634. doi: 10.1523/JNEUROSCI.22-21-09626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida C.G., Tampellini D., Takahashi R.H., Greengard P., Lin M.T., Snyder E.M., Gouras G.K. β-Amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol. Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Aylward E.H., Li Q., Honeycutt N.A., Warren A.C., Pulsifer M.B., Barta P.E., Chan M.D., Smith P.D., Jerram M., Pearlson G.D. MRI volumes of the hippocampus and amygdala in adults with Down’s syndrome with and without dementia. Am. J. Psychiatry. 1999;156:564–568. doi: 10.1176/ajp.156.4.564. [DOI] [PubMed] [Google Scholar]
- Bannerman D.M., Rawlins J.N., Good M.A. The drugs don’t work-or do they? Pharmacological and transgenic studies of the contribution of NMDA and GluR-A-containing AMPA receptors to hippocampal-dependent memory. Psychopharmacology. 2006;188:552–566. doi: 10.1007/s00213-006-0403-6. [DOI] [PubMed] [Google Scholar]
- Barker G.R., Warburton E.C., Koder T., Dolman N.P., More J.C., Aggleton J.P., Bashir Z.I., Auberson Y.P., Jane D.E., Brown M.W. The different effects on recognition memory of perirhinal kainate and NMDA glutamate receptor antagonism: Implications for underlying plasticity mechanisms. J. Neurosci. 2006;26:3561–3566. doi: 10.1523/JNEUROSCI.3154-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belichenko P.V., Masliah E., Kleschevnikov A.M., Villar A.J., Epstein C.J., Salehi A., Mobley W.C. Synaptic structural abnormalities in the Ts65Dn mouse model of Down Syndrome. J. Comp. Neurol. 2004;480:281–298. doi: 10.1002/cne.20337. [DOI] [PubMed] [Google Scholar]
- Belichenko P.V., Kleschevnikov A.M., Salehi A., Epstein C.J., Mobley W.C. Synaptic and cognitive abnormalities in mouse models of Down syndrome: Exploring genotype-phenotype relationships. J. Comp. Neurol. 2007;504:329–345. doi: 10.1002/cne.21433. [DOI] [PubMed] [Google Scholar]
- Bliss T.V., Collingridge G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bower A., Hayes A. Short-term memory deficits and Down’s syndrome: A comparative study. Down Syndrome Res. Practice. 1994;2:47–50. [Google Scholar]
- Bredt D.S., Nicoll R.A. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Brock J., Jarrold C. Serial order reconstruction in Down syndrome: Evidence for a selective deficit in verbal short-term memory. J. Child Psychol. Psychiatry. 2005;46:304–316. doi: 10.1111/j.1469-7610.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- Brown J.H., Johnson M.H., Paterson S.J., Gilmore R., Longhi E., Karmiloff-Smith A. Spatial representation and attention in toddlers with Williams syndrome and Down syndrome. Neuropsychologia. 2003;41:1037–1046. doi: 10.1016/s0028-3932(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Carlesimo G.A., Marotta L., Vicari S. Long-term memory in mental retardation: Evidence for a specific impairment in subjects with Down’s syndrome. Neuropsychologia. 1997;35:71–79. doi: 10.1016/s0028-3932(96)00055-3. [DOI] [PubMed] [Google Scholar]
- Caycho L., Gunn P., Siegal M. Counting by children with Down syndrome. Am. J. Ment. Retard. 1991;95:575–583. [PubMed] [Google Scholar]
- Chapman R.S., Hesketh L.J. Behavioral phenotype of individuals with Down syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2000;6:84–95. doi: 10.1002/1098-2779(2000)6:2<84::AID-MRDD2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Chapman R.S., Hesketh L.J. Language, cognition, and short-term memory in individuals with Down syndrome. Downs Syndr. Res. Pract. 2001;7:1–7. doi: 10.3104/reviews.108. [DOI] [PubMed] [Google Scholar]
- Costa A.C., Grybko M.J. Deficits in hippocampal CA1 LTP induced by TBS but not HFS in the Ts65Dn mouse: A model of Down syndrome. Neurosci. Lett. 2005;382:317–322. doi: 10.1016/j.neulet.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Davis H.P., Squire L.R. Protein synthesis and memory: A review. Psychol. Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Dewachter I., Filipkowski R.K., Priller C., Ris L., Neyton J., Croes S., Terwel D., Gysemans M., Devijver H., Borghgraef P., et al. Deregulation of NMDA-receptor function and down-stream signaling in APP[V717I] transgenic mice. Neurobiol. Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Emptage N.J., Carew T.J. Long-term synaptic facilitation in the absence of short-term facilitation in Aplysia neurons. Science. 1993;262:253–256. doi: 10.1126/science.8211146. [DOI] [PubMed] [Google Scholar]
- Escorihuela R.M., Fernandez-Teruel A., Vallina I.F., Baamonde C., Lumbreras M.A., Dierssen M., Tobena A., Florez J. A behavioral assessment of Ts65Dn mice: A putative Down syndrome model. Neurosci. Lett. 1995;199:143–146. doi: 10.1016/0304-3940(95)12052-6. [DOI] [PubMed] [Google Scholar]
- Fernandez F., Garner C.C. Object recognition memory is conserved in Ts1Cje, a mouse model of Down syndrome. Neurosci. Lett. 2007;421:137–141. doi: 10.1016/j.neulet.2007.04.075. [DOI] [PubMed] [Google Scholar]
- Fernandez F., Garner C.C. Episodic-like memory in Ts65Dn, a mouse model of Down syndrome. Behav. Brain Res. 2008;188:233–237. doi: 10.1016/j.bbr.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F., Morishita W., Zuniga E., Nguyen J., Blank M., Malenka R.C., Garner C.C. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat. Neurosci. 2007;10:411–413. doi: 10.1038/nn1860. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S. Regional and cellular fractionation of working memory. Proc. Natl. Acad. Sci. 1996;93:13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor P., Reeves R.H., Jabs E.W., Yang X., Dackowski W., Rochelle J.M., Brown R.H., Haines J.L., O’Hara B.F., Uhl G.R., et al. Chromosomal localization of glutamate receptor genes: Relationship to familial amyotrophic lateral sclerosis and other neurological disorders of mice and humans. Proc. Natl. Acad. Sci. 1993;90:3053–3057. doi: 10.1073/pnas.90.7.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans D.R., Clayton D.A., Coultrap S.J., Browning M.D. Analysis of glutamate receptor surface expression in acute hippocampal slices. Sci. STKE. 2002;2002:PL8. doi: 10.1126/stke.2002.137.pl8. [DOI] [PubMed] [Google Scholar]
- Hattori M., Fujiyama A., Taylor T.D., Watanabe H., Yada T., Park H.S., Toyoda A., Ishii K., Totoki Y., Choi D.K., et al. The DNA sequence of human chromosome 21. Nature. 2000;405:311–319. doi: 10.1038/35012518. [DOI] [PubMed] [Google Scholar]
- Hebb D.O. The organization of behavior: A neuropsychological theory. Wiley; New York: 1949. [Google Scholar]
- Holtzman D.M., Santucci D., Kilbridge J., Chua-Couzens J., Fontana D.J., Daniels S.E., Johnson R.M., Chen K., Sun Y., Carlson E., et al. Developmental abnormalities and age-related neurodegeneration in a mouse model of Down syndrome. Proc. Natl. Acad. Sci. 1996;93:13333–13338. doi: 10.1073/pnas.93.23.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H., Boehm J., Sato C., Iwatsubo T., Tomita T., Sisodia S., Malinow R. AMPAR removal underlies Aβ-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti A.M., Megias M., Crespo D., Cruz-Orive L.M., Dierssen M., Vallina I.F., Insausti R., Florez J. Hippocampal volume and neuronal number in Ts65Dn mice: A murine model of Down syndrome. Neurosci. Lett. 1998;253:175–178. doi: 10.1016/s0304-3940(98)00641-7. [DOI] [PubMed] [Google Scholar]
- Izquierdo I., Medina J.H., Vianna M.R., Izquierdo L.A., Barros D.M. Separate mechanisms for short- and long-term memory. Behav. Brain Res. 1999;103:1–11. doi: 10.1016/s0166-4328(99)00036-4. [DOI] [PubMed] [Google Scholar]
- Janus C. Search strategies used by APP transgenic mice during navigation in the Morris water maze. Learn. Mem. 2004;11:337–346. doi: 10.1101/lm.70104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrold C., Baddeley A.D. Short-term memory in Down syndrome: Applying the working memory model. Downs Syndr. Res. Pract. 2001;7:17–23. doi: 10.3104/reviews.110. [DOI] [PubMed] [Google Scholar]
- Jarrold C., Baddeley A.D., Phillips C. Long-term memory for verbal and visual information in Down syndrome and Williams syndrome: Performance on the Doors and People test. Cortex. 2007;43:233–247. doi: 10.1016/s0010-9452(08)70478-7. [DOI] [PubMed] [Google Scholar]
- Jones M.W., Errington M.L., French P.J., Fine A., Bliss T.V., Garel S., Charnay P., Bozon B., Laroche S., Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat. Neurosci. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Clark R.E., Thompson R.F. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav. Neurosci. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Kleschevnikov A.M., Belichenko P.V., Villar A.J., Epstein C.J., Malenka R.C., Mobley W.C. Hippocampal long-term potentiation suppressed by increased inhibition in the Ts65Dn mouse, a genetic model of Down syndrome. J. Neurosci. 2004;24:8153–8160. doi: 10.1523/JNEUROSCI.1766-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt M.A., Kafa M.I., Dierssen M., Davies D.C. Deficits of neuronal density in CA1 and synaptic density in the dentate gyrus, CA3 and CA1, in a mouse model of Down syndrome. Brain Res. 2004;1022:101–109. doi: 10.1016/j.brainres.2004.06.075. [DOI] [PubMed] [Google Scholar]
- Lanfranchi S., Cornoldi C., Vianello R. Verbal and visuospatial working memory deficits in children with Down syndrome. Am. J. Ment. Retard. 2004;109:456–466. doi: 10.1352/0895-8017(2004)109<456:VAVWMD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lejeune J., Gautier M., Turpin R. [Study of somatic chromosomes from 9 mongoloid children.] C. R. Hebd. Seances Acad. Sci. 1959;248:1721–1722. [PubMed] [Google Scholar]
- Lorenzi H.A., Reeves R.H. Hippocampal hypocellularity in the Ts65Dn mouse originates early in development. Brain Res. 2006;1104:153–159. doi: 10.1016/j.brainres.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Maatta T., Tervo-Maatta T., Taanila A., Kaski M., Iivanainen M. Mental health, behaviour and intellectual abilities of people with Down syndrome. Downs Syndr. Res. Pract. 2006;11:37–43. doi: 10.3104/reports.313. [DOI] [PubMed] [Google Scholar]
- Malinow R., Malenka R.C. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Martin S.J., Morris R.G. New life in an old idea: The synaptic plasticity and memory hypothesis revisited. Hippocampus. 2002;12:609–636. doi: 10.1002/hipo.10107. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L. Time-dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- Morris R.G., Garrud P., Rawlins J.N., O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- O’Doherty A., Ruf S., Mulligan C., Hildreth V., Errington M.L., Cooke S., Sesay A., Modino S., Vanes L., Hernandez D., et al. An aneuploid mouse strain carrying human chromosome 21 with Down syndrome phenotypes. Science. 2005;309:2033–2037. doi: 10.1126/science.1114535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J., Nadel L. The hippocampus as a cognitive map. Oxford University Press; Oxford: 1978. [Google Scholar]
- Oliver C., Holland T., Hall S., Crayton L. Effects of increasing task load on memory impairment in adults with Down syndrome. Am. J. Ment. Retard. 2005;110:339–345. doi: 10.1352/0895-8017(2005)110[339:EOITLO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Olton D.S., Papas B.C. Spatial memory and hippocampal function. Neuropsychologia. 1979;17:669–682. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- Patterson D., Gardiner K., Kao F.T., Tanzi R., Watkins P., Gusella J.F. Mapping of the gene encoding the β-amyloid precursor protein and its relationship to the Down syndrome region of chromosome 21. Proc. Natl. Acad. Sci. 1988;85:8266–8270. doi: 10.1073/pnas.85.21.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington B.F., Moon J., Edgin J., Stedron J., Nadel L. The neuropsychology of Down syndrome: evidence for hippocampal dysfunction. Child Dev. 2003;74:75–93. doi: 10.1111/1467-8624.00522. [DOI] [PubMed] [Google Scholar]
- Phillips R.G., LeDoux J.E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pinter J.D., Brown W.E., Eliez S., Schmitt J.E., Capone G.T., Reiss A.L. Amygdala and hippocampal volumes in children with Down syndrome: A high-resolution MRI study. Neurology. 2001;56:972–974. doi: 10.1212/wnl.56.7.972. [DOI] [PubMed] [Google Scholar]
- Plath N., Ohana O., Dammermann B., Errington M.L., Schmitz D., Gross C., Mao X., Engelsberg A., Mahlke C., Welzl H., et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Reeves R.H. Down syndrome mouse models are looking up. Trends Mol. Med. 2006;12:237–240. doi: 10.1016/j.molmed.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Reeves R.H., Irving N.G., Moran T.H., Wohn A., Kitt C., Sisodia S.S., Schmidt C., Bronson R.T., Davisson M.T. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat. Genet. 1995;11:177–184. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- Reisel D., Bannerman D.M., Schmitt W.B., Deacon R.M., Flint J., Borchardt T., Seeburg P.H., Rawlins J.N. Spatial memory dissociations in mice lacking GluR1. Nat. Neurosci. 2002;5:868–873. doi: 10.1038/nn910. [DOI] [PubMed] [Google Scholar]
- Sago H., Carlson E.J., Smith D.J., Kilbridge J., Rubin E.M., Mobley W.C., Epstein C.J., Huang T.T. Ts1Cje, a partial trisomy 16 mouse model for Down syndrome, exhibits learning and behavioral abnormalities. Proc. Natl. Acad. Sci. 1998;95:6256–6261. doi: 10.1073/pnas.95.11.6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sago H., Carlson E.J., Smith D.J., Rubin E.M., Crnic L.S., Huang T.T., Epstein C.J. Genetic dissection of region associated with behavioral abnormalities in mouse models for Down syndrome. Pediatr. Res. 2000;48:606–613. doi: 10.1203/00006450-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Schafe G.E., LeDoux J.E. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J. Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt W.B., Deacon R.M., Seeburg P.H., Rawlins J.N., Bannerman D.M. A within-subjects, within-task demonstration of intact spatial reference memory and impaired spatial working memory in glutamate receptor-A-deficient mice. J. Neurosci. 2003;23:3953–3959. doi: 10.1523/JNEUROSCI.23-09-03953.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt W.B., Arianpour R., Deacon R.M., Seeburg P.H., Sprengel R., Rawlins J.N., Bannerman D.M. The role of hippocampal glutamate receptor-A-dependent synaptic plasticity in conditional learning: the importance of spatiotemporal discontiguity. J. Neurosci. 2004;24:7277–7282. doi: 10.1523/JNEUROSCI.1093-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt W.B., Sprengel R., Mack V., Draft R.W., Seeburg P.H., Deacon R.M., Rawlins J.N., Bannerman D.M. Restoration of spatial working memory by genetic rescue of GluR-A-deficient mice. Nat. Neurosci. 2005;8:270–272. doi: 10.1038/nn1412. [DOI] [PubMed] [Google Scholar]
- Scoville W.B., Milner B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatr. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siarey R.J., Stoll J., Rapoport S.I., Galdzicki Z. Altered long-term potentiation in the young and old Ts65Dn mouse, a model for Down syndrome. Neuropharmacology. 1997;36:1549–1554. doi: 10.1016/s0028-3908(97)00157-3. [DOI] [PubMed] [Google Scholar]
- Siarey R.J., Villar A.J., Epstein C.J., Galdzicki Z. Abnormal synaptic plasticity in the Ts1Cje segmental trisomy 16 mouse model of Down syndrome. Neuropharmacology. 2005;49:122–128. doi: 10.1016/j.neuropharm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Siarey R.J., Kline-Burgess A., Cho M., Balbo A., Best T.K., Harashima C., Klann E., Galdzicki Z. Altered signaling pathways underlying abnormal hippocampal synaptic plasticity in the Ts65Dn mouse model of Down syndrome. J. Neurochem. 2006;98:1266–1277. doi: 10.1111/j.1471-4159.2006.03971.x. [DOI] [PubMed] [Google Scholar]
- Silverman W. Down syndrome: Cognitive phenotype. Ment. Retard. Dev. Disabil. Res. Rev. 2007;13:228–236. doi: 10.1002/mrdd.20156. [DOI] [PubMed] [Google Scholar]
- Snyder E.M., Nong Y., Almeida C.G., Paul S., Moran T., Choi E.Y., Nairn A.C., Salter M.W., Lombroso P.J., Gouras G.K., et al. Regulation of NMDA receptor trafficking by amyloid-β. Nat. Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Squire L.R. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stasko M.R., Costa A.C. Experimental parameters affecting the Morris water maze performance of a mouse model of Down syndrome. Behav. Brain Res. 2004;154:1–17. doi: 10.1016/j.bbr.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Sullivan E.V., Sagar H.J. Double dissociation of short-term and long-term memory for nonverbal material in Parkinson’s disease and global amnesia. A further analysis. Brain. 1991;114:893–906. doi: 10.1093/brain/114.2.893. [DOI] [PubMed] [Google Scholar]
- Tully T., Gold D. Differential effects of dunce mutations on associative learning and memory in Drosophila. J. Neurogenet. 1993;9:55–71. doi: 10.3109/01677069309167275. [DOI] [PubMed] [Google Scholar]
- Uecker A., Mangan P.A., Obrzut J.E., Nadel L. Down syndrome in neurobiological perspective: An emphasis on spatial cognition. J. Clin. Child Psychol. 1993;22:266–276. [Google Scholar]
- Vicari S., Bellucci S., Carlesimo G.A. Evidence from two genetic syndromes for the independence of spatial and visual working memory. Dev. Med. Child Neurol. 2006;48:126–131. doi: 10.1017/S0012162206000272. [DOI] [PubMed] [Google Scholar]
- Warrington E.K., Shallice T. The selective impairment of auditory verbal short-term memory. Brain. 1969;92:885–896. doi: 10.1093/brain/92.4.885. [DOI] [PubMed] [Google Scholar]
- Wishart J.G. The development of learning difficulties in children with Down’s syndrome. J. Intellect. Disabil. Res. 1993;37:389–403. doi: 10.1111/j.1365-2788.1993.tb00882.x. [DOI] [PubMed] [Google Scholar]