Abstract
Transcription is a critical component for consolidation of long-term memory. However, relatively few transcriptional mechanisms have been identified for the regulation of gene expression in memory formation. In the current study, we investigated the activity of one specific member of the NF-κB transcription factor family, c-Rel, during memory consolidation. We found that contextual fear conditioning elicited a time-dependent increase in nuclear c-Rel levels in area CA1 and DG of hippocampus. These results suggest that c-rel is active in regulating transcription during memory consolidation. To identify the functional role of c-Rel in memory formation, we characterized c-rel−/− mice in several behavioral tasks. c-rel−/− mice displayed significant deficits in freezing behavior 24 h after training for contextual fear conditioning but showed normal freezing behavior in cued fear conditioning and in short-term contextual fear conditioning. In a novel object recognition test, wild-type littermate mice exhibited a significant preference for a novel object, but c-rel−/− mice did not. These results indicate that c-rel−/− mice have impaired hippocampus-dependent memory formation. To investigate the role of c-Rel in long-term synaptic plasticity, baseline synaptic transmission and long-term potentiation (LTP) at Schaffer collateral synapses in c-rel−/− mice was assessed. c-rel−/− slices had normal baseline synaptic transmission but exhibited significantly less LTP than did wild-type littermate slices. Together, our results demonstrate that c-Rel is necessary for long-term synaptic potentiation in vitro and hippocampus-dependent memory formation in vivo.
Memory is a process through which learned information is stored, and newly formed memories are susceptible to disruption and must be stabilized for long-term storage through a process referred to as memory consolidation (McGaugh 2000). Consolidation of explicit long-term memory requires a series of hippocampus-dependent molecular processes, including gene transcription. The necessity for gene transcription to sustain long-term memory has been demonstrated in several animal models using transcription inhibitors (Thut and Lindell 1974; Kandel 2001). However, the identity and role of transcriptional mediators in long-term memory formation has yet to be fully addressed. So far, a relatively few transcription factors such as CREB, C/EBP, c-fos, and Zif268/egr-1 have been implicated in the consolidation of long-term memory (Yin et al. 1995; Jones et al. 2001; Chen et al. 2003; Fleischmann et al. 2003). Therefore in a previous study, we identified a list of candidate transcription factors that are potentially involved in the process of long-term memory consolidation using expression array profiling and a bioinformatics approach (Levenson et al. 2004a). Through these studies, the c-Rel transcription factor was identified as the most probable candidate for contributing to memory consolidation from among this novel list of transcription factors, because its DNA-binding motif is highly enriched upstream of memory associated genes.
c-Rel is a member of the nuclear factor κB (NF-κB) transcription factor family. The NF-κB family of dimeric transcription factors consists of four other mammalian members, RelA (p65), RelB, NF-κB1 (p105/p50), and NF-κB2 (p100/p52) (Verma et al. 1995). NF-κB transcription factors were initially identified as κ light chain enhancer binding proteins (Sen and Baltimore 1986), and most NF-κB studies have focused on the immune system. In the immune system, NF-κB plays critical roles in cell survival and proliferation, as well as innate and adaptive immune responses (Li and Verma 2002). A growing body of literature supports a role for NF-κB in the brain, related to immune and inflammatory activities in the central nervous system (CNS) (Memet 2006; O’Mahony et al. 2006; Sheridan et al. 2007; Bracchi-Ricard et al. 2008). Recently, an increasing number of studies have provided evidence for the involvement of NF-κB specifically in the regulation of neuronal plasticity and memory formation (Meffert and Baltimore 2005; Romano et al. 2006). These studies demonstrated that p65- or p50-containing NF-κB complexes are active during synaptic activation and regulated during synaptic plasticity and memory formation (Wellmann et al. 2001; Yeh et al. 2002; Freudenthal et al. 2004). Indeed, p65-deficient mice have a spatial learning deficit (Meffert et al. 2003), and p50-deficient mice also exhibit a learning deficit in an active avoidance test (Kassed et al. 2002). More recently, our studies showed that the NF-κB pathway and its upstream regulators are necessary for memory reconsolidation via regulating post-translational modification of histone H3 (Lubin and Sweatt 2007). In preliminary experiments using nonlittermate controls, we also observed that c-Rel knockout animals appeared to have a deficit in contextual fear conditioning (Levenson et al. 2004a).
Although recent studies have implicated the p65 and p50 in synaptic plasticity and memory formation, little is known about the regulation of other members of the NF-κB family in these processes. In the case of c-Rel, previous studies suggest that c-Rel is regulated by glutamate receptor activation and is necessary for mGluR-LTD (Lubin et al. 2005; O’Riordan et al. 2006). However, it is still uncertain whether c-Rel is necessary for various forms of hippocampus-dependent long-term memory formation and long-term potentiation (LTP). In the present study we provide biochemical, physiologic, and behavioral evidence for the involvement of the NF-κB family member c-Rel in hippocampus-dependent long-term synaptic plasticity and long-term memory formation.
Results
Increase of nuclear levels of c-Rel in hippocampus during fear memory formation
Most NF-κB dimers are present in the cytoplasm in association with IκB inhibitory proteins, which prevent nuclear translocation (Ghosh et al. 1998). In response to a variety of inducers, IκB proteins are phosphorylated by the IκB–kinase complex and degraded via the ubiquitination pathway. The degradation of IκB allows NF-κB dimers to translocate to the nucleus and activate the transcription of target genes (Li and Verma 2002). Thus far, a number of studies have demonstrated that NF-κB is translocated to the nucleus by synaptic activation and is activated during memory formation (Wellmann et al. 2001; Yeh et al. 2002; Meffert et al. 2003; Freudenthal et al. 2005; Lubin et al. 2005). However, less is known about nuclear translocation of the NF-κB family member c-Rel during long-term memory formation. We hypothesized that if c-Rel is involved in hippocampus-dependent memory formation, then the level of c-Rel in the nucleus should increase during long-term memory formation.
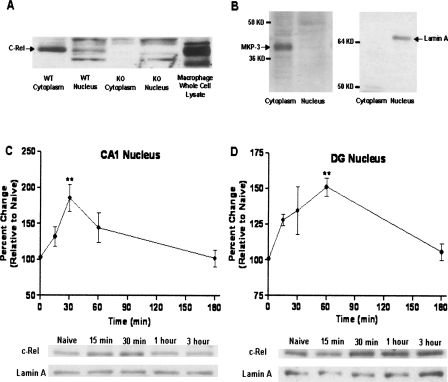
Before testing this hypothesis, c-Rel protein expression in area CA1 in the hippocampus was analyzed by Western blotting. c-Rel protein was expressed predominantly in the cytoplasm of the CA1 region of the hippocampus and slightly in the nucleus (Fig. 1A,B). c-rel−/− mice do not express c-Rel in the cytoplasm or nucleus (Fig. 1A). In further studies, mice were trained using the contextual fear conditioning paradigm, which is widely used to test hippocampus-dependent long-term memory formation (Atkins et al. 1998; Fanselow 2000). Mice were sacrificed at various times after training (15 min, 30 min, 1 h, and 3 h), time points corresponding to the consolidation phase of long-term contextual fear memory (Igaz et al. 2002). Nuclear fractions were prepared from CA1 and the dentate gyrus (DG) of the hippocampus, and nuclear levels of c-Rel were assessed by immunoblotting. Nuclear c-Rel levels were significantly increased 30 min after contextual fear conditioning in area CA1 (185.7 ± 18.40% of control [0 min]; P < 0.01, n = 5) and 60 min after training in the DG (151.2 ± 6.28% of control [0 min]; P < 0.01, n = 4). Nuclear c-Rel levels returned to basal levels 3 h after fear conditioning in both CA1 and DG regions (Fig. 1C,D).
Figure 1.
Temporal change of nuclear levels of c-Rel during long-term memory formation. (A) c-Rel protein expression in area CA1 in the hippocampus was analyzed by Western blotting. c-Rel is expressed predominantly in the cytoplasm of the CA1 region of the hippocampus and slightly in the nucleus. c-rel−/− mice do not express c-Rel in either the cytoplasm or the nucleus. (B) Cytoplasmic marker MKP-3 and nuclear marker lamin A were used to show the purity of cytoplasmic and nuclear extracts. (C) Mice were trained using a contextual fear conditioning paradigm and sacrificed at different times after training (15 min, 30 min, 1 h, and 3 h). Nuclear fraction was prepared from area CA1 and the DG of the hippocampus, and amount of c-Rel protein was assessed using Western blotting. Nuclear marker protein lamin A was used as a loading control. Level of c-Rel in the nucleus was significantly increased at 30 min after training in area CA1 (F[4.19] = 5.505; P < 0.01; n = 5); **P < 0.01. (D) Level of c-Rel in the nucleus was significantly increased at1 h after fear conditioning in DG (F[4.15] = 5.900; P < 0.01; n = 4); **P < 0.01.
This increase of nuclear levels of c-Rel during memory formation is consistent with the accepted paradigm of NF-κB regulation, in which activated NF-κB dimer is translocated from the cytoplasm to the nucleus as part of the transcriptional activation mechanism. Therefore, our results suggest that c-Rel is transcriptionally active in the hippocampus during hippocampus-dependent memory formation.
Stereological analysis of hippocampal volume and total numbers of neurons in the CA1
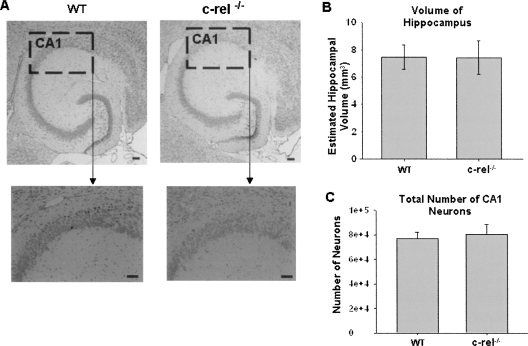
To identify the functional role of c-Rel in long-term memory formation, we used a gene knockout approach to test whether c-Rel is necessary for memory formation. As a control for subsequent behavioral testing in these mice, cresyl violet-stained hippocampal sections from wild-type and knockout mice were examined for differences in hippocampal volume and total number of pyramidal neurons in area CA1. Despite the absence of c-Rel, c-rel−/− mice did not demonstrate gross changes in hippocampal volume or total number of pyramidal neurons in CA1 compared with wild-type littermates (Fig. 2).
Figure 2.
Stereological analysis of total numbers of pyramidal neurons in hippocampal area CA1 and quantification of hippocampal volume. (A) Cresyl violet-stained horizontal section of hippocampus from c-rel−/− and wild-type littermate mice were compared for the possible presence of gross morphological changes in c-rel−/− mice. We found no gross morphological changes or differences in the hippocampus between c-rel−/− and wild-type littermate mice. Total hippocampal volume (B) and CA1 pyramidal neuron number (C) were determined from horizontal cresyl violet sections. The hippocampal volume and CA1 neuron numbers were not different between c-rel−/− and wild-type littermate mice. n = 3 wild-type and 3 c-rel−/− (male only). Scale bars, 100 μm.
Basal behavioral characterization of c-rel−/− mice
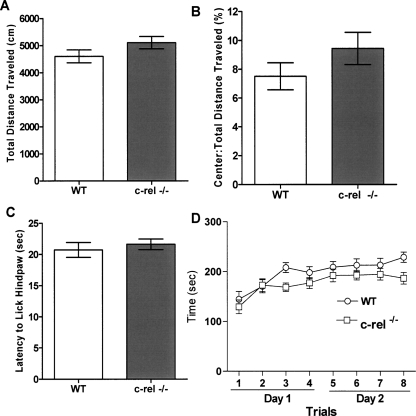
We initially undertook a basal behavioral characterization of c-rel−/− mice and wild-type littermates for control purposes. By use of the open field task, c-rel−/− mice exhibited similar levels of spontaneous locomotor activity compared with wild-type littermates, as assessed by total distance traveled (Fig. 3A). They also exhibited similar levels of basal anxiety relative to wild-type littermates as assessed by the ratio of the center:total distance traveled in the open field task (Fig. 3B). Thermal sensitivity, as assessed by the hot-plate test, revealed no significant differences between c-rel−/− and wild-type littermates (Fig. 3C). Moreover, c-rel−/− mice exhibited similar levels of motor coordination and learning compared with wild-type littermates as assessed using the rotarod task (Fig. 3D).
Figure 3.
Basal behavioral characterization of c-rel−/− mice. Several behavioral tasks were performed in both c-rel−/− and wild-type littermate mice to measure their overall levels of locomotor activity, anxiety, nociception, and motor learning. (A) c-rel−/− mice have similar levels of locomotor activity relative to wild-type littermates as assessed by total distance traveled during a 15 min session in the open field task. n = 25 wild-type (14 male, 11 female) and 27 c-rel−/− (18 male, nine female). (B) c-rel−/− mice have similar levels of basal anxiety relative to wild-type littermate as assessed by the ratio of the center:total distance traveled in the open field task. n = 25 wild-type (14 male, 11 female) and 27 c-rel−/− (18 male, nine female). (C) No significant difference was seen in thermal sensitivity between c-rel−/− and wild-type littermates, indicating that nociception is comparable between the two groups of mice. n = 25 wild-type (14 male, 11 female) and 27 c-rel−/− (18 male, nine female). (D) Motor learning of c-rel−/− mice was measured using an accelerating rotarod test. In this test, c-rel−/− mice did not show motor learning deficits compared with wild-type littermates. n = 18 wild-type (11 male, seven female) and 20 c-rel−/− (13 male, seven female).
Impaired hippocampus-dependent fear memory in c-rel−/− mice
To determine whether c-rel−/− mice have deficits in long-term memory formation, we used two types of fear-conditioning paradigms. Amygdala-dependent long-term memory formation was assessed via a cued fear conditioning paradigm, and hippocampus-dependent long-term memory formation was assessed via a contextual fear conditioning paradigm (Phillips and LeDoux 1992). Mice were trained to a single pairing of tone and shock. The next day, for assessing long-term memory formation, freezing behavior was measured in the two different kinds of environments. For the contextual test, mice were tested for freezing 24 h after training by re-placing them in the training environment, but without the tone. Subsequently, for the cue test, mice were tested for freezing 4 h after the contextual test in a novel environment with or without tone.
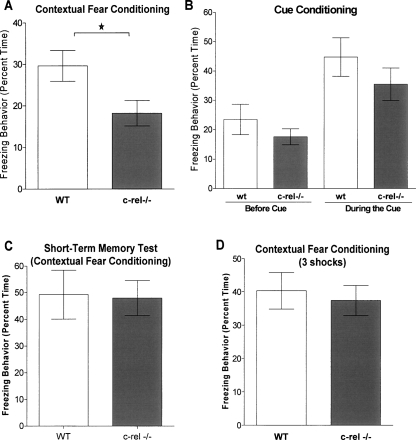
In this modest training paradigm, c-rel−/− mice had a significant deficit in freezing behavior compared with wild-type littermates in the contextual test (P < 0.05) (Fig. 4A). However, in the cue test, c-rel−/− mice displayed similar freezing behavior to wild-type littermates (Fig. 4B). These results indicate that c-rel−/− mice have impaired hippocampus-dependent memory formation. The lack of a significant difference between wild-type and c-rel−/− animals in the cued test suggests that they have normal amygdala-dependent memory formation, although there is a nonsignificant trend toward diminished freezing in this test as well. c-rel−/− mice exhibited no diminution of short-term (1 h) contextual fear memory (Fig. 4C).
Figure 4.
c-rel−/− mice have impaired hippocampus-dependent fear memory. c-rel−/− and wild-type littermate mice were trained in two different fear conditioning paradigms and then tested for freezing behavior. Hippocampus-dependent long-term memory formation was assessed via a contextual fear conditioning paradigm, and amygdala-dependent long-term memory formation was assessed via a cued fear conditioning paradigm. Mice were trained with modest training protocol (one exposure to cue and shock), and freezing behavior was measured 24 h after training. (A) In the contextual test, c-rel−/− mice had a significant deficit in freezing behavior assessed 24 h after training (*P < 0.05). n = 9 wild-type (seven male, two female) and 9 c-rel−/− (seven male, two female). (B) In the cued test, no significant difference was seen in freezing behavior between c-rel−/− and wild-type littermates when mice were tested in the absence or the presence of an auditory cue 24 h after training. n = 9 wild-type (seven male, two female) and 9 c-rel−/− (seven male, two female). (C) To assess short-term contextual memory in c-rel−/− mice, in a parallel experiment, freezing behavior was measured 1 h after training via a context re-exposure test. c-rel−/− mice did not show any deficits in short-term fear memory in this test. n = 6 wild-type (four male, two female) and 7 c-rel−/− (five male, two female). (D) c-rel−/− mice showed no deficits in long-term fear memory with a more robust training paradigm consisting of three training trials instead of one. n = 12 wild-type (seven male, five female) and 13 c-rel−/− (eight male, five female).
To determine whether long-term contextual fear memory could be recovered in c-rel−/− mice, mice were exposed to a robust training paradigm consisting of three mild footshocks spaced 2 min apart. c-rel−/− mice exhibited similar levels of freezing behavior compared with wild-type littermates 24 h after the robust training procedure (Fig. 4D), indicating that loss of c-Rel did not abolish the ability for animals to form a new memory, and is consistent with our previous findings suggesting that loss of c-Rel does not result in profound changes in hippocampal ultrastructure or function. These data also illustrate that c-rel−/− mice are capable of normal levels of freezing behavior when exposed to a more intense training paradigm. This observation also provides an interesting parallel to prior studies in which memory deficits in genetically engineered mice could be overcome with more robust training protocols. Thus, like c-rel−/− mice, CaMKII T286A mutants (Irvine et al. 2005), CREB hypomorphs (Hebda-Bauer et al. 2005), and transgenic Alzheimer’s disease model mice (Dineley et al. 2002) all exhibit long-term memory deficits that can be overcome with additional training. Whether this compensatory memory capacity occurs at the molecular, cellular, or circuit level is unclear at this time.
Impaired novel object recognition memory in c-rel−/− mice
We next evaluated whether c-rel−/− mice have deficits using another test for hippocampus-dependent memory formation. Novel object recognition is in part a hippocampus-dependent memory test based on the natural tendency of mice to investigate a novel object rather than a familiar object, when both objects are simultaneously present in an open field (Rampon et al. 2000; Broadbent et al. 2004; Hammond et al. 2004). This test does not involve primary reinforcement such as food or electric shocks, making it comparable to memory tests currently used in humans (Ennaceur and Delacour 1988).
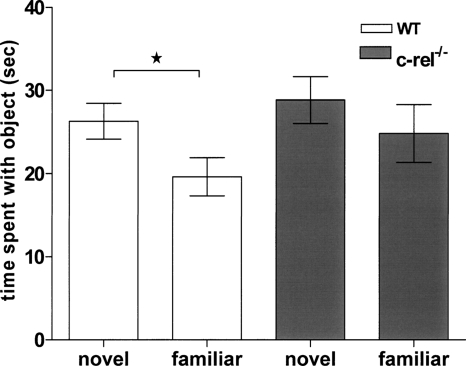
Novel object recognition training and testing was performed over five consecutive days. Mice were individually habituated to the training/testing environment and to Lego blocks over the first 3 d. On day four, two identical objects made from Legos were placed near the corners in opposite sides of the training/testing box. Each mouse was then placed in the middle of the box and allowed to freely investigate the objects for 10 min. On day 5, one of the familiar objects was replaced with a novel object. Wild-type littermate animals showed a significant preference for the novel object (P < 0.05). However, c-rel−/− mice did not exhibit a preference for the novel object (Fig. 5). These results indicate that c-Rel is necessary for the formation of long-term object recognition memory in mice.
Figure 5.
Impaired novel object recognition memory in c-rel−/− mice. The object recognition task is based on the natural tendency of mice to investigate a novel object rather than a familiar object, and object recognition memory also depends on hippocampus. During the training day, two identical objects were placed in the open field box. Each mouse was then placed in the middle of box and allowed to freely investigate the objects for 10 min. On the test day (24 h after training), one of the familiar objects was replaced with a novel object, and the amount of time the mouse explored each object was recorded. In this test, wild-type littermate showed statistical significant preference to the novel object (*P < 0.05). However, c-rel−/− mice did not distinguish the novel object from the familiar object. These results indicate that c-rel−/− mice have a deficit in hippocampus-dependent object recognition memory. n = 13 wild-type (nine male, four female) and 14 c-rel−/− (10 male, four female).
Impairment of long-term synaptic plasticity in c-rel−/− mice
Synaptic plasticity is thought to be a key mechanism contributing to learning and memory (Whitlock et al. 2006). The best-studied form of synaptic plasticity is LTP, which is a robust and long-lasting increase in synaptic strength. To investigate whether hippocampus-dependent memory deficits coincided with alterations in hippocampal synaptic plasticity, we studied LTP and baseline synaptic transmission in the CA1 region of hippocampal slices. LTP can be divided into at least two temporally distinct forms. The first stage is early LTP (E-LTP), lasting from 30 min to ∼90 min, and the second phase is late LTP (L-LTP), lasting several hours in vitro and up to a few weeks in vivo (Adams and Sweatt 2002). Transcriptional regulation is a critical mechanism for induction of L-LTP, and mRNA synthesis inhibitor studies have provided evidence of a transcription requirement for L-LTP (Nguyen et al. 1994; Levenson et al. 2004a). Therefore, we hypothesized that loss of the transcription factor c-Rel might affect L-LTP induction.
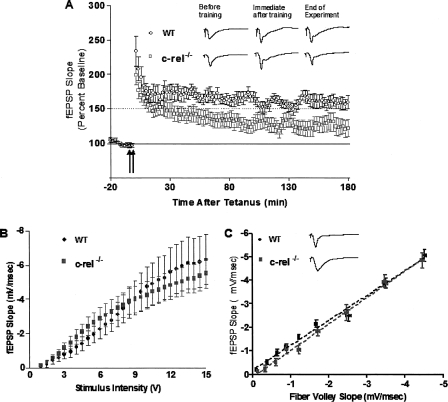
To test this hypothesis, LTP was measured at Schaffer collateral synapses in hippocampal slice from c-rel−/− mice and wild-type littermates for 3 h after tetanic stimulation. LTP in c-rel−/− slices was compared to that in wild-type littermates by inducing LTP using high-frequency stimulation consisting of two trains of 100-Hz stimulation for 1 sec, separated by 20 sec. LTP induced by this high-frequency stimulation protocol is NMDA receptor dependent and transcription dependent (Selcher et al. 2003; Levenson et al. 2004b). LTP in c-rel−/− slices exhibited similar levels of LTP versus wild-type littermate slices until ∼30 min after LTP induction. However, after that time point, levels of synaptic efficacy in c-rel−/− slices were significantly less than wild-type littermates (P < 0.0001) (Fig. 6A). These results indicate that L-LTP in hippocampus of c-rel−/− mice is impaired. Furthermore, these results are consistent with the hippocampus-dependent memory deficits we observed in c-rel−/− mice and are in agreement with our hypothesis that c-Rel is involved in transcriptional regulation mechanisms for long-term synaptic plasticity.
Figure 6.
Impaired short-term and long-term synaptic plasticity in c-rel−/− mice. (A) LTP at Schaffer collateral synapses in hippocampal slices of c-rel−/− mice was compared with wild-type littermate controls, after two trains of 100-Hz stimulation for 1 sec separated by 20 sec at 30°C. c-rel−/− slices exhibited significantly less potentiation after LTP induction relative to wild-type littermate slices. F[1,1504] = 433.00, P < 0.0001; n = 10 wild-type and 9 c-rel−/− (male only). (B) To analyze basal synaptic transmission of c-rel−/− mice, input/output functions for stimulus intensity versus fEPSP was measured. The input/output function in c-rel−/− mice was not different from wild-type littermate. (C) Basal synaptic transmission was also assessed by examining the correlation between the slopes of fEPSP and fiber volleys, known as the input/output relationship. c-rel−/− mice and wild-type littermate had comparable input/output relationships.
In order to determine whether c-rel−/− mice exhibited normal baseline neurotransmitter release, we investigate input/output functions for stimulus intensity versus evoked field excitatory post-synaptic potentials (fEPSP); that is, we measured the relationship between the slopes of fEPSPs versus fiber volleys in c-rel−/− and control mice. c-rel−/− mice and wild-type littermates had comparable input/output functions (Fig. 6B,C). This finding indicates that there is no difference in baseline synaptic function between c-rel−/− mice versus wild-type littermates. A previous study (O’Riordan et al. 2006) showed that the input/output relation of hippocampal Schaeffer collateral-CA1 transmission, as determined by the ratio of fEPSP slope versus fiber volley slope, was reduced at high stimulus strengths in animals lacking c-Rel versus littermate controls. In the present study we limited our stimulus intensities to the lower, linear range of stimulus strengths, where differences between knockouts and wild types are not present (see Fig. 6B,C).
Identification of memory-associated genes potentially regulated by c-Rel
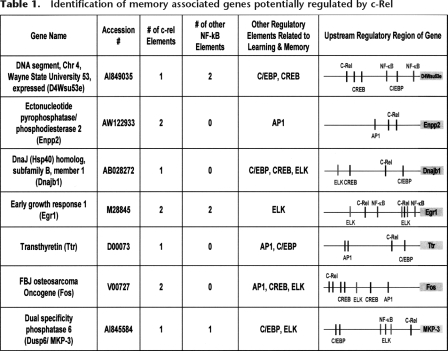
Our behavioral and electrophysiological data strongly suggest that the transcription factor c-Rel is involved in hippocampus-dependent long-term memory formation and synaptic plasticity. Therefore, as a next step in our studies, we determined genes potentially regulated by c-Rel during long-term memory formation. In a prior microarray study, expression of genes in area CA1 was analyzed at 1 h, 2 h, 4 h, and 6 h after fear conditioning, and a total of 35 genes were identified as memory-associated genes based on having their transcription altered after contextual fear conditioning. Upstream regions of genes that were identified in our previous expression array profiling and bioinformatics study were further analyzed for the presence of the c-Rel regulatory binding site using the program MatInspector (Levenson et al. 2004a). In the present study, we identified the memory-associated genes that contained likely c-Rel elements in their DNA upstream region. Interestingly, c-Rel elements were detected in seven out of eight genes regulated at 1 h after fear conditioning (see Table 1; Levenson et al. 2004a). Among memory-associated genes regulated at other time points after fear conditioning, <45% of genes contained putative c-Rel elements. This finding is consistent with our biochemical data, which indicate that nuclear levels of c-Rel are significantly increased within 1 h after fear conditioning. In addition, we screened for regulatory elements in randomly selected genes expressed in the hippocampus. Among these 35 randomly selected genes expressed in the hippocampus, only eight genes contained putative c-Rel elements. This result confirmed that identification of c-Rel elements in memory-associated genes at 1 h after fear conditioning is not due to random chance (P < 0.05, Fisher’s exact test).
Table 1.
Identification of memory associated genes potentially regulated by c-Rel
From our current bioinformatics analysis, we hypothesize that these genes (containing c-Rel elements) are regulated by c-Rel during memory consolidation in the hippocampus. The upstream regions (1 kilobase) of these seven genes were further analyzed using the program MatInspector to identify other putative NF-κB elements and binding elements for other transcription factors related to memory formation, such as CREB, C/EBP, ELK, and AP1 (Table 1). All seven candidate memory genes contained likely regulatory elements for additional memory-associated transcription factors as well.
In addition, we investigated which genes were already identified as bona fide NF-κB target gives in cell biological studies of the immune system. Reassuringly, four out of seven genes in our list (Egr1, Enpp2, Fos, and MKP-3) have already been identified as known NF-κB target genes in other studies (Li et al. 2001, 2002; Carayol et al. 2006), and even though Dnajb1 has not been directly shown to be an NF-κB target, Dnajb1 gene expression is closely associated with NF-κB activation (Fribley et al. 2004). The other two genes (transthyretin and D4Wsu53e) of our list of seven have not previously been associated with NF-κB function. The function of D4Wsu53e is unknown, but our study identifies it as a novel candidate for a c-Rel-dependent memory-associated gene. Interestingly, three of the genes, egr1/Zif268, transthyretin, and fos, have been directly implicated in hippocampus-dependent memory based on other studies of genetically engineered mice (Jones et al. 2001; Fleischmann et al. 2003; Sousa et al. 2007). Overall, our studies suggest these genes are memory associated targets of c-Rel regulation.
Discussion
One of the most intriguing questions in contemporary neuroscience is the interplay between environment and molecular genetic processes within neurons to determine an animal’s behavior. This is especially the case because it is becoming increasingly clear that altered gene expression is a typical molecular response in the CNS triggered by environmental stimuli. Such molecular processes are associated with changes in synaptic strength within behavior-mediating neuronal circuits, and these changes subsequently result in altered behavior. Our prior gene expression array and bioinformatics study showed how memory-inducing environmental stimuli could trigger complex changes in gene expression in mouse hippocampus, and identified several novel transcription factors that might be involved in consolidation of long-term memory (Levenson et al. 2004a). Based on our findings in this earlier bioinformatics study, we hypothesized that the transcription factor c-Rel might be involved in synaptic plasticity and memory formation. The present study provides evidence that the NF-κB family transcription factor c-Rel plays an important role in long-term memory formation at the molecular level and is necessary for plasticity in neuronal circuitry and in animal behavior.
Previous work (Levenson et al. 2004a; O’Riordan et al. 2006), has also supported the hypothesis of a role for c-Rel in synaptic plasticity and memory formation. O’Riordan et al. (2006) demonstrated that c-Rel-deficient animals have decreased metabotropic glutamate receptor-dependent LTD at Schaffer/collateral synapses, and a deficit in passive avoidance memory. In additional preliminary studies using nonlittermate animals, Levenson et al. (2004a) also found that c-Rel-deficient animals had a deficit in contextual fear conditioning. These preliminary studies prompted us to undertake the more thorough study described in this article, using littermate control animals assessed using several behavioral learning paradigms and evaluating the leading candidate for a cellular mechanism mediating long-term memory and LTP at Schaffer/collateral synapses. In the present studies, we observed deficits in hippocampal LTP, novel object recognition, and contextual fear conditioning in knockout animals lacking the c-Rel transcription factor.
Consolidation of long-term memory requires new mRNA synthesis. Therefore, in order to start transcription of new mRNAs, signals must be transferred from activated synapses to the nucleus. p50 and p65 NF-κB, other members of the Rel transcription factor family, have already been identified as candidates for synapse-to-nucleus messenger molecules. Several pioneering studies have supported this idea. In cerebellar granule neurons, NMDA-receptor activation regulates the p65 isoform of NF-κB (Guerrini et al. 1995), and LTP-induced PKC activation facilitates nuclear translocalization of p65 and p50 in rat hippocampus (Meberg et al. 1996). In addition, Meffert et al. (2003) observed p65 movement from activated synapses to the nucleus in hippocampal neuronal cell culture. Our prior results using behavioral training and gene chip analysis suggested that a specific member of the NF-κB superfamily, c-Rel, played a critical role in gene regulation in long-term memory formation; this was based on our finding that c-Rel DNA-binding elements were highly enriched upstream of memory-associated genes in the hippocampus.
However, until the present study little was known about nuclear translocation of c-Rel during memory formation and the role of this transcription factor in transcriptional regulation during memory consolidation. Our study indicates that the nuclear levels of c-Rel increase during consolidation of long-term memory, and this result is consistent with our hypothesis that c-Rel translocates to the nucleus to regulate altered gene transcription necessary for consolidation of long-term memory. One interesting point from our data is that nuclear levels of c-Rel significantly increased within 1 h in both CA1 and DG after treatment and returned to the basal level 3 h after training (Fig. 1). This finding is consistent with several studies of memory-associated gene expression, which indicate that many long-term memory associated genes are unregulated ∼1 h after behavioral treatments or LTP induction (Levenson et al. 2004a; von Hertzen and Giese 2005; Park et al. 2006). Furthermore, our earlier bioinformatics and expression array analysis showed that most memory-associated genes regulated at 1 h after behavioral training contain putative c-Rel elements in their DNA upstream regions, within 1 kb of their initiation start site. In addition, Freudenthal et al. (2005) investigated a temporal course of hippocampal NF-κB DNA-binding activity after inhibitory avoidance training. The temporal pattern of NF-κB DNA-binding activity in their study is very similar to that of nuclear levels of c-Rel in our study. Therefore, these parallel studies suggest that c-Rel is an attractive candidate for a transcription factor that is critical for the regulation of long-term memory formation. The present studies, wherein we investigated LTP and memory formation in c-Rel deficient animals, support this hypothesis.
In a previous study (Levenson et al. 2004a), we performed pilot behavior experiments with c-rel−/− mice investigating basic behaviors such as open field mobility, hotplate responses, and fear conditioning using robust training. The pilot data from those studies suggested that c-rel−/− animals were deficient in contextual fear conditioning and modestly hypoactive in the open field test relative to age-matched wild-type mice from a similar background strain. Our previous use of nonlittermate age- and strain-matched wild-type control animals necessitated repeating and greatly extending our behavioral characterization of c-Rel-deficient animals using appropriate controls and a much wider battery of behavioral tests. In our pilot studies, c-rel−/− mice had impaired fear memory as assessed using a robust-training contextual fear conditioning paradigm. However, in the present experiments with littermate wild-type controls, there were no significant differences between c-rel−/− mice and littermate wild type in the same test. We should note here that these differences did not appear to arise from changes in the behavior of the c-rel−/− mice, which exhibited comparable levels of freezing in both studies, but rather appear to be due to differences in performance between age-/strain-matched and littermate wild-type controls. Thus, the behavioral indexes of c-rel−/− mice from the previous and the present study were almost identical, while the wild-type control data were different. An additional difference in the data obtained in the present study versus our prior study was exhibited in the open field test. In open-field behavioral testing, c-rel−/− mice were hypoactive relative to the age-/strain-matched wild-types in our earlier pilot study (Levenson et al. 2004a). However, in the present experiments with littermate wild types, c-rel−/− mice exhibited similar levels of locomotor activity relative to controls. Overall, these results demonstrate the importance of littermate controls in behavioral experiments, where differences in baseline behaviors between strain-matched controls versus true littermate controls may complicate the interpretation of results.
The present study suggested that c-Rel is necessary for hippocampus-dependent memory formation but not amygdala-dependent memory formation. However, some previous studies demonstrated a requirement of NF-κB activation in amygdala-dependent fear memory formation (Yeh et al. 2002, 2004). In these studies using amygdala tissue (Yeh et al. 2002), the p65 and p50 subunits, but not c-Rel, increased their DNA-binding activity during an amygdala-dependent memory task. Therefore, a comparison of these results suggests that c-Rel is involved more specifically in hippocampus-dependent memory formation versus other NF-κB subunits.
NF-κB family proteins are comprised of homo- and heterodimers of five family members. So far, at least 12 dimers have been identified as having affinity for κB recognition sequences (Hoffmann et al. 2006). Indeed, there are different dimerization preferences between the five family members. The c-Rel homodimer appears to be more stable than the p65 homodimer. The p65/p50 heterodimer and c-Re homodimer are more abundant than c-Rel/p50 heterodimer or others based on studies in many cell types. However, the relative abundance of different NF-κB dimers differs between each cell type, to allow specificity in NF-κB pathway signaling in specific cell types (Hoffmann et al. 2006). Even though the different dimer configurations have broad sequence recognition specificities that largely overlap, the different dimmers have differential affinity for κB binding sequences, and each of the NF-κB proteins also has nonredundant functions. For instance, electrophoretic mobility shift assays show that c-Rel/p65 heterodimer binds to κB-like sequences (5′-CGGAGTTTCC-3′) but p65/p50 heterodimer does not (Parry and Mackman 1994). Using a fluorescence polarization assay, Huang et al. (2001) observed that c-Rel homodimer binds to the IL-2 CD28RE (CD28 response element) with higher affinities than to other κB consensus sequences. In contrast, p50 homodimer and p65/p50 heterodimer bind to CD28RE weakly. In addition, each distinct member has its own selective DNA-binding activity for specific target genes (Sanjabi et al. 2005), and the DNA-binding activity of each NF-κB member can be directly and selectively modified by phosphorylation and acetylation (Chen et al. 2001; Martin et al. 2001; Yeh et al. 2004). Consistent with the idea of differential roles and regulation of individual dimer subtypes, the available NF-κB gene-knockout animal models indicate distinct roles for each NF-κB family member in immune system function (Li and Verma 2002; Liou and Hsia 2003). Overall, these considerations indicate that c-Rel has its own specific target genes and c-Rel DNA-binding activity, and can be directly modulated by unique post-translational modifications, suggesting that c-Rel could play a unique role in long-term synaptic plasticity and memory formation. Indeed, the present results indicate that the presence of the other NF-κB family members in our c-rel−/− mice is not sufficient to compensate for the unique absence of c-Rel.
Several prior studies had suggested that NF-κB transcription factors might play an important role in synaptic plasticity. A study using NF-κB decoy DNA treatment showed that inhibition of NF-κB prevented induction of LTD and significantly reduced LTP expression (Albensi and Mattson 2000). Additionally, overexpression of the NF-κB super repressor (IκBα-AA) caused an impairment of LTD and L-LTP (Kaltschmidt et al. 2006). Based on these prior studies, an important point to keep in mind is the fact that inhibition of NF-κB leads to an impairment of LTD as well as LTP. In the present study, we found that loss of c-Rel led to an impairment of L-LTP, and a previous study demonstrated that c-Rel plays a role in the induction of mGluR-LTD in the hippocampus (O’Riordan et al. 2006). In addition, previous studies using nonisoform selective inhibitors of NF-κB signaling also attenuated both LTP and LTD (Albensi and Mattson 2000; Kaltschmidt et al. 2006). Taken together, our results and others demonstrate that NF-κB family members generally, and c-Rel specifically, are involved in bidirectional regulation of synaptic function in the hippocampus.
How does one single molecule participate in both forms of plasticity, which alter synaptic strength in opposite directions? When we consider the bidirectional characteristics of NF-κB signaling in apoptosis and anti-apoptosis, this Janus face of NF-κB in synaptic plasticity is not surprising (Silverman and Maniatis 2001; Li and Verma 2002; Kucharczak et al. 2003). NF-κB proteins can suppress apoptosis by promoting the expression of anti-apoptotic genes, while they can induce apoptosis in response to certain death-inducing signals. The type of stimulus and the duration of stimulus determine NF-κB’s destiny as a promoter or protector of neuronal cell death (Kaltschmidt et al. 2002; Pizzi et al. 2002). The role of c-Rel in LTP or LTD is likely to depend on the type of stimulus, the duration of stimulus, and specific signaling pathways that activate c-Rel. In our studies, c-Rel participates in formation of LTP via NMDA receptor-dependent signaling pathway and in the formation of LTD via mGluR receptor-dependent signaling pathway.
One question worth considering is whether the deficits in synaptic plasticity and memory formation that we observed in c-rel−/− mice originate from a neuro-developmental defect. Thus far, several studies have documented the participation of NF-κB in neural development (Gutierrez et al. 2005; Memet 2006). According to these earlier studies, general NF-κB signaling regulates neurite growth and cell survival in the developing nervous system. However, neurite growth and neural cell survival were changed via regulating of expression of p65 or degradation of IκB. Therefore, the specific function of c-Rel in the developing nervous system is still unknown. Schmidt-Ullrich et al. (1996) investigated the pattern of NF-κB activity during mouse development using lacZ reporter construct. In their study, lacZ expression in mouse hippocampus was not observed until 2 wk after birth, and changes of NF-κB activity in the cortex were not observed until 3 wk after birth. Recently, Gray et al. (2004) analyzed the expression of over 1000 transcription factors and their coregulated genes during development in the mouse brain, and according to their study, among NF-κB transcription factors only p50 was expressed in E13.5 embryos or in P0 mice. c-Rel and other NF-κB members were not expressed during mouse brain development but rather were only present in older animals. In addition, previous studies with c-rel−/− mice have suggested that c-rel−/− mice do not have generalized developmental defects in lymphocyte and macrophage function (Köntgen et al. 1995; Tumang et al. 1998; Liou and Hsia 2003). Moreover, our histological data indicate that there are no gross morphological changes or differences in neuron number in the hippocampus of c-rel−/− mice compared with wild-type littermates (Fig. 2), and our basal behavioral studies also suggest that c-rel−/− mice do not have any appreciable baseline defects (Fig. 4). All these results suggest that the impaired long-term synaptic plasticity and memory formation that we observe in c-rel−/− mice were not caused by developmental or structural defects.
In summary, in these studies we investigated the role of the transcription factor c-Rel in long-term memory formation using molecular, cellular, and behavioral approaches. We found that nuclear levels of c-Rel were regulated in the hippocampus during long-term memory formation. In addition, c-rel−/− mice showed impaired long-term synaptic plasticity and behavioral deficits in hippocampus-dependent long-term memory formation. Furthermore, using a bioinformatics approach, we have been able to predict several bona fide memory-associated genes (fos, Zif268/egr-1, and transthyretin) potentially regulated by c-Rel. Thus, c-Rel can be added to a small but growing list of transcription factors that are critical in mediating changes in gene expression that are involved in hippocampal synaptic plasticity and memory formation.
Materials and Methods
Animals
C57BL/6J (6-8weeks old) mice were obtained from the Baylor College of Medicine transgenic mouse facility. Generation of c-rel−/− mice has been previously described (Tumang et al. 1998). Animals were housed under a 12-h light/dark cycle and were given ad libitum access to food and water. All procedures performed with the approval of the Baylor College of Medicine Institutional Animal Care and Use Committee and according to national guidelines and policies. All behavioral experiments were done with the experimenter blind to genotype. Wild-type littermate controls from heterozygote breeding were used in all behavioral and electrophysiological experiments.
Fear conditioning
Mice were allowed to acclimate to the testing room at least 2 h prior to use. Mice were trained in one of two training paradigms, a modest or intense contextual training paradigm. In modest training, mice were allowed to explore the training chamber for 1.5 min and then were exposed to a cue, a white noise tone (90 dB, 30 sec). During the last 2 sec of the tone, a single mild footshock (0.5 mA) was applied to the floor grid, and mice were removed from the training chamber 1 min after footshock. Freezing behavior was measured by observing mice for 1 sec every 5 sec. Contextual learning was assessed 24 h after training by measuring freezing behavior in the training chamber for 3 min. Cued fear learning was assessed 4 h after the contextual test by placing mice in a novel test cage with altered cage floor, dimension, color, and odor. Mice were exposed to the novel context for 3 min prior to a 3-min presentation of the cue used during training. Freezing behavior was measured during the entire 6-min period of cue testing. The robust contextual training was performed as previously described (Levenson et al. 2004a). Mice were allowed to explore the training chamber for the first 2 min and then received three mild footshocks (1 sec, 0.5 mA) spaced 2 min apart. Mice were removed from the training chamber 1 min after the last footshock. Contextual learning was assessed 24 h after training by re-exposing mice to the same training chamber for 7 min.
Western blotting
C57BL/6 (6–8 wk) mice were trained using the robust contextual fear conditioning paradigm and sacrificed at four different times after exposure to the training (15 min, 30 min, 1 h, and 3 h). For preparation of naïve samples, i.e., controls, mice were allowed to acclimate to the testing room for 2 h and then sacrificed without training or exposure to a novel context. Nuclear extracts were prepared using NE-PER nuclear and cytoplasmic extraction reagents (Pierce) from hippocampal subregions: area CA1 and the DG according to the manufacturer’s instructions. Briefly, small pieces of tissue samples were homogenized in ice-cold cytoplasmic extraction reagent I with a Dounce homogenizer. The sample was vortexed for 15 sec, followed by 10-min incubation on ice, addition of ice-cold cytoplasmic extraction reagent II, and centrifugation at 16,000g for 5 min at 4°C. The supernatant (cytoplasmic extract) was immediately transferred to a clean prechilled tube. The nuclear pellet was resuspended with ice-cold nuclear extraction reagent, vortexed for 15 sec, and incubated on ice for 40 min. The extract was centrifuged at 16,000g for 10 min at 4°C, and the supernatant (nuclear extract) was frozen at −70°C. Nuclear extracts were resolved on 10% SDS-polyacrylamide gels and transferred to PVDF membranes (Immobilon, Millipore) membranes. The membranes were blocked in 3% BSA and 5% skim milk in TTBS buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.05% Tween 20) for 1 h and then incubated in the primary antibody overnight at 4°C. Primary antibodies used were c-Rel (1:100), MKP-3 (1:300, Santa Cruz Biotechnology), and Lamin A/C (1:500, Upstate Biotechnology). The membranes were washed in TTBS buffer and then incubated in HRP-conjugated anti-rabbit or anti-mouse secondary antibody (Jackson ImmunoResearch) for 1 h at room temperature. The membranes were detected with either ECL (Amersham Bioscience) or Super Signal (Pierce). The immunoblots were quantified by densitometry using Scion image software (Scion Corporation) and levels of c-Rel were normalized to levels of nuclear marker protein, lamin A.
Cresyl violet staining and unbiased stereology
Mice were deeply anesthetized with 0.2% avertin and then transcardially perfused with ice-cold PBS (0.01 M phosphate, 0.15 M sodium chloride) followed by 4% paraformaldehyde (PFA) in PBS. Whole brains were extracted and post-fixed overnight at 4°C in 4.0% PFA. Tissue was processed for paraffin embedding and sagittally sectioned at 10 μm. Sections were deparaffinized, rehydrated, and stained with 0.5% cresyl violet. After thorough rinsing, sections were dehydrated and mounted with Permount (Fisher Scientific). CA1 pyramidal neuron number and total hippocampal volume were determined from horizontal cresyl violet sections taken from the entire extent of the hippocampus using a point counting method and optical fractionator methods with the aid of a computerized stereology system powered by Stereologer software (Systems Planning and Analysis Inc.) and hardware consisting of a Zeiss Axioskop microscope, motorized XYZ stage (Applied Scientific Instrumentation), Sony CCD video camera, TARGA video card, and personal computer/monitor.
Basal behavioral studies
For the open field test, a mouse was placed in the center of a clear Plexiglas box (43 × 43 cm), and locomotor activity was measured for 15 min by an automatic video tracking system (Limelight; Actimetrics). A thermal sensitivity test was performed using a hotplate Analgesia Meter (Columbus Instruments) at 53°C to measure the latency to lick a hind paw. The accelerating rotarod task was used to measure motor coordination, balance, and motor learning. Each 5-min trial began with a 30-sec acclimation period at 4 rpm followed by gradual acceleration to a maximum of 40 rpm over the next 5 min. Performance was measured as the latency for an animal to fall off of the rod. Four rotarod trials per day were performed over two consecutive days.
Novel object recognition
The novel object recognition task was performed in the open field apparatus for five consecutive days. Mouse movement and locations were tracked and recorded by an automatic video tracking system (Limelight; Actimetrics). During the first day, each mouse was placed in the empty open field and allowed to freely explore the testing arena for 15 min. During days 2 and 3, each mouse was placed in an open field with eight single Duplo Lego blocks for 10 min. Legos of various colors (blue, green, yellow, and red) were equally spaced 5 cm from the edge of the open field such that each color was present in a corner and the center of a wall. Days 1 through 3 allowed the animals to familiarize themselves with the apparatus and with experimenter handling. In day 4, two identical objects made of Legos were placed in two opposite corners of the box. Each mouse was then placed in the middle of box and allowed to freely investigate the objects for 10 min. The time spent to explore each object was recorded. Exploration of an object was defined as directing the nose to the object within a distance of 2 cm. Some behaviors such as turning around on or sitting on an object were not considered as exploration. In day 5, one of the familiar objects was replaced with a novel object and object exploration was recorded.
Electrophysiology
Hippocampal slices were prepared as previously described (O’Riordan et al. 2006). Extracellular field potential recordings in area CA1 are utilized to monitor synaptic transmission at the Schaffer collateral inputs. Four hundred–micrometer hippocampal slices were placed in a Fine Science Tools interface chamber, maintained at 30°C, and perfused with aerated ACSF (∼1 mL/min). An ACSF-filled glass recording electrode was placed in the stratum radiatum of area CA1, and a stimulating electrode was positioned along the Schaffer collateral afferents from CA3. Input/output (I/O) functions for stimulus intensity versus EPSP magnitude were recorded in response to increasing intensities of stimulation (0.5–15 V). For the remainder of the experiment, the test stimulus intensity was set to elicit an EPSP that was 50% of the maximum response recorded during the I/O measurements. Following I/O curve generation, paired-pulse facilitation was measured with various interstimulus intervals (10, 20, 50, 100, 200, and 300 msec). A minimum of 20 min of baseline stimulation (0.05 Hz) was recorded before the LTP induction. LTP was induced via high-frequency (100-Hz) stimulation, which consisted of two trains of 1-sec, 100-Hz stimulation with an intertrain interval of 20 sec.
Transcription factor bioinformatic analysis
Transcription factor analysis was performed as previously described (Levenson et al. 2004a). Briefly, upstream sequence information of each gene in Table 1 was obtained from the University of California, Santa Cruz Genome Browser (http://www.genome.ucsc.edu/). To identify putative transcription factor binding sites, 1 kbp upstream of the immediate upstream sequence was examined using MatInspector (Geomatix) (Cartharius et al. 2005). Putative binding sites for c-Rel and other memory related transcription factors were identified using an optimized matrix thresholds setting, with core similarity at >0.90 and matrix similarity at >0.80 to reduce false positives.
Statistical analysis
Comparison of nuclear levels of c-Rel in CA1 and the DG among four different times after training was made using one-way ANOVA with Bonferroni post-hoc test. The behavioral characterization of c-rel−/− mice in the open field, thermal sensitivity, and fear conditioning was analyzed using a two-tailed student’s t-test, and statistical significance in accelerating rotarod task and LTP study was determined by repeated-measured two-way ANOVA with Bonferroni post-hoc test. In the novel object recognition test, we used a two-tailed t-test to compare the preferences for novel or familiar objects in each group of micel, i.e., wild types versus knockouts were analyzed separately to assess whether each group overall learned the objects. In all tests, data were considered significant at P < 0.05.
Acknowledgments
We thank Dr. Richard E. Paylor for help with statistical analysis of the novel object recognition test. Grant support was from MH57014, NS13546, P30 NS047466, and P30 NS057098, McKnight Brain Research Foundation.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.866408.
References
- Adams J.P., Sweatt J.D. Molecular psychology: Roles for the ERK MAP kinase cascade in memory. Annu. Rev. Pharmacol. Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- Albensi B.C., Mattson M.P. Evidence for the involvement of TNF and NF-κB in hippocampal synaptic plasticity. Synapse. 2000;35:151–159. doi: 10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Atkins C.M., Selcher J.C., Petraitis J.J., Trzaskos J.M., Sweatt J.D. The MAPK cascade is required for mammalian associative learning. Nat. Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Bracchi-Ricard V., Brambilla R., Levenson J., Hu W.-H., Bramwell A., Sweatt J.D., Green E.J., Bethea J.R. Astroglial nuclear factor-κB regulates learning and memory synaptic plasticity in female mice. J. Neurochem. 2008;104:611–623. doi: 10.1111/j.1471-4159.2007.04993.x. [DOI] [PubMed] [Google Scholar]
- Broadbent N.J., Squire L.R., Clark R.E. Spatial memory, recognition memory, and the hippocampus. Proc. Natl. Acad. Sci. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carayol N., Chen J., Yang F., Jin T., Jin L., States D., Wang C.Y. A dominant function of IKK/NF-κB signaling in global lipopolysaccharide-induced gene expression. J. Biol. Chem. 2006;281:31142–31151. doi: 10.1074/jbc.M603417200. [DOI] [PubMed] [Google Scholar]
- Cartharius K., Frech K., Grote K., Klocke B., Haltmeier M., Klingenhoff A., Frisch M., Bayerlein M., Werner T. MatInspector and beyond: Promoter analysis based on transcription factor binding sites. Bioinformatics. 2005;21:2933–2942. doi: 10.1093/bioinformatics/bti473. [DOI] [PubMed] [Google Scholar]
- Chen L., Fischle W., Verdin E., Greene W.C. Duration of nuclear NF-κB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- Chen A., Muzzio I.A., Malleret G., Bartsch D., Verbitsky M., Pavlidis P., Yonan A.L., Vronskaya S., Grody M.B., Cepeda I., et al. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron. 2003;39:655–669. doi: 10.1016/s0896-6273(03)00501-4. [DOI] [PubMed] [Google Scholar]
- Dineley K.T., Xia X., Bui D., Sweatt J.D., Zheng H. Accelerated plaque accumulation, associative learning deficits, and up-regulation of α7 nicotinic receptor protein in transgenic mice co-expressing mutant human presenilin 1 and amyloid precursor proteins. J. Biol. Chem. 2002;277:22768–22780. doi: 10.1074/jbc.M200164200. [DOI] [PubMed] [Google Scholar]
- Ennaceur A., Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Fanselow M.S. Contextual fear, gestalt memories, and the hippocampus. Behav. Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Fleischmann A., Hvalby O., Jensen V., Strekalova T., Zacher C., Layer L.E., Kvello A., Reschke M., Spanagel R., Sprengel R., et al. Impaired long-term memory and NR2A-type NMDA receptor-dependent synaptic plasticity in mice lacking c-Fos in the CNS. J. Neurosci. 2003;23:9116–9122. doi: 10.1523/JNEUROSCI.23-27-09116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenthal R., Romano A., Routtenberg A. Transcription factor NF-κB activation after in vivo perforant path LTP in mouse hippocampus. Hippocampus. 2004;14:677–683. doi: 10.1002/hipo.20020. [DOI] [PubMed] [Google Scholar]
- Freudenthal R., Boccia M.M., Acosta G.B., Blake M.G., Merlo E., Baratti C.M., Romano A. NF-κB transcription factor is required for inhibitory avoidance long-term memory in mice. Eur. J. Neurosci. 2005;21:2845–2852. doi: 10.1111/j.1460-9568.2005.04126.x. [DOI] [PubMed] [Google Scholar]
- Fribley A., Zeng Q., Wang C.Y. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol. Cell. Biol. 2004;24:9695–9704. doi: 10.1128/MCB.24.22.9695-9704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., May M.J., Kopp E.B. NF-κB and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- Gray P.A., Fu H., Luo P., Zhao Q., Yu J., Ferrari A., Tenzen T., Yuk D.I., Tsung E.F., Cai Z., et al. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Guerrini L., Blasi F., Denis-Donini S. Synaptic activation of NF-κB by glutamate in cerebellar granule neurons in vitro. Proc. Natl. Acad. Sci. 1995;92:9077–9081. doi: 10.1073/pnas.92.20.9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez H., Hale V.A., Dolcet X., Davies A. NF-κB signalling regulates the growth of neural processes in the developing PNS and CNS. Development. 2005;132:1713–1726. doi: 10.1242/dev.01702. [DOI] [PubMed] [Google Scholar]
- Hammond R.S., Tull L.E., Stackman R.W. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol. Learn. Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hebda-Bauer E.K., Watson S.J., Akil H. Cognitive performance is highly sensitive to prior experience in mice with a learning and memory deficit: Failure leads to more failure. Neurobiol. Learn. Mem. 2005;12:461–471. doi: 10.1101/lm.94105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A., Natoli G., Ghosh G. Transcriptional regulation via the NF-κB signaling module. Oncogene. 2006;25:6706–6716. doi: 10.1038/sj.onc.1209933. [DOI] [PubMed] [Google Scholar]
- Huang D.B., Chen Y.Q., Ruetsche M., Phelps C.B., Ghosh G. X-ray crystal structure of proto-oncogene product c-Rel bound to the CD28 response element of IL-2. Structure. 2001;9:669–678. doi: 10.1016/s0969-2126(01)00635-9. [DOI] [PubMed] [Google Scholar]
- Igaz L.M., Vianna M.R., Medina J.H., Izquierdo I. Two time periods of hippocampal mRNA synthesis are required for memory consolidation of fear-motivated learning. J. Neurosci. 2002;22:6781–6789. doi: 10.1523/JNEUROSCI.22-15-06781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine E.E., Vernon J., Giese K.P. αCaMKII autophosphorylation contributes to rapid learning but is not necessary for memory. Nat. Neurosci. 2005;8:411–412. doi: 10.1038/nn1431. [DOI] [PubMed] [Google Scholar]
- Jones M.W., Errington M.L., French P.J., Fine A., Bliss T.V., Garel S., Charnay P., Bozon B., Laroche S., Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat. Neurosci. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt B., Heinrich M., Kaltschmidt C. Stimulus-dependent activation of NF-κB specifies apoptosis or neuroprotection in cerebellar granule cells. Neuromolecular Med. 2002;2:299–309. doi: 10.1385/NMM:2:3:299. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt B., Ndiaye D., Korte M., Pothion S., Arbibe L., Prullage M., Pfeiffer J., Lindecke A., Staiger V., Israel A., et al. NF-κB regulates spatial memory formation and synaptic plasticity through protein kinase A/CREB signaling. Mol. Cell. Biol. 2006;26:2936–2946. doi: 10.1128/MCB.26.8.2936-2946.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E.R. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kassed C.A., Willing A.E., Garbuzova-Davis S., Sanberg P.R., Pennypacker K.R. Lack of NF-κB p50 exacerbates degeneration of hippocampal neurons after chemical exposure and impairs learning. Exp. Neurol. 2002;176:277–288. doi: 10.1006/exnr.2002.7967. [DOI] [PubMed] [Google Scholar]
- Köntgen F., Grumont R.J., Strasser A., Metcalf D., Li R., Tarlinton D., Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes & Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- Kucharczak J., Simmons M.J., Fan Y., Gelinas C. To be, or not to be: NF-κB is the answer—role of Rel/NF-κB in the regulation of apoptosis. Oncogene. 2003;22:8961–8982. doi: 10.1038/sj.onc.1207230. [DOI] [PubMed] [Google Scholar]
- Levenson J.M., Choi S., Lee S.Y., Cao Y.A., Ahn H.J., Worley K.C., Pizzi M., Liou H.C., Sweatt J.D. A bioinformatics analysis of memory consolidation reveals involvement of the transcription factor c-Rel. J. Neurosci. 2004a;24:3933–3943. doi: 10.1523/JNEUROSCI.5646-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson J.M., O’Riordan K.J., Brown K.D., Trinh M.A., Molfese D.L., Sweatt J.D. Regulation of histone acetylation during memory formation in the hippocampus. J. Biol. Chem. 2004b;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Li Q., Verma I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Li J., Peet G.W., Balzarano D., Li X., Massa P., Barton R.W., Marcu K.B. Novel NEMO/IκB kinase and NF-κB target genes at the pre-B to immature B cell transition. J. Biol. Chem. 2001;276:18579–18590. doi: 10.1074/jbc.M100846200. [DOI] [PubMed] [Google Scholar]
- Li X., Massa P.E., Hanidu A., Peet G.W., Aro P., Savitt A., Mische S., Li J., Marcu K.B. IKKα, IKKβ, and NEMO/IKKγ are each required for the NF-κB-mediated inflammatory response program. J. Biol. Chem. 2002;277:45129–45140. doi: 10.1074/jbc.M205165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou H.C., Hsia C.Y. Distinctions between c-Rel and other NF-κB proteins in immunity and disease. Bioessays. 2003;25:767–780. doi: 10.1002/bies.10306. [DOI] [PubMed] [Google Scholar]
- Lubin F.D., Sweatt J.D. The IκB kinase regulates chromatin structure during reconsolidation of conditioned fear memories. Neuron. 2007;55:942–957. doi: 10.1016/j.neuron.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin F.D., Johnston L.D., Sweatt J.D., Anderson A.E. Kainate mediates nuclear factor-κB activation in hippocampus via phosphatidylinositol-3 kinase and extracellular signal-regulated protein kinase. Neuroscience. 2005;133:969–981. doi: 10.1016/j.neuroscience.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Martin A.G., San-Antonio B., Fresno M. Regulation of nuclear factor κB transactivation. Implication of phosphatidylinositol 3-kinase and protein kinase Cζ in c-Rel activation by tumor necrosis factor α. J. Biol. Chem. 2001;276:15840–15849. doi: 10.1074/jbc.M011313200. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L. Memory—a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Meberg P.J., Kinney W.R., Valcourt E.G., Routtenberg A. Gene expression of the transcription factor NF-κB in hippocampus: Regulation by synaptic activity. Brain Res. 1996;38:179–190. doi: 10.1016/0169-328x(95)00229-l. [DOI] [PubMed] [Google Scholar]
- Meffert M.K., Baltimore D. Physiological functions for brain NF-κB. Trends Neurosci. 2005;28:37–43. doi: 10.1016/j.tins.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Meffert M.K., Chang J.M., Wiltgen B.J., Fanselow M.S., Baltimore D. NF-κB functions in synaptic signaling and behavior. Nat. Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- Memet S. NF-κB functions in the nervous system: From development to disease. Biochem. Pharmacol. 2006;72:1180–1195. doi: 10.1016/j.bcp.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Nguyen P.V., Abel T., Kandel E.R. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- O’Mahony A., Raber J., Montano M., Foehr E., Han V., Lu S.-M., Kwon H., LeFevour A., Chakraborty-Sett S., Greene W.C. NF-κB/Rel regulates inhibitory and excitatory neuronal function and synaptic plasticity. Mol. Cell. Biol. 2006;19:7283–7298. doi: 10.1128/MCB.00510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Riordan K.J., Huang I.C., Pizzi M., Spano P., Boroni F., Egli R., Desai P., Fitch O., Malone L., Ahn H.J., et al. Regulation of nuclear factor κB in the hippocampus by group I metabotropic glutamate receptors. J. Neurosci. 2006;26:4870–4879. doi: 10.1523/JNEUROSCI.4527-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.S., Gong R., Stuart J., Tang S.J. Molecular network and chromosomal clustering of genes involved in synaptic plasticity in the hippocampus. J. Biol. Chem. 2006;281:30195–30211. doi: 10.1074/jbc.M605876200. [DOI] [PubMed] [Google Scholar]
- Parry G.C., Mackman N. A set of inducible genes expressed by activated human monocytic and endothelial cells contain κB-like sites that specifically bind c-Rel-p65 heterodimers. J. Biol. Chem. 1994;269:20823–20825. [PubMed] [Google Scholar]
- Phillips R.G., LeDoux J.E. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pizzi M., Goffi F., Boroni F., Benarese M., Perkins S.E., Liou H.C., Spano P. Opposing roles for NF-κB/Rel factors p65 and c-Rel in the modulation of neuron survival elicited by glutamate and interleukin-1beta. J. Biol. Chem. 2002;277:20717–20723. doi: 10.1074/jbc.M201014200. [DOI] [PubMed] [Google Scholar]
- Rampon C., Tang Y.P., Goodhouse J., Shimizu E., Kyin M., Tsien J.Z. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat. Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- Romano A., Freudenthal R., Merlo E., Routtenberg A. Evolutionarily conserved role of the NF-κB transcription factor in neural plasticity and memory. Eur. J. Neurosci. 2006;24:1507–1516. doi: 10.1111/j.1460-9568.2006.05022.x. [DOI] [PubMed] [Google Scholar]
- Sanjabi S., Williams K.J., Saccani S., Zhou L., Hoffmann A., Ghosh G., Gerondakis S., Natoli G., Smale S.T. A c-Rel subdomain responsible for enhanced DNA-binding affinity and selective gene activation. Genes & Dev. 2005;19:2138–2151. doi: 10.1101/gad.1329805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ullrich R., Memet S., Lilienbaum A., Feuillard J., Raphael M., Israel A. NF-κB activity in transgenic mice: Developmental regulation and tissue specificity. Development. 1996;122:2117–2128. doi: 10.1242/dev.122.7.2117. [DOI] [PubMed] [Google Scholar]
- Selcher J.C., Weeber E.J., Christian J., Nekrasova T., Landreth G.E., Sweatt J.D. A role for ERK MAP kinase in physiologic temporal integration in hippocampal area CA1. Learn. Mem. 2003;10:26–39. doi: 10.1101/lm.51103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Sheridan G.K., Pickering M., Twomey C., Moynagh P.N., O’Connor J.J., Murphy K.J. NF-κB activity in distinct neural subtypes of the rat hippocampus: Influence of time and GABA antagonism in acute slice preparations. Learn. Mem. 2007;14:525–532. doi: 10.1101/lm.590007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman N., Maniatis T. NF-κB signaling pathways in mammalian and insect innate immunity. Genes & Dev. 2001;15:2321–2342. doi: 10.1101/gad.909001. [DOI] [PubMed] [Google Scholar]
- Sousa J.C., Marques F., Dias-Ferreira E., Cerqueira J.J., Sousa N., Palha J.A. Transthyretin influences spatial reference memory. Neurobiol. Learn. Mem. 2007;88:381–385. doi: 10.1016/j.nlm.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Thut P.D., Lindell T.J. α-Amanitin inhibition of mouse brain form II ribonucleic acid polymerase and passive avoidance retention. Mol. Pharmacol. 1974;10:146–154. [PubMed] [Google Scholar]
- Tumang J.R., Owyang A., Andjelic S., Jin Z., Hardy R.R., Liou M.L., Liou H.C. c-Rel is essential for B lymphocyte survival and cell cycle progression. Eur. J. Immunol. 1998;28:4299–4312. doi: 10.1002/(SICI)1521-4141(199812)28:12<4299::AID-IMMU4299>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Verma I.M., Stevenson J.K., Schwarz E.M., Van Antwerp D., Miyamoto S. Rel/NF-κB/IκB family: Intimate tales of association and dissociation. Genes & Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- von Hertzen L.S., Giese K.P. Memory reconsolidation engages only a subset of immediate-early genes induced during consolidation. J. Neurosci. 2005;25:1935–1942. doi: 10.1523/JNEUROSCI.4707-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmann H., Kaltschmidt B., Kaltschmidt C. Retrograde transport of transcription factor NF-κB in living neurons. J. Biol. Chem. 2001;276:11821–11829. doi: 10.1074/jbc.M009253200. [DOI] [PubMed] [Google Scholar]
- Whitlock J.R., Heynen A.J., Shuler M.G., Bear M.F. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Yeh S.H., Lin C.H., Lee C.F., Gean P.W. A requirement of nuclear factor-κB activation in fear-potentiated startle. J. Biol. Chem. 2002;277:46720–46729. doi: 10.1074/jbc.M206258200. [DOI] [PubMed] [Google Scholar]
- Yeh S.H., Lin C.H., Gean P.W. Acetylation of nuclear factor-κB in rat amygdala improves long-term but not short-term retention of fear memory. Mol. Pharmacol. 2004;65:1286–1292. doi: 10.1124/mol.65.5.1286. [DOI] [PubMed] [Google Scholar]
- Yin J.C., Del Vecchio M., Zhou H., Tully T. CREB as a memory modulator: Induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]