Abstract
Oocytes are held in meiotic arrest in prophase I until ovulation, when gonadotropins trigger a subpopulation of oocytes to resume meiosis in a process termed “maturation.” Meiotic arrest is maintained through a mechanism whereby constitutive cAMP production exceeds phosphodiesterase-mediated degradation, leading to elevated intracellular cAMP. Studies have implicated a constitutively activated Gαs-coupled receptor, G protein-coupled receptor 3 (GPR3), as one of the molecules responsible for maintaining meiotic arrest in mouse oocytes. Here we characterized the signaling and functional properties of GPR3 using the more amenable model system of Xenopus laevis oocytes. We cloned the X. laevis isoform of GPR3 (XGPR3) from oocytes and showed that overexpressed XGPR3 elevated intraoocyte cAMP, in large part via Gβγ signaling. Overexpressed XGPR3 suppressed steroid-triggered kinase activation and maturation of isolated oocytes, as well as gonadotropin-induced maturation of follicle-enclosed oocytes. In contrast, depletion of XGPR3 using antisense oligodeoxynucleotides reduced intracellular cAMP levels and enhanced steroid- and gonadotropin-mediated oocyte maturation. Interestingly, collagenase treatment of Xenopus oocytes cleaved and inactivated cell surface XGPR3, which enhanced steroid-triggered oocyte maturation and activation of MAPK. In addition, human chorionic gonadotropin-treatment of follicle-enclosed oocytes triggered metalloproteinase-mediated cleavage of XGPR3 at the oocyte cell surface. Together, these results suggest that GPR3 moderates the oocyte response to maturation-promoting signals, and that gonadotropin-mediated activation of metalloproteinases may play a partial role in sensitizing oocytes for maturation by inactivating constitutive GPR3 signaling.
NORMAL FEMALE fertility requires precise regulation of oocyte meiosis. Immature oocytes are arrested at prophase I of meiosis until just before ovulation, when gonadotropin-induced signals trigger a subpopulation of oocytes to resume meiosis and progress to metaphase II (1,2). At this stage meiotic progression is again arrested until after ovulation and fertilization. One of the best-established models of oocyte maturation comes from Xenopus laevis, in which gonadotropin-mediated steroid production triggers meiotic progression (2,3). Although many steroids promote Xenopus oocyte maturation in vitro, androgens likely serve as the physiological trigger in vivo, signaling through classical androgen receptors in a transcription-independent (nongenomic) fashion (4,5).
Interestingly, steroids trigger Xenopus oocyte maturation in a release of inhibition fashion whereby oocytes are held in meiotic arrest by constitutive G protein signals that stimulate adenylyl cyclase to elevate intracellular cAMP (4,6,7,8). Both Gαs and Gβγ are important for maintaining elevated intracellular cAMP and meiotic arrest, and rapid suppression of Gβγ signaling occurs upon addition of steroid (9). Suppression of Gβγ signaling (and possibly Gαs) leads to lower intracellular cAMP levels, resulting in the activation of multiple downstream signals, including MAPK and cyclin-dependent kinase 1 (CDK1), that ultimately promote germinal vesicle breakdown and meiotic progression.
A major goal in the field of oocyte maturation has been to identify potential G protein-coupled receptors (GPRs) that could be stimulating this inhibitory G protein signaling. In fact, a novel family of Gαs-coupled receptors that includes GPRs 3 and 12 has been shown to participate in maintaining meiotic arrest in mammalian oocytes (10,11,12,13). These proteins are orphan receptors that appear to constitutively increase cAMP levels when overexpressed in a variety of cells. GPR3 is present in mouse oocytes, and studies using a GPR3 knockout mouse demonstrate spontaneous resumption of meiosis in oocytes within Gpr3−/− antral follicles (11,14). Importantly, female Gpr3−/− null mice have smaller than normal litter sizes, with premature ovarian failure; however, they are still fertile (14). Furthermore, anywhere from 10–30% of the antral oocytes in Gpr3−/− null mice remain in meiotic arrest (11,14), and a similar number of approximately 20–30% of follicle-enclosed oocytes remain in meiotic arrest after being injected with short interfering RNAs directed against GPR3 mRNA (15). These observations suggest that GPR3 is important, but not essential, for maintaining meiotic arrest in vivo. Unfortunately, direct evaluation of the effect of GPR3 on intracellular signals that accompany maturation, including changes in cAMP levels, activation of protein kinases, and detection of receptor on the cell surface, have been difficult due to the limited numbers of mouse oocytes that can be cleanly isolated and examined.
To study the signaling and biological properties of GPR3 in a more amenable model of meiosis, we examined the role of GPR3 in regulating X. laevis oocyte maturation. We generated a cDNA encoding the X. laevis isoform of GPR3 (XGPR3) with a FLAG tag at the amino terminus. Overexpressed XGPR3 was present on the oocyte cell surface and abrogated its response to steroid- and human chorionic gonadotropin (hCG)-triggered maturation. In contrast, knockdown of endogenous XGPR3 expression enhanced steroid- and hCG-triggered maturation. Surprisingly, collagenase cleaved and partially inactivated XGPR3 on the surfaces of isolated Xenopus oocytes, which enhanced steroid-triggered maturation. Furthermore, hCG treatment of follicles triggered cleavage of XGPR3 on the oocyte cell surface. These results demonstrate that XGPR3 participates in maintaining meiotic inhibition in Xenopus oocytes and suggest that gonadotropin-induced metalloproteinase activation may inactivate GPR3 during meiosis.
RESULTS
Cloning of Xenopus GPR3
A cDNA encoding a 340-amino acid isoform of X. laevis GPR3 protein was cloned from oocyte RNA using reverse-transcription and nested PCR techniques. A FLAG epitope tag at the amino terminus of XGPR3 was added to allow for detection of the protein (Fig. 1A). XGPR3 (GenBank accession no. ABS19626; also known as GPRx) shares approximately 42% overall identity and 65% homology with murine GPR3 and GPR12 (Fig. 1B). Most of the differences in sequence are located in the 50-amino acid extracellular amino-terminal portion of the protein. A thorough search of all GenBank and Xenopus (accessed via Xenbase) databases revealed no other similar X. laevis G protein-coupled receptors. Furthermore, the first 50 matches on GenBank homology searches are either GPR3 or GPR12. These results indicate that the clone identified encodes the only currently known member of the GPR3/12 family in X. laevis.
Figure 1.
Schematic and Sequence of XGPR3
A, XGPR3 is a seven-transmembrane domain protein with a predicted 30-amino acid carboxyl-terminal intracellular tail and 50-amino acid amino-terminal extracellular domain. For these studies, an amino-terminal FLAG sequence has been added. B, Sequence comparison of XGPR3 with the mouse GPR3 and GPR12 (mGPR3 and mGPR12). Amino acids that are identical between all three proteins (42%) are in boxes. XGPR3 shares 49% and 54% identity with mGPR3 and mGPR12, respectively. XGPR3 shares approximately 70% sequence similarity with both mouse receptors. AA, Amino acid.
Transfection of a cDNA encoding XGPR3 into COS-7 cells resulted in significant cell surface expression of XGPR3, as well as elevation of intracellular cAMP (data not shown), confirming that the Xenopus isoform of GPR3 was functioning as expected.
Overexpression of XGPR3 in Xenopus laevis Oocytes Increased cAMP and Inhibited Steroid-Mediated Intracellular Signaling and Maturation
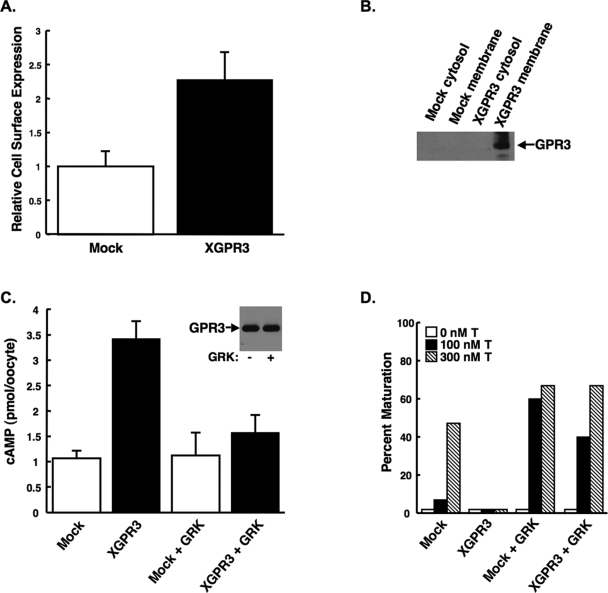
The effects of XGPR3 on steroid-triggered signaling in X. laevis oocytes were explored. Injection of cRNA encoding FLAG-tagged XGPR3 into Xenopus oocytes resulted in substantial cell surface expression of protein, as detected using an anti-FLAG antibody in a cell surface ELISA (Fig. 2A). Membrane expression of XGPR3 was confirmed by Western blot using the anti-FLAG antibody, where protein was present in total membrane, but not cytosolic, oocyte fractions (Fig. 2B).
Figure 2.
Overexpression of XGPR in Xenopus Oocytes Increased Intracellular cAMP, in Part, by Stimulating Gβγ Signaling
A, Oocytes injected with cRNA encoding XGPR3 expressed high levels of XGPR3 on the cell surface relative to mock-injected oocytes as determined by an anti-FLAG ELISA on fixed, intact oocytes. Values are the average ± sd (n = 3). B, XGPR3 was expressed in total membrane, but not cytoplasmic, oocyte fractions, as detected by Western blot using an anti-FLAG antibody. C, Overexpression of XGPR3 increased intracellular cAMP in Xenopus oocytes relative to mock-injected cells. Coexpression of the carboxyl tail of GRK1 (GRK) abrogated this XGPR-mediated increase in cAMP, suggesting that that Gβγ at least partially mediates XGPR signaling. Values are the average ± sd (n = 5). Equal amounts of XGPR3 were detected by Western blot both with and without GRK coexpression (inset). D, Overexpression of the GRK peptide enhanced testosterone-mediated oocyte maturation in both mock-injected oocytes and oocytes overexpressing XGPR3. T, Testosterone. All experiments were performed at least three times with nearly identical results.
Overexpressed murine GPR3 has been shown to increase cAMP levels in somatic cells (10) and oocytes (13), and to block oocyte maturation (11,12,13). To confirm that XGPR3 would similarly increased intracellular cAMP, we measured cAMP levels in Xenopus oocytes injected with buffer or cRNA encoding XGPR3. Oocytes overexpressing XGPR3 contained significantly more intracellular cAMP relative to mock-injected oocytes (Fig. 2C). This suggested that, as described in somatic cells and mouse oocytes, overexpressed XGPR3 might be stimulating Gαs to promote adenylyl cyclase activity. However, substantial evidence demonstrates that, in Xenopus oocytes, both Gαs and Gβγ act in concert to elevate intracellular cAMP levels and maintain meiotic arrest (4,7,8). Furthermore, overexpression of the carboxyl-G protein receptor-coupled kinase (GRK)1 peptide in frog oocytes is known to enhance steroid-triggered oocyte maturation by suppressing the constitutive Gβγ signaling that holds oocytes in meiotic arrest (8,16). Therefore, to determine whether part of the effects of XGPR3 on intracellular cAMP might be mediated by Gβγ, oocytes were injected with cRNAs encoding both XGPR3 and the Gβγ scavenger carboxyl-GRK1 peptide. Interestingly, the carboxyl-GRK1 peptide had minimal effect on cAMP levels in mock-injected oocytes but markedly attenuated the XGPR3-mediated rise in intracellular cAMP (Fig. 2C). Furthermore, the carboxyl-GRK1 peptide significantly enhanced testosterone-mediated oocyte maturation in both mock-injected and XGPR3-expressing cells (Fig. 2D). XGPR3 expression was not affected by coexpression of the GRK peptide (Fig. 2C, inset). These results suggest that, unlike in mouse oocytes, XGPR3 might, in fact, require Gβγ signaling to fully stimulate adenylyl cyclase and elevate cAMP levels in Xenopus oocytes.
Because overexpressed XGPR3 increased intracellular cAMP, it would be predicted to inhibit steroid-triggered oocyte maturation. Indeed, overexpression of XGPR3 in denuded frog oocytes by cRNA injection almost completely abrogated testosterone-induced maturation as compared with mock-injected control oocytes (Figs. 2D and 3A). As mentioned, this nearly complete XGPR3-mediated inhibition of maturation was rescued by sequestration of Gβγ with the GRK1 carboxyl peptide (Fig. 2D), suggesting that Gβγ is a significant mediator of XGPR3 signaling. XGPR3 also completely abrogated steroid-triggered activation of MAPK, an important signal associated with oocyte maturation, as evidenced by the loss of testosterone-induced phosphorylation of p42 protein (Fig. 3B). XGPR3 also suppressed steroid-mediated activation of CDK1/cdc2, an important cyclin that promotes meiotic progression and is dephosphorylated during activation. As shown in Fig. 3B, overexpression of XGPR3 blocked testosterone-induced de-phosphorylation of the CDK1 protein. Finally, overexpression of XGPR3 in follicle-enclosed, manually defolliculated oocytes significantly inhibited both testosterone- and hCG-mediated oocyte maturation (Fig. 3C). Together, these data suggest that XGPR3-mediated increases in intracellular cAMP under overexpression conditions are sufficient to maintain Xenopus oocytes in meiotic arrest, even in the presence of amounts of steroid or hCG that would normally initiate maturation.
Figure 3.

Overexpression of XGPR3 in Xenopus Oocytes Inhibited Maturation
A and B, Overexpression of XGPR3 markedly inhibited testosterone-mediated oocyte maturation (A) and activation of MAPK and CDK1 (B) in denuded oocytes. Maturation was determined by examining oocytes for Germinal Vesicle Breakdown (GVBD) after overnight incubation with testosterone. After 6 h with testosterone, MAPK activation was detected by Western blot for increased phosphorylation of p42, whereas CDK1 activation was determined by Western blot for decreased phosphorylation of CDK1. Total p42 and MAPK expression in the same samples are also shown. 100T, 100 nm testosterone; 1000T, 1000 nm testosterone. C, Overexpression of XGPR3 markedly inhibited hCG (150 Units/ml)- and testosterone (500 nm)-induced oocyte maturation in follicle-enclosed oocytes. All studies were performed at least three times with similar results.
Notably, overexpressed XGPR3 lacking the FLAG tag still stimulated cAMP production and almost completely suppressed testosterone-induced activation of MAPK, CDK1, and maturation (data not shown). This confirms that the FLAG tag was not significantly contributing to the constitutive activity of exogenous XGPR3.
Reduction of Endogenous XGPR3 Expression in X. laevis Oocytes Enhanced Testosterone- and hCG-Mediated Intracellular Signaling and Maturation
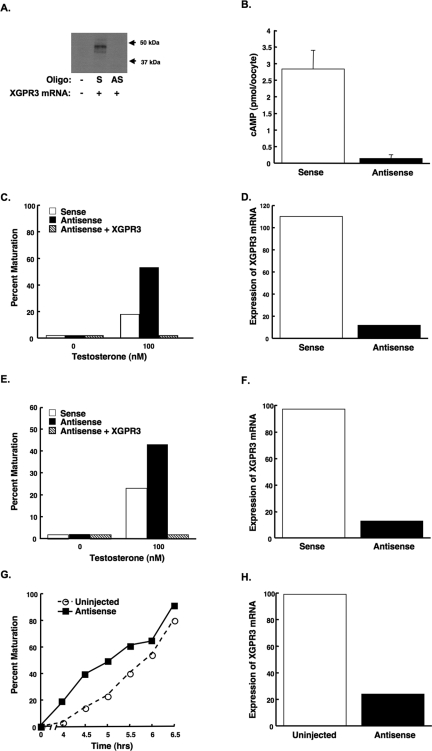
If XGPR3 plays a physiological role in repressing maturation in response to endogenous signals, then its removal would be predicted to enhance the maturation response to these triggers. The lack of anti-GPR3 antibodies precluded demonstrating the loss of endogenous XGPR3 protein expression. However, injection of antisense, but not sense, phosphothiorated oligodeoxynucleotides directed against mRNA encoding XGPR3 completely suppressed expression of exogenous FLAG-tagged XGPR3 protein in denuded oocytes (Fig. 4A). This result indicates that the antisense oligodeoxynucleotides were capable of specifically abrogating XGPR3 protein expression.
Figure 4.
Knockdown of Endogenous XGPR3 Expression Enhanced Oocyte Maturation
A, Injection of antisense, but not sense, oligonucleotides prevented the expression of exogenous XGPR3. Denuded oocytes were injected with buffer (−), sense (S), or antisense (AS) oligodeoxynucleotides, as well as mRNA encoding FLAG-tagged XGPR3. After 48 h, oocytes were lysed, and equal amounts of extracts were loaded in each lane. FLAG-tagged XGPR3 was then detected by Western blot. B and C, Injection of denuded oocytes (without attached follicle cells) with an antisense, but not sense, oligodeoxynucleotide directed against XGPR3 mRNA reduced intracellular cAMP (B) and enhanced testosterone-mediated oocyte maturation (C). Values in panel B represent the average ± sd (n = 3). Similarly, injection of manually defolliculated oocytes (still with attached follicle cells) with antisense, but not sense, oligodeoxynucleotide directed against XGPR3 mRNA enhanced testosterone-mediated oocyte maturation (E). Importantly, coinjection of cRNA encoding XGPR3 rescued this enhancement in both instances (C and E). Oocytes were incubated with 100 nm testosterone for 16 h (C) or 7 h (E), and oocytes were then scored for germinal vesicle breakdown. Expression of XGPR3 mRNA was reduced by approximately 90% in both cases, as determined by real-time PCR (D, denuded; and F, defolliculated). G, Manually defolliculated oocytes (still with attached follicle cells) that were injected with the antisense oligodeoxynucleotide directed against XGPR3 while still in ovarian fragments matured more quickly in response to hCG (150 units/ml) relative to uninjected oocytes. Expression of XGPR3 mRNA was reduced by approximately 75% in these oocytes, as determined by real-time PCR (H). All experiments were repeated at least twice with similar results.
Injection of oocytes with the antisense oligodeoxynucleotides directed against mRNA encoding XGPR3 reduced intracellular cAMP relative to oocytes injected with the sense oligodeoxynucleotide (Fig. 4B), confirming that endogenous XGPR3 plays a role in stimulating cAMP production. Although denuded oocytes injected with the antisense oligodeoxynucleotide did not spontaneously mature (up to 72 h after injection), they became significantly more sensitive to testosterone-triggered maturation (Fig. 4C). Similarly, follicle-enclosed oocytes injected with the antisense oligodeoxynucleotide became more sensitive to testosterone-mediated maturation (Fig. 4E). Quantitative PCR confirmed that XGPR3 mRNA levels were significantly decreased in response to the injected antisense, but not sense, oligodeoxynucleotides (Fig. 4, D and F). The ability of overexpressed XGPR3 to rescue the effects of the antisense oligodeoxynucleotides in both oocyte preparations (Fig. 4, C and E), as well as the inability of a nonspecific sense oligodeoxynucleotide to enhance maturation, confirmed the specificity of the antisense-mediated depletion. Finally, depletion of XGPR3 mRNA by injection of the antisense oligodeoxynucleotide into oocytes still enclosed in their follicles within pieces of cultured ovary (Fig. 4H) enhanced the rate of subsequent oocyte maturation mediated by hCG, the initial in vivo signal for maturation (Fig. 4G). Together, these data suggest that endogenous XGPR3 plays at least a partial role in maintaining meiotic arrest in Xenopus oocytes.
The Metalloproteinase Collagenase Cleaves and Inactivates Cell Surface GPR3
Because GPR3 appears to be a constitutively activated G protein-coupled receptor, we postulated that inactivation of GPR3 might play a role permitting meiosis to progress in follicle-enclosed oocytes. Specifically, we hypothesized that proteases may be capable of inactivating GPR3 at the cell surface. Interestingly, treatment of manually defolliculated oocytes with collagenase, a technique commonly used to remove follicular cells from Xenopus oocytes, markedly enhanced testosterone-mediated oocyte maturation (Fig. 5A). This maturation-enhancing effect was seen using every preparation of collagenase tested (data not shown), including the highly purified collagenase preparation shown in Fig. 5A. These results suggest that the collagenase itself, rather than a contamination, was mediating this enhancement.
Figure 5.
Collagenase Cleaved Cell Surface GPR3 and Enhanced Testosterone-Mediated Oocyte Maturation
A, Manually defolliculated oocytes (some follicle cells still attached) were more sensitive to testosterone after treatment with highly pure collagenase type IV (1 mg/ml). B, The amino-termini of both FLAG-tagged XGPR3 and mouse GPR3 (mGPR3) expressed in COS cells were cleaved by collagenase A (0.8 mg/ml for 30 min), whereas the amino terminus of the M1R was unaffected. C, The amino terminus of XGPR3 expressed in Xenopus oocytes was cleaved by collagenase A (0.8 mg/ml for 30 min). For panels B and C, cleavage was measured as the loss of cell surface FLAG expression by ELISA. Values are presented as cell surface expression relative to mock-transfected, untreated cells and are the average ± sd (n = 3). D, Collagenase treatment of oocytes botinjected with cRNA encoding XGPR3 abrogated the mRNA-dependent rise in intracellular cAMP, while having no significant effect on mock-injected oocytes. Values represent the mean ± sd (n = 3). E, Collagenase A (0.8 mg/ml) partially rescued the inhibitory effects of overexpressed XGPR3 on testosterone-mediated oocyte maturation (E, overnight treatment) and activation of MAPK (F, 6-h treatment). All experiments were repeated at least three times with nearly identical results.
To determine whether collagenase was enhancing maturation by cleaving and inactivating GPR3 on the cell surface, we first examined the effects of collagenase treatment for 30 min on GPR3 cell surface expression in COS cells. Compared with mock-transfected cells, cells transfected with cDNAs encoding XGPR3 and our positive control, M1R, both of which contain an amino-terminal FLAG tag, revealed significant cell surface expression. However, after 30 min of collagenase treatment, detectable levels of the FLAG-epitope markedly decreased in the XGPR3-, but not the M1R-expressing cells, suggesting that collagenase cleaved the amino terminus of XGPR3 in a receptor-specific fashion (Fig. 5B). Importantly, mouse FLAG-GPR3 cell surface expression was similarly reduced by collagenase, demonstrating that collagenase-mediated cleavage of the amino terminus of GPR3 is not specific for only the frog isoform of GPR3.
Repeating the collagenase-cleavage experiment in Xenopus oocytes also showed a rapid collagenase-mediated reduction of overexpressed XGPR3 cell surface expression (Fig. 5C). Although the cell surface levels of overexpressed FLAG-tagged XGPR3 were decreased, total XGPR3 expression, as well as the size of the detected FLAG-tagged XGPR3, was unaffected, as determined by Western blot using an anti-FLAG antibody (data not shown). This observation suggests that significant levels of intact overexpressed GPR3 are present inside cells and therefore protected from the collagenase.
To test the effects of collagenase-mediated cleavage of GPR3 on oocyte maturation, denuded oocytes were isolated with collagenase to both remove follicle cells and to cleave endogenous XGPR3. As expected, subsequent overexpression of XGPR3 increased intracellular cAMP (Fig. 5D) and almost completely blocked testosterone-mediated maturation and activation of MAPK (Fig. 5, E and F). Importantly, collagenase treatment of oocytes overexpressing XGPR3 reduced intracellular cAMP (Fig. 5D) and partially restored steroid-triggered oocyte maturation and activation of MAPK (Fig. 5, E and F), indicating that, similar to reducing endogenous XGPR3 expression, collagenase-mediated cleavage and inactivation of XGPR3 sensitizes oocytes to testosterone. Notably, collagenase did not completely restore steroid sensitivity in oocytes overexpressing XGPR3 to that seen in mock-injected oocytes, most likely due to the large amounts of overexpressed intracellular full-length XGPR3 that are still detected by Western blot and may be stimulating sufficient intracellular cAMP production to prevent maturation.
Treatment of Follicle-Enclosed Oocytes with hCG Triggers Matrix Metalloproteinase-Mediated Cleavage of XGPR3 at the Oocyte Cell Surface
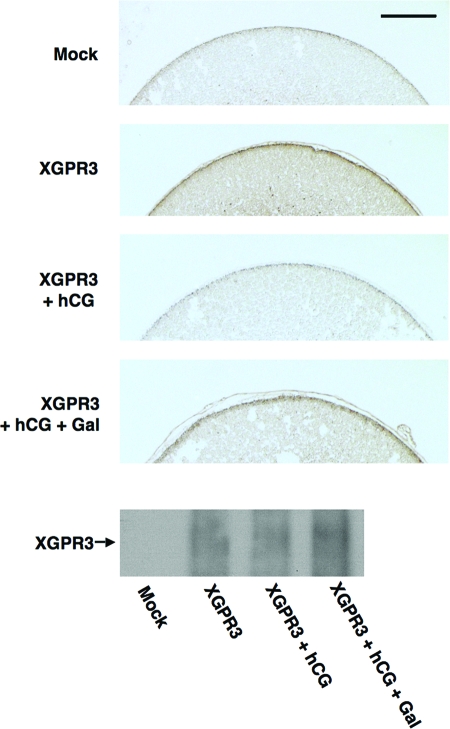
To determine whether XGPR3 was being cleaved in follicles during gonadotropin-induced oocyte maturation and ovulation, follicle-enclosed oocytes were injected with cRNA encoding FLAG-tagged XGPR3, followed by treatment with hCG for 4 h. Individual oocytes enclosed in follicle cells were then fixed and mounted on slides for immunohistochemical analysis using an anti-FLAG antibody. As expected, oocytes injected with the cRNA encoding XGPR3 expressed significant amounts of protein at the cell surface relative to mock-injected cells (Fig. 6, top two panels). Intriguingly, treatment of oocytes overexpressing XGPR3 with hCG reduced cell surface expression of the FLAG epitope (Fig. 6, third panel from top), suggesting that the amino terminus of XGPR3 was being cleaved in response to hCG. Furthermore, the metalloproteinase inhibitor Galardin partially abrogated the hCG-induced cleavage of XGPR3 at the cell surface (Fig. 6, fourth panel from the top), suggesting that hCG may be activating a matrix metalloproteinase to degrade XGPR3. Notably, Galardin blocked hCG-mediated oocyte maturation in follicle-enclosed oocytes. However, Galardin also reduced hCG-induced steroid production (data not shown), thus complicating interpretation of this result. Western blot analysis of oocyte lysates from all four conditions confirmed that FLAG-tagged XGPR3 was appropriately expressed in oocytes injected with cRNA encoding the receptor. Note that the total FLAG-XGPR3 levels were unchanged in response to hCG, despite the observed loss of expression at the cell surface. This observation again suggests that significant levels of intact overexpressed XGPR3 are present inside cells and therefore protected from proteolysis at the cell surface.
Figure 6.
hCG Treatment of Follicle-Enclosed Oocytes Triggered Matrix Metalloproteinase-Mediated Cleavage of XGPR3 at the Oocyte Cell Surface
Oocytes were injected with either vehicle (Mock) or 20 ng cRNA encoding the FLAG-tagged XGPR3. After 24 h, oocytes were treated for 4 h with either MBSH, MBSH containing either 5 IU hCG, or MBSH with 5 IU hCG and 50 μm Galardin. Oocytes receiving Galardin treatment were pretreated with 50 μm Galardin in MBSH for 1 h. The oocytes were then fixed in paraffin, sectioned, and mounted on slides for immunohistochemistry with an anti-FLAG antibody. Slides were photographed using a Zeiss Axioskop 2 scope at ×10 and digital camera using 36-msec exposure for all pictures. The black bar represents 100 μm. Note that oocytes injected with the mRNA encoding FLAG-XGPR3 demonstrated significant expression of XGPR3 at the oocyte cell surface relative to mock-injected oocyte. Treatment with hCG reduced cell surface expression of FLAG-tagged XGPR3, but addition of Galardin partially abrogated the effects of hCG. Photos are representative of three experiments with similar results. Western blot with the anti-FLAG antibody confirms equal levels of overexpressed Flag-XGPR3 compared with mock-injected oocytes after the indicated treatments. Gal, Galardin.
DISCUSSION
The studies presented here were designed to carefully characterize the signaling properties of GPR3 in the X. laevis model system. Advantages of the Xenopus model over the mouse system are the relative ease in isolating large numbers of oocytes, overexpressing and depleting proteins, and measuring signals associated with maturation, including changes in cAMP and activation of MAPK and CDK1 (2,3,17). In addition, the physiological trigger for mouse oocyte maturation is still not known, and mouse oocytes spontaneously mature upon removal from follicles via mechanisms that are also not well understood (1,18). In contrast, Xenopus oocytes remain in meiotic arrest after removal from the ovary until stimulated by steroid (2); thus, timed responses to the physiological agonist testosterone (5) can be readily followed with Xenopus oocytes. Finally, detection of overexpressed mouse GPR3 has been hampered by the lack of anti-GPR3 antibodies. By engineering the XGPR3 protein to contain a FLAG sequence at its amino terminus, overexpressed XGPR3 could be detected by Western blot of oocyte extracts, whereas cell surface XGPR3 expression could be measured with a whole-cell ELISA strategy.
Using the Xenopus model, we have confirmed and extended our knowledge regarding GPR3 signaling. First, we cloned a Xenopus isoform of GPR3 using X. laevis oocyte mRNA, demonstrating the conservation of GPR3 expression in lower vertebrate oocytes. Notably, the cloned X. laevis GPR3 bears similar homology to both mouse GPR3 and GPR12, both of which appear to promote adenylyl cyclase activity in the absence of ligand (12). In fact, these receptors seem to have differential effects in mice vs. rats, because injection of morpholino oligonucleotides directed against GPR3, but not GPR12, into isolated mouse oocytes held in meiotic arrest with hypoxanthine resulted in increased maturation, whereas injection of morpholino oligonucleotides directed against GPR12, but not GRP3, into isolated rat oocytes held in meiotic arrest with hypoxanthine enhanced maturation. For the studies described here, the X. laevis clone was arbitrarily designated XGPR3; however, although none to date have been found in existing sequence databases, Xenopus oocytes may also express other still unknown members of this constitutively active G protein-coupled receptor family.
Second, we demonstrated that overexpressed XGPR3 localized to the cell surface and inhibited maturation induced by both gonadotropin and the direct physiological trigger, testosterone. Furthermore, we showed that XGPR3 increased intraoocyte cAMP in isolated oocytes and inhibited kinase signals associated with maturation, including MAPK and CDK1. Importantly, overexpressed XGPR3 increased intraoocyte cAMP in the absence of any known ligand, confirming that XGPR3 likely inhibits steroid-triggered maturation by constitutively increasing intracellular cAMP.
Finally, we demonstrated that reduction of endogenous XGPR3 expression using antisense oligodeoxynucleotides lowered intracellular cAMP and enhanced both gonadotropin- and testosterone-triggered oocyte maturation. These findings confirm the studies in GPR3 null mice (11,14) and demonstrate that, unlike in mice, endogenous XGPR3-mediated signaling plays a role in maintaining meiotic arrest in Xenopus oocytes even after they have been removed from the ovary. Notably, despite significant reductions in XGPR3 mRNA using antisense oligodeoxynucleotides, Xenopus oocytes did not spontaneously mature, even 72 h after injection. This may be due, in part, to incomplete loss of endogenous XGPR3 protein, which could not be accurately quantified due to the lack of an anti-XGPR3 antibody. However, another possibility is that XGPR3 may not be the only signal maintaining meiotic arrest. For example, other members of the GPR3/GPR12 family of constitutively activated G protein-coupled receptors, novel G protein receptors such as the membrane progesterone receptor (mPR) family of steroid receptors (19), or receptor-independent G protein signaling, may be stimulating adenylyl cyclase to elevate intracellular cAMP and prevent oocyte maturation.
How is GPR3 stimulating adenylyl cyclase in Xenopus oocytes? As mentioned, an unusual feature of Xenopus oocytes is that Gβγ and Gαs appear to signal together to stimulate adenylyl cyclase, elevate intracellular cAMP, and hold oocytes in meiotic arrest (4,6,7,8). This differs from mouse oocytes, in which Gβγ signaling may, in fact, inhibit adenylyl cyclase and promote oocyte maturation (20). The difference between these species is likely due to the presence of adenylyl cyclase VII in Xenopus (7,21), but not mouse, oocytes. Adenylyl cyclase VII is stimulated by both Gβγ and Gαs (22); thus, the G protein signaling that maintains meiotic arrest in frog oocytes may be more powerful than that in mice. In fact, the additional Gβγ-mediated stimulation of adenylyl cyclase in Xenopus, but not mouse, oocytes may partially explain why Xenopus oocytes uniquely remain in meiotic arrest after removal from the ovary. Interestingly, sequestration of Gβγ by overexpression of the carboxyl-terminal GRK1 markedly reduced XGPR3-mediated elevation of intracellular cAMP (Fig. 2C) and almost completely rescued GPR3-mediated inhibition of testosterone-induced maturation (Fig. 2D). These observations suggest that XGPR3 is likely activating both Gαs and Gβγ in Xenopus oocytes to stimulate adenylyl cyclase and maintain meiotic arrest in Xenopus oocytes, with Gβγ being the dominant signal regulating these processes. Further studies will be needed to confirm whether GPR3 stimulates Gβγ in other cells or whether this Gβγ-stimulatory effect is specific only to cells containing adenylyl cyclase VII; however, recent observations that Gαs signaling may be relatively unchanged during LH-induced oocyte maturation in mouse oocytes suggests that other G protein-coupled signaling pathways may indeed be involved (23).
How do steroids inhibit G protein signaling in Xenopus oocytes? Evidence suggests that androgens bind to classical androgen receptors to attenuate G protein signaling, perhaps via the scaffold molecule called the modulator of nongenomic steroid responses (MNAR) (4,24,25). In fact, reducing MNAR expression in Xenopus oocytes enhances testosterone-mediated maturation and reduces the ability of the Gβγ-coupled M2R receptor to trigger calcium mobilization (24). These observations indicate that MNAR may be enhancing Gβγ signaling in Xenopus oocytes, even in the absence of steroid, and suggest the intriguing possibility that MNAR might enhance GPR3 signaling.
Recent work in GPR3 null mice have clearly demonstrated that GPR3 plays an important role in maintaining meiotic arrest in mouse oocytes, because the majority of oocytes in Gpr3−/− antral follicles prematurely progress through meiosis (11,14). However, 10–30% of oocytes in the Gpr3−/− preantral follicles remain in meiotic arrest, and female GPR3 null mice are still fertile. Furthermore, wild-type mouse oocytes removed from follicles spontaneously mature despite the continued presence of constitutively activated GPR3 (1,13). Together, these observations indicate that, as we now confirm in Xenopus oocytes, GPR3 is very important but is neither necessary nor sufficient to maintain meiotic arrest in all oocytes.
To reconcile the results in the mouse and frog models, we propose that GPR3 indeed plays a partial role in elevating intracellular cAMP and maintaining meiotic arrest, and that protease-mediated inactivation of GPR3 may be one of several signals that contribute to gonadotropin-induced oocyte maturation (Fig. 7). Our data show that the metalloproteinase collagenase readily cleaves both Xenopus and mouse GPR3, removing the amino terminus from the cell surface. The exact nature of this proteolysis is not known because collagenase cleaves proteins at several hydrophobic residues (26,27). However, the sequences 6A7V8S and 5A6V7G are conserved between Xenopus and mouse GPR3, respectively, and could be serving as a target. In addition, the hydrophobic residues “13L14L” in XGPR3 and “14A15G” in mouse GPR3 may serve as collagenase targets.
Figure 7.
GPR3 Is Partially Responsible for Maintaining Meiotic Arrest in Xenopus Oocytes
XGPR3, as well as other unknown factors, stimulate both Gαs and Gβγ to elevate cAMP and maintain meiotic arrest. Stimulation of follicles with gonadotropin may lead to the activation of proteinases that cleave XGPR3 and partially reduce the inhibitory signals maintaining meiotic arrest. Testosterone then suppresses the other sources of Gβγ signaling, resulting in significant reduction of intracellular cAMP, activation of MAPK and CDK1, and meiotic progression. AR, Androgen receptor.
Similar to the knockdown experiments using antisense oligodeoxynucleotides, treatment of manually defolliculated oocytes with collagenase enhanced testosterone-mediated maturation without triggering spontaneous maturation. In addition, collagenase partially rescued the inhibitory effects of overexpressed XGPR3. These data suggest that collagenase-mediated proteolysis may inactivate cell surface GPR3. Because LH is known to activate multiple metalloproteinases, including collagenase, during ovulation (28,29,30,31), we postulate that LH-induced activation of ovarian metolloproteinases may prime oocytes for maturation by inactivating GPR3 and eliminating one of the signals holding cells in meiotic arrest (Fig. 7). Accordingly, we demonstrated that hCG treatment of follicle-enclosed oocytes led to at least partial metalloproteinase-mediated (Galardin-sensitive) cleavage of cell surface XGPR3 (Fig. 6). Further studies will be needed to determine the physiological importance of this finding and to identify the specific metalloproteinases that might be inactivating GPR3 in vivo.
On a final note regarding collagenase and GPR3, collagenase is commonly used to isolate Xenopus oocytes for maturation studies. The studies presented here highlight an important caveat with this technique: collagenase treatment will make oocytes more sensitive to steroid just after isolation; however, with time, translation of new cell surface GPR3 protein may lead to decreased sensitivity. This issue needs to be taken into account when comparing steroid sensitivities between different populations of oocytes.
MATERIALS AND METHODS
Cloning of the Xenopus GPR3
The XGPR3 coding sequence was isolated by nested PCR techniques using the following primers: forward (outside), GACAGAGCTGGAGACGGAGGA; forward (inside), CGGATCCAGCCATGCTTCACCAGCCCTGCAGTC; reverse (outside), CTCCAAACATACAGTCCCGGA; reverse (inside), GGCGGCCGCGAATTCTTATACGTCACTGGAAGTTCT. The cDNA encoding XGPR3 was ligated into the mammalian expression vector pEF2-FlmugR-DNM, which contains the sequence encoding an amino-terminal FLAG tag (a generous gift from Mark Kahn, University of Pennsylvania, Philadelphia, PA). We then used the forward primer CGCCCGGGCCAGCCATGCGACCGACGCTGCTGTGGTCG to insert the complete N-terminal FLAG-tagged XGPR3 cDNA clone into the pGEM-HE vector (from L. Jan, University of California, San Francisco, CA). During the course of these studies, a clone matching ours, designated ABS19626, was submitted by others to the GenBank database and is designated GPRx.
Oocyte Preparation
All frogs were treated in accord with accepted NIH and University of Texas standards of humane animal care. Oocytes were prepared using two different methods as indicated in the figure legends. To isolate denuded oocytes, ovaries were harvested from female X. laevis (Nasco, Fort Atkinson, WI) and treated as described elsewhere (16). Briefly, follicle cells were removed by incubation of ovaries in 0.8 mg/ml collagenase A (Roche Applied Science, Indianapolis, IN) in modified Barth’s solution (MBSH) without Ca2+ for 3–4 h. Oocytes were then washed and incubated overnight at 16 C in MBSH containing 1 mg/ml Ficoll, 1 mg/ml BSA, 100 U/ml penicillin, and 0.1 U/ml streptomycin. Testosterone (Steraloids, Newport, RI)-induced maturation assays were performed on stage V/VI oocytes from each preparation to determine sensitivity to steroid, because this varies considerably with each batch of oocytes. Maturation was scored as germinal vesicle breakdown, which was visualized as a white spot on the animal pole of the oocyte. Twenty oocytes were used for each data point in all experiments.
In the second method, stage V/VI oocytes were manually defolliculated from female X. laevis, injected with oligodeoxynucleotides or cRNA, as discussed below and in the figure legends, and incubated in oocyte culture medium (OCM) as described elsewhere (32). Oocytes were then stimulated for maturation using either testosterone or human chorionic gonadotropin (hCG) (Intervet, Millsboro, DE). Maturation was scored as germinal vesicle breakdown.
RNA Synthesis and Injections
The pGEM-HE plasmid containing the FLAG-tagged XGPR3 cDNA sequence was linearized with AatII or SphI. Capped cRNA was transcribed in vitro with T7 RNA polymerase according to the manufacturer’s protocol (Ambion, Inc., Austin, TX). RNA was suspended in injection buffer (10 mm HEPES, pH 7.4) or water, and Stage V/VI oocytes were injected with the amounts of cRNA indicated in the figure legends using a Drummond or Harvard Apparatus automatic injector. The sequences of the sense and antisense XGPR3 HPLC purified oligodeoxynucleotides were G*C*A*TATAGCAATGCTTCA*C*C*A and T*A*G*GGTGGCCAGTTC*A*C*T, respectively, with phosphorthiorated bonds indicated by asterisks.
Oocytes were injected with oligodeoxynucleotides as indicated in the figure legends. For the rescue studies, XGPR3 cRNA was injected with the oligodeoxynucleotides as indicated. After all injections, oocytes were incubated at least 36–48 h before any assay was begun.
For injection of intraovarian oocytes, ovaries were harvested from adult, nonvirgin female X. laevis that had previously laid eggs and cut into pieces containing approximately 30–50 large oocytes. Intraovarian stage V/VI oocytes were injected with a mixture of either XGPR3 antisense oligodeoxynucleotide and the lineage marker fluorescein-lysine-dextran, or water and fluorescein-lysine-dextran. The ovarian pieces were incubated at 18 C in OCM for 4 d. After incubation, the lineage-labeled oocytes were manually defolliculated under a fluorescent microscope (Nikon SMZ1500; Nikon, Melville, NY). The oocytes were then stimulated with 150 units/ml hCG and scored for germinal vesicle breakdown every 30 min at room temperature.
Analysis of Gene Expression using Real-Time PCR
Real-time RT-PCR was performed using total RNA extraction of two oocytes as described elsewhere (33). cDNA was synthesized using approximately one sixth oocyte equivalent and oligo deoxythymidine primers. Real-time RT-PCR and quantification were performed using the LightCycler System version 3.5 (Roche) as described previously (34). Briefly, relative expressive levels were calculated using a standard curve, generated by a dilution series of control oocyte cDNA. Samples were normalized to a housekeeping gene, ornithine decarboxylase, to serve as a loading control.
The primer sequences for the XGPR3 gene are: forward, 5′-CTGGGGCTCATTGTGAATTT-3′; and reverse, 5′-GTGGTAGGTGAGGGCATTGT-3′. The ornithine decarboxylase primer sequences are: forward, 5′-GCCATTGTGAAGACTCTCTCCATTC-3′; and reverse, 5′-TTCGGGTGATTCCTTGCCAC-3′ (33).
Testosterone-Mediated Maturation Assays
Maturation assays were conducted by incubating 20 oocytes per condition with the indicated concentration of testosterone (Steraloids) or ethanol in MBSH or OCM for 12–16 h, at which time oocytes were scored for germinal vesicle breakdown. Dilutions were performed such that ethanol concentration remained at 0.1%.
Western Blots
Oocytes were incubated with steroid or vehicle, permeabilized in 20 μl/oocyte lysis buffer (1% Triton X-100; 50 mm Tris-HCl, pH 7.6; 150 mm NaCl; 2 mm EDTA; 2 mm NaF; 0.5 mm sodium vanadate; 100 μg/ml phenylmethylsulfonyl fluoride), and microcentrifuged at 14,000 × g for 10 min to remove yolk and other debris. The cleared supernatants were then mixed 1:1 with 2× sodium dodecyl sulfate sample buffer (16). The equivalent of 0.5 oocytes was loaded in each lane for SDS-PAGE, transferred to Immobilon-P membranes (Millipore Corp., Bedford, MA), blocked in 5% Tris-buffered saline-Tween 20-milk for 1 h, and then incubated with primary antibody overnight at 4 C (1:5000 for anti-FLAG M1, 1:5000 for anti-FLAG M2, 1:2000 for antiphospho-p42 and antitotal p42, 1:2000 for antiphospho- and antitotal CDK1). Membranes were then incubated with horseradish peroxidase-conjugated secondary antibody for 1 h, and signal was detected by ECL Plus (Amersham Biosciences, Piscataway, NJ). Anti-Flag M1 and M2 were obtained from Sigma-Aldrich (St. Louis, MO), antiphospho-CDC2, and antitotal-CDC2, antiphospho-p44/42 MAPK, and antitotal p44/42 MAPK were from Cell Signaling Technology (Beverly, MA).
Membrane Preparations
Crude oocyte membrane and cytosplasmic fractions were prepared as described elsewhere (16). For the Western blots using these fractions, approximately 0.5 oocyte equivalent was added to each lane.
Cell Culture and Transfection
COS-7 cells (American Type Culture Collection, Manassas, VA) were maintained at 37 C in DMEM (Fisher Scientific, Pittsburgh, PA) containing 10% fetal bovine serum, 100 IU/ml penicillin, and 0.1 mg/ml streptomycin (Invitrogen, Carlsbad, CA). Transfections were performed in six-well plates using Lipofectamine reagent (Invitrogen). Each well was transfected with 1 μg of total DNA as indicated. After 48 h incubation in 10% serum, cells were washed two times with ice-cold PBS (pH 7.4), and permeabilized in 300 μl of oocyte lysis buffer. Wells were scraped, cell debris was removed by centrifugation, and the cleared lysates were mixed 1:1 with 2× sodium dodecyl sulfate sample buffer and immunoblotted as described above.
cAMP Competitive ELISA
COS-7 cells were transfected with XGPR3 plasmid or vehicle, and oocytes were injected with XGPR3 cRNA or vehicle as described previously. The cells and oocytes were treated with 0.1 m HCl 48 h after transfection/injection to prevent endogenous phosphodiesterase activity and microcentrifuged at 0.6 rcf to remove debris. The samples were then added to a 96-well plate, and cAMP levels were measured using a colorimetric assay per manufacturer’s instructions (Endogen, Inc., Woburn, MA).
Cell Surface Expression Assay
COS cells were transfected with XGPR3 plasmid, and oocytes were injected with XGPR3 cRNA as described. Cells were washed 48 h after transfection/injection and then incubated for 1 h with the M2 antibody at 1:1000 dilution in DMEM (cells) or the M1 antibody at 1:1000 dilution in modified Barth’s solution (oocytes). After several washes with PBS, the cells were incubated with an antimouse secondary antibody (Bio-Rad Laboratories, Inc., Hercules, CA) at 1:2000. To assess the relative values of GPR3 cell surface expression levels, 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid), known as ATBS solution (Pierce Chemical Co., Rockford, IL) was added, and absorption was measured by a spectrophotometer (BioTek Instruments, Inc., Winooski, VT) at 405 nm.
Collagenase Treatment
Cells or oocytes were transfected or injected with XGPR3 as described. For the cell surface assays, COS-7 cells were treated with either serum free media alone or serum free media containing 0.8 mg/ml collagenase A (Roche Applied Science) for 30 min. Injected oocytes were treated with either MBSH alone or with 0.8 mg/ml collagenase A for 30 min. Immediately after collagenase treatment, cell surface expression of FLAG-tagged XGPR3 was measured as described above. For maturation and MAPK assays, isolated oocytes injected with cRNA encoding XGPR3 were incubated MBSH with the indicated concentration of testosterone or ethanol, plus or minus 0.8 mg/ml collagenase A. To measure phosphorylation of p42-ERK, oocytes were incubated for 6 h followed by lysis and Western blot as described above. To follow oocyte maturation, oocytes were treated for 12–16 h and scored for germinal vesicle breakdown.
For Fig. 5A, manually defolliculated oocytes were treated with Collagenase Type IV (generous gift from Worthington Biochemical Corp., Freehold, NJ) at 1 mg/ml in 1× Mark’s Modified Ringer (MMR) solution (1 m NaCl, 20 mm KCl, 20 mm CaCl2, 10 mm MgCl2, 150 mm HEPES). Before treatment with collagenase, the oocytes were washed five times in 1× MMR for 5 min. The oocytes were treated with 1 mg/ml collagenase solution for 1 h 45 min at room temperature. After treatment, oocytes were washed three times in 1× MMR for 5 min, and then three times in OCM for 5 min. Collagenase-treated oocytes were then incubated in OCM for 24 h, followed by treatment with testosterone as indicated in the figure legends. After 16 h incubation, oocytes were scored for germinal vesicle breakdown.
Immunohistochemistry
Oocytes were injected with either vehicle or 20 ng of XGPR3 cRNA. After 24 h, the oocytes were treated with either 5 IU hCG/ml in MBSH, 5 IU hCG with 50 μm Galardin (Calbiochem, La Jolla, CA) in MBSH, 0.8 mg/ml Collagenase A in MBSH, or MBSH alone for 4 h. The oocytes receiving Galardin were pretreated with 50 μm Galardin in MBSH for 1 h. The oocytes were then fixed in paraffin, sectioned, and mounted on slides (Molecular Pathology Core Facility, University of Texas Southwestern). The immunohistochemistry was carried out as previously described (35) using 1:200 dilution of horse serum containing an anti-FLAG mouse monoclonal antibody.
Footnotes
This work was supported by National Institutes of Health Grant DK59913, the Welch Foundation (Grant I-1506), and the Cincinnati Children’s Hospital Research Foundation.
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 29, 2008
Abbreviations: CDK1, Cyclin-dependent kinase 1; GPR3, G-protein coupled receptor 3; GRK, G protein receptor-coupled kinase; hCG, human chorionic gonadotropin; M1R, M1 muscarinic receptor; MBSH, modified Barth’s solution; MMR solution, Mark’s Modified Ringer solution; MNAR, modulator of nongenomic steroid responses; OCM, oocyte culture medium; XGPR3, X. laevis isoform of GPR3.
References
- Albertini DF, Carabatsos MJ 1998 Comparative aspects of meiotic cell cycle control in mammals. J Mol Med 76:795–799 [DOI] [PubMed] [Google Scholar]
- Maller JL, Krebs EG 1980 Regulation of oocyte maturation. Curr Top Cell Regul 16:271–311 [DOI] [PubMed] [Google Scholar]
- Hammes SR 2003 The further redefining of steroid-mediated signaling. Proc Natl Acad Sci USA 100:2168–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz LB, Cole LM, Gupta MK, Kwist KW, Auchus RJ, Hammes SR 2001 Evidence that androgens are the primary steroids produced by Xenopus laevis ovaries and may signal through the classical androgen receptor to promote oocyte maturation. Proc Natl Acad Sci USA 98:13728–13733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes SR 2004 Steroids and oocyte maturation—a new look at an old story. Mol Endocrinol 18:769–775 [DOI] [PubMed] [Google Scholar]
- Gallo CJ, Hand AR, Jones TL, Jaffe LA 1995 Stimulation of Xenopus oocyte maturation by inhibition of the G-protein α S subunit, a component of the plasma membrane and yolk platelet membranes. J Cell Biol 130:275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Y, Montplaisir V, Liu XJ 2005 Co-operation of Gsα and Gβγ in maintaining G2 arrest in Xenopus oocytes. J Cell Physiol 202:32–40 [DOI] [PubMed] [Google Scholar]
- Sheng Y, Tiberi M, Booth RA, Ma C, Liu XJ 2001 Regulation of Xenopus oocyte meiosis arrest by G protein βγ subunits. Curr Biol 11:405–416 [DOI] [PubMed] [Google Scholar]
- Evaul K, Jamnongjit M, Bhagavath B, Hammes SR 2007 Testosterone and progesterone rapidly attenuate plasma membrane Gβγ-mediated signaling in Xenopus laevis oocytes by signaling through classical steroid receptors. Mol Endocrinol 21:186–196 [DOI] [PubMed] [Google Scholar]
- Eggerickx D, Denef JF, Labbe O, Hayashi Y, Refetoff S, Vassart G, Parmentier M, Libert F 1995 Molecular cloning of an orphan G-protein-coupled receptor that constitutively activates adenylate cyclase. Biochem J 309:837–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA 2004 The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science 306:1947–1950 [DOI] [PubMed] [Google Scholar]
- Hinckley M, Vaccari S, Horner K, Chen R, Conti M 2005 The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol 287:249–261 [DOI] [PubMed] [Google Scholar]
- Freudzon L, Norris RP, Hand AR, Tanaka S, Saeki Y, Jones TL, Rasenick MM, Berlot CH, Mehlmann LM, Jaffe LA 2005 Regulation of meiotic prophase arrest in mouse oocytes by GPR3, a constitutive activator of the Gs G protein. J Cell Biol 171:255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent C, Demeestere I, Blum D, Petermans J, Hamalainen T, Smits G, Vassart G 2005 Premature ovarian aging in mice deficient for Gpr3. Proc Natl Acad Sci USA 102:8922–8926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM 2005 Oocyte-specific expression of Gpr3 is required for the maintenance of meiotic arrest in mouse oocytes. Dev Biol 288:397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz LB, Kim B, Jahani D, Hammes SR 2000 G protein β γ subunits inhibit nongenomic progesterone-induced signaling and maturation in Xenopus laevis oocytes. Evidence for a release of inhibition mechanism for cell cycle progression. J Biol Chem 275:41512–41520 [DOI] [PubMed] [Google Scholar]
- Rasar MA, Hammes SR 2006 The physiology of the Xenopus laevis ovary. Methods Mol Biol 322:17–30 [DOI] [PubMed] [Google Scholar]
- Jamnongjit M, Hammes SR 2005 Oocyte maturation: the coming of age of a germ cell. Semin Reprod Med 23:234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsberg Ben-Yehoshua L, Lewellyn AL, Thomas P, Maller JL 2007 The role of Xenopus membrane progesterone receptor β in mediating the effect of progesterone on oocyte maturation. Mol Endocrinol 21:664–673 [DOI] [PubMed] [Google Scholar]
- Gill A, Hammes SR 2007 Gβγ signaling reduces intracellular cAMP to promote meiotic progression in mouse oocytes. Steroids 72:117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman L, Romo X, Grandy R, Soto X, Montecino M, Hinrichs M, Olate J 2005 A Gβγ stimulated adenylyl cyclase is involved in Xenopus laevis oocyte maturation. J Cell Physiol 202:223–229 [DOI] [PubMed] [Google Scholar]
- Federman AD, Conklin BR, Schrader KA, Reed RR, Bourne HR 1992 Hormonal stimulation of adenylyl cyclase through Gi-protein β γ subunits. Nature 356:159–161 [DOI] [PubMed] [Google Scholar]
- Norris RP, Freudzon L, Freudzon M, Hand AR, Mehlmann LM, Jaffe LA 2007 A G(s)-linked receptor maintains meiotic arrest in mouse oocytes, but luteinizing hormone does not cause meiotic resumption by terminating receptor-G(s) signaling. Dev Biol 310:240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D, White SN, Lutz LB, Rasar M, Hammes SR 2005 The modulator of nongenomic actions of the estrogen receptor (MNAR) regulates transcription-independent androgen receptor-mediated signaling: evidence that MNAR participates in G protein-regulated meiosis in Xenopus laevis oocytes. Mol Endocrinol 19:2035–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz LB, Jamnongjit M, Yang WH, Jahani D, Gill A, Hammes SR 2003 Selective modulation of genomic and nongenomic androgen responses by androgen receptor ligands. Mol Endocrinol 17:1106–1116 [DOI] [PubMed] [Google Scholar]
- Wu H, Byrne MH, Stacey A, Goldring MB, Birkhead JR, Jaenisch R, Krane SM 1990 Generation of collagenase-resistant collagen by site-directed mutagenesis of murine pro α 1(I) collagen gene. Proc Natl Acad Sci USA 87:5888–5892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk BE, Huang LL, Piro ET, Cantley LC 2001 Determination of protease cleavage site motifs using mixture-based oriented peptide libraries. Nat Biotechnol 19:661–667 [DOI] [PubMed] [Google Scholar]
- Tsafriri A 1995 Ovulation as a tissue remodelling process. Proteolysis and cumulus expansion. Adv Exp Med Biol 377:121–140 [DOI] [PubMed] [Google Scholar]
- Lind AK, Dahm-Kahler P, Weijdegard B, Sundfeldt And K, Brannstrom M 2006 Gelatinases and their tissue inhibitors during human ovulation: increased expression of tissue inhibitor of matrix metalloproteinase-1. Mol Hum Reprod 12:725–736 [DOI] [PubMed] [Google Scholar]
- LeMaire WJ 1989 Mechanism of mammalian ovulation. Steroids 54:455–469 [DOI] [PubMed] [Google Scholar]
- Richards JS, Hernandez-Gonzalez I, Gonzalez-Robayna I, Teuling E, Lo Y, Boerboom D, Falender AE, Doyle KH, LeBaron RG, Thompson V, Sandy JD 2005 Regulated expression of ADAMTS family members in follicles and cumulus oocyte complexes: evidence for specific and redundant patterns during ovulation. Biol Reprod 72:1241–1255 [DOI] [PubMed] [Google Scholar]
- Zuck MV, WYlie CC, Heasman J 1998 Maternal mRNAs in Xenopus embryos: an antisense approach. In: Richter JD, ed. A comparative methods approach to the study of oocytes and embryos. Oxford, UK: Oxford University Press; 341–354 [Google Scholar]
- Kofron M, Klein P, Zhang F, Houston DW, Schaible K, Wylie C, Heasman J 2001 The role of maternal axin in patterning the Xenopus embryo. Dev Biol 237:183–201 [DOI] [PubMed] [Google Scholar]
- Standley HJ, Destree O, Kofron M, Wylie C, Heasman J 2006 Maternal XTcf1 and XTcf4 have distinct roles in regulating Wnt target genes. Dev Biol 289:318–328 [DOI] [PubMed] [Google Scholar]
- Rasar M, DeFranco DB, Hammes SR 2006 Paxillin regulates steroid-triggered meiotic resumption in oocytes by enhancing an all-or-none positive feedback kinase loop. J Biol Chem 281:39455–39464 [DOI] [PubMed] [Google Scholar]