Abstract
Smallie (slie), a spontaneous, autosomal-recessive mutation causes dwarfing and infertility in mice. The purpose of this study was to determine and characterize the underlying molecular genetic basis for its phenotype. The slie locus was mapped to chromosome 1, and fine-structure mapping narrowed the slie allele within 2 Mb between genetic markers D1Mit36 and Mpz. To pinpoint the underlying mutation quantitative real-time PCR was used to measure the relative expression levels for the genes residing within this region. Expression of one gene, Ddr2, which encodes discoidin domain receptor 2 (DDR2), was absent in slie homozygote mice. Genomic sequencing analysis detected a 150-kb deletion that extended into the Ddr2 gene transcript. Detailed phenotype analysis revealed that gonadal dysregulation underlies infertility in slie mice because all females were anovulatory and most adult males lacked spermatogenesis. The pituitary gland of prepubertal slie mice was smaller than in wild-type mice. The basal levels and gene expression for pituitary and hypothalamic hormones, and gene expression for hypothalamic-releasing hormones, were not significantly different between slie and wild-type mice. Circulating levels of IGF-1 did not differ in slie mice despite lower Igf-1 mRNA expression in the liver. After exogenous gonadotropin administration, the levels of secreted steroid hormones in both male and female adult slie mice were blunted compared to adult wild-type, but was similar to prepubertal wild-type mice. Taken together, our results indicate that the absence of DDR2 leads to growth retardation and gonadal dysfunction due to peripheral defects in hormonal-responsive pathways in slie mice.
MANY CONCEPTUAL foundations in endocrinology, such as homeostatic systems governing hormones, physiology, and behavior, have been built upon studies of mutant organisms. For instance, growth-retarded mice provide a valuable model system to dissect the molecular mechanisms that interplay among body size, reproduction, and the neuroendocrine axis. Some spontaneous genetic mutations in mice cause dwarfism by altering neuroendocrine systems, such as Snell dwarf (dw), Ames dwarf (df ), and little (lit) mice (1,2,3,4). These mutants were critical to unravel the mechanisms of GH regulation and the role of transcription factors such as Prop1 (5) and Pit1 (6) as regulators underlying neuroendocrine axis organization and activation. Genetic mutations in these two factors cause defects in one or more anterior pituitary cell types, resulting in little or no production of GH, prolactin, and TSH, but normal basal levels of FSH and LH (3,7,8,9,10).
Mice homozygous for these mutations also manifest infertility (11). Infertility in Snell dwarf mice appears to be due to gonadal dysfunction arising from a lack of neuroendocrine axis activation (11,12,13) in both sexes. It has been proposed that decreased GH levels directly lead to diminished circulating insulin and IGF-I, both of which are necessary for normal body size and aging in Snell, as well as Ames, dwarf mice (5,14,15,16,17,18,19).
However, dwarfism is not limited to disorders of the hypothalamus and pituitary gland. In addition to central mechanisms, peripheral defects yield growth retardation, as seen in hypothyroid (hyt), congenital goiter (cog), growth-retarded (grt), thyroid peroixdase (tpo), and Pax8 homozygous mutant mice (20,21,22,23,24). Thus, detailed analysis of novel dwarf mutant models can generate further insights into cellular and molecular pathways integrating central and peripheral endocrine systems.
We discovered a new spontaneous, autosomal-recessive mutation in our mouse colony, which results in dwarfing and sterility, called smallie (slie). slie homozygote mutant mice have mild craniofacial deformities, such as protuberant eyes and snub noses. This constellation of phenotypes is similar to a rare form of human dwarfism, known as Levi type (25). Given its effects on growth, body size, and reproduction, and its potential relevance to human disease, we initiated experiments to determine the molecular genetic basis for the slie phenotype. We identified and characterized the underlying mutation for the slie phenotype, a deletion of the Ddr2 gene. DDR2 (discoidin domain receptor 2) is a nonintegrin receptor tyrosine kinase activated by fibrillar collagen (26,27). Similar to slie homozygote mice, targeted deletion of Ddr2 in mice also leads to dwarfism; however, no fertility tests were conducted (28).
In this study, we report novel observations of infertility and endocrine function associated with loss of DDR2 function. These mice provide a new model with which to investigate the role of collagen receptors regulating reproduction, as well as its involvement in growth.
RESULTS
Dwarfism Phenotype of slie Mutant Mice
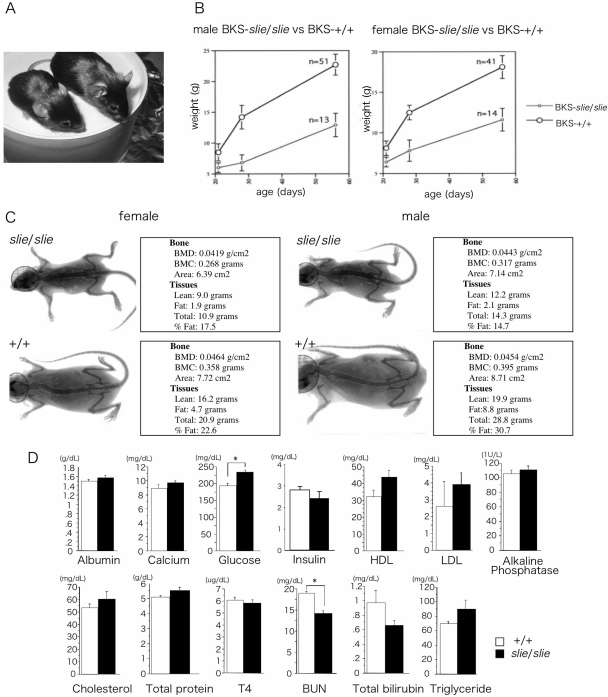
A spontaneous, autosomal-recessive mutation arose in our mouse colony. It was named smallie because affected mice exhibited overt dwarfism (Fig. 1A). At birth, slie mutants are indistinguishable from wild-type mice, but by adulthood slie mutant mice have minor craniofacial abnormalities, such as protruding eyes and shortened snout (Fig. 1A). After weaning, their weight gain is slower and they lack the juvenile growth spurt observed in control littermates (Fig. 1B). Whereas total body mass is reduced in slie homozygotes, as assessed by dual x-ray absorptiometry, the percentage of body fat is significantly reduced, with an increase in lean mass, compared with wild-type mice at 5–6 months of age (Fig. 1C and Table 1; fat ANOVA: F3,82 = 49.7; P < 0.001; lean ANOVA: F3,82 = 64.7; P < 0.0001). Likewise, bone mineral content (BMC), but not density, is reduced in slie homozygotes compared with wild-type (Table 1, BMC ANOVA, F3,82 = 29.5; P < 0.001). Plasma analyte levels were typically not altered, except for glucose levels, which were significantly increased, and blood urea nitrogen, which was significantly decreased in slie mutant mice (Fig. 1D).
Figure 1.
Body Composition and Blood Biochemistries in Wild-Type and slie Mutant Mice %A, The 4-month-old wild-type and slie mutant mice. Growth curve (panel B)and representative images of male and female wild-type and slie mutant mice (panel C). D, Blood biochemistry panel from 10-wk-old mice. HDL, High-density lipoprotein; LDL, low-density lipoprotein; BUN, blood urea nitrogen; BMD, bone mineral density. All data represent mean and sem. *, P < 0.05; **, P < 0.01.
Table 1.
Body Composition and Bone Morphometrics in 22- to 24-wk-old slie and Control Mice
| Group | Lean Tissue
|
Fat Tissue
|
Bone Mineral Density (g/cm2) | BMC (g) | Area (cm2) | ||
|---|---|---|---|---|---|---|---|
| Mass (g) | % | Mass (g) | % | ||||
| Male | |||||||
| +/+ (n = 18) | 19.3 ± 0.2 | 72.2 ± 0.6 | 7.5 ± 0.2 | 27.8 ± 0.6 | 0.00445 ± 0.0004 | 0.00402 ± 0.006 | 8.8 ± 0.2 |
| slie/slie (n = 9) | 15.5 ± 1.2a | 81.3 ± 1.5a | 3.8 ± 0.7a | 18.7 ± 1.5a | 0.00437 ± 0.0004 | 0.345 ± 0.018a | 7.7 ± 0.4 |
| Female | |||||||
| +/+ (n = 16) | 14.9 ± 0.1 | 75.8 ± 0.6 | 4.7 ± 0.8 | 24.2 ± 0.6 | 0.00443 ± 0.0001 | 0.348 ± 0.004 | 7.9 ± 0.1 |
| slie/slie (n = 8) | 12.5 ± 0.6 | 81.2 ± 0.7a | 2.9 ± 0.9 | 18.8 ± 0.7a | 0.00433 ± 0.0006 | 0.309 ± 0.006a | 7.2 ± 0.2 |
P < 0.01;bP < 0.05.
Genetics, Chromosomal Mapping, and Cloning of slie Allele
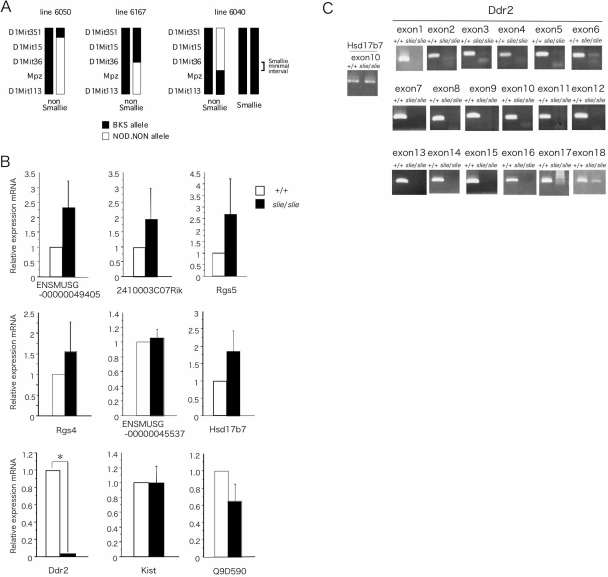
Genetic intercross demonstrated that dwarfism appeared only in the F2 generation, and the dwarfism phenotype was inherited in an autosomal-recessive Mendelian 3:1 ratio of normal-mutant progeny (58:20). A significant association for the slie locus was detected with marker D1Mit353 on chromosome 1, approximately 92 centiMorgans from the centromere. A high-resolution genetic map of the region was constructed using the flanking markers D1Mit36 and Mpz on 796 F2 progeny from a BKS(HRS)-slie/Jng homozygote × NOD.NON-H2nb1 F1 intercross and their associated phenotype (Fig. 2A). The fine structure genetic map encompassed an estimated 1.94-Mb physical region on chromosome 1 containing the slie mutation. According to annotations in the Mus musculus genome assembly version 34 (http://www.ensembl.org) the slie critical region contained 23 gene loci. Of these candidates, the coding sequences of 14 genes were compared, and no mutations were identified (data not shown). Nine remaining genes were analyzed by quantitative real-time PCR (qPCR) on brain cDNA from both wild-type and slie homozygotes. The relative levels of Ddr2 gene expression were quite low in slie mutants, and no significant expression differences in eight other genes were observed (Fig. 2B). Whereas Ddr2 expression was readily detected in wild-type mice, exons 1–17 of Ddr2 did not amplify from slie mutant genomic DNA by PCR (Fig. 2C). Both exon 18 of Ddr2 and the last exon of Hsd17b7, a gene proximal to Ddr2, were amplified from both slie homozygote mutant and control genomic DNA (Fig. 2C). Thus, a large deletion of approximately 150 kb in length encompasses the majority of the Ddr2 gene in slie mutant mice. Thus, the mutation results in a loss-of-function allele of Ddr2, officially called BKS(HRS)-Ddr2slie/JngJ.
Figure 2.
Genetic Mapping and Positional Cloning of slie %A, Genotype of recombinant progeny and markers defining the minimal interval are indicated. Test matings confirmed genotype with mouse 6040. B, Relative expression levels of candidate genes within the D1Mit36 to D1Mit353 interval as determined by qPCR, n = 3/genotype. C, Identification of exons from the Ddr2 gene. A deletion from exon 1 to exon 17 was detected in genomic DNA of slie mutant mice. All data represent mean and sem. *, P < 0.01.
Fertility and Gonad Histology of slie Mutants
Matings between male and female slie mutants failed to produce offspring, and in vitro fertilization had to be employed to generate the F2 intercross animals for mapping the slie mutation, as well as to produce heterozygous carriers for colony maintenance (see Materials and Methods). Female homozygous slie mutants mated to either homozygous slie or wild-type males result in no pregnancies or live pups, even when sperm plugs have been present. Occasionally, a young slie male has been found to sire a few litters with wild-type or heterozygous females, but such slie males are too infrequent to maintain the mouse colony.
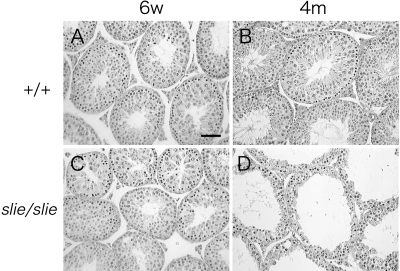
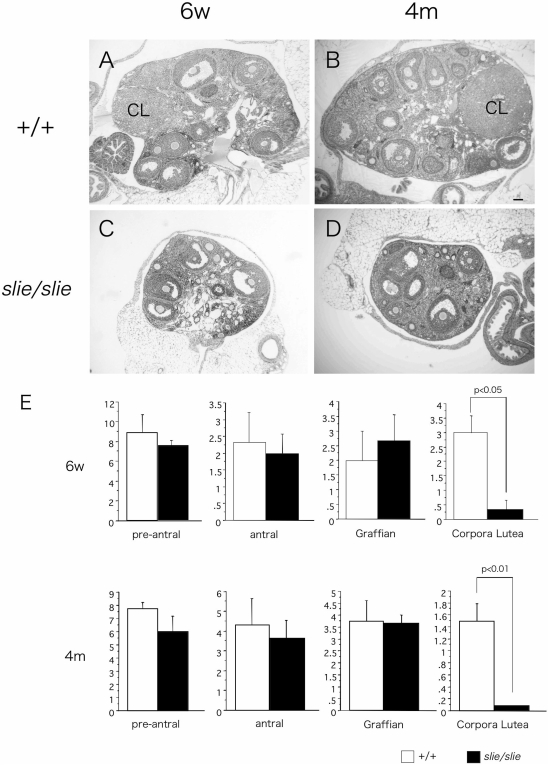
To further investigate the physiological and morphological consequences of a Ddr2 deficiency, the major organs of homozygous wild-type and slie mutant mice were compared at 6 wk and 4 months of age. Most slie homozygous testes appeared similar at 6 wk of age (Fig. 3C). However, by 4 months, remarkably fewer spermatids along with atrophy of spermatogonia, Serotoli, and Leydig cells occurred in slie homozygous males (Fig. 3D). The foremost difference in ovaries from slie mutant mice was the striking absence of corpora lutea in both 6-wk-old and 4-month-old slie homozygotes, as compared with wild type (Fig. 4). Conversely, no significant deviation was found in the amount of preantral, antral, and Graffian follicles at these ages (P < 0.05, Fig. 4). No gross morphological abnormalities were observed in any other organs and tissues examined (data not shown).
Figure 3.
Gonadal Abnormalities in Male slie Mutant Mice %Cross-sections of testes from 6-wk-old and 4-month-old wild-type (A and B) and slie mutant (C and D) mice stained with hematoxylin and eosin (n = 3/genotype). Scale bar, 50 μm. 6w, 6 wk old; 4m, 4 months old.
Figure 4.
Anovulation in Female slie Mutants %Wild-type 6-wk-old and 4-month-old (A and C) and mutant (B and D) ovaries sections stained with hematoxylin and eosin depicting follicular development (n = 3/genotype). CL, Corpus lutea; 6w, 6 wk; 4m, 4 months. Scale bar, 50 μm.
Neuroendocrine Axis of slie Mutants
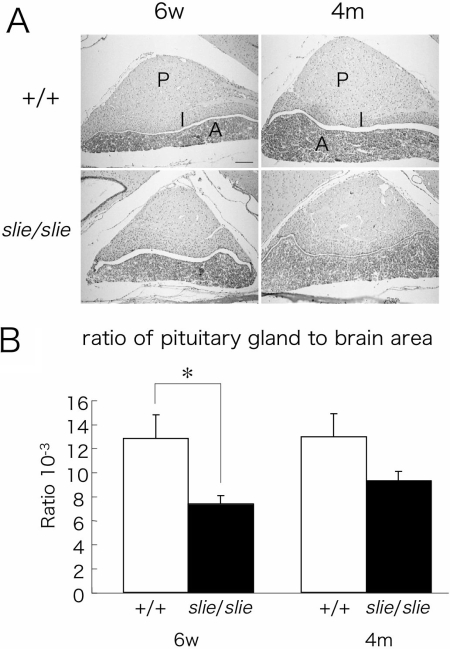
Given the phenotypes of dwarfism and infertility, we tested the hypothesis that primary hypopituitarism might be underlying the slie mutant phenotype. Histological examination revealed that trilaminar structure of the pituitary gland was intact in slie mutant mice at both ages (Fig. 5A), but the pituitary gland appeared smaller in slie mutants (Fig. 5A). In a detailed analysis using brain and pituitary histological sections, the proportion of the pituitary gland area to whole-brain area in slie mutant mice was significantly smaller (P < 0.05) compared with wild-type mice at 4 wk of age (Fig. 5B), but this difference disappeared by adulthood.
Figure 5.
Pituitary Morphology in slie Mutant and Wild-Type Mice %A, Representative sections from 6-wk-old and 4-month-old from wild-type and slie mutant mice (n = 3/group) depicting maximum height and width. Scale bar, 100 μm. B, Comparison of pituitary gland area/brain area ratio between control and slie mutant mice at 6 wk and 4 months of age (n = 4/group). P, Posterior lobe; I, intermediate lobe; A, anterior lobe; 6w, 6 wk; 4m, 4 month. *, P < 0.01; scale bar, 100 μm.
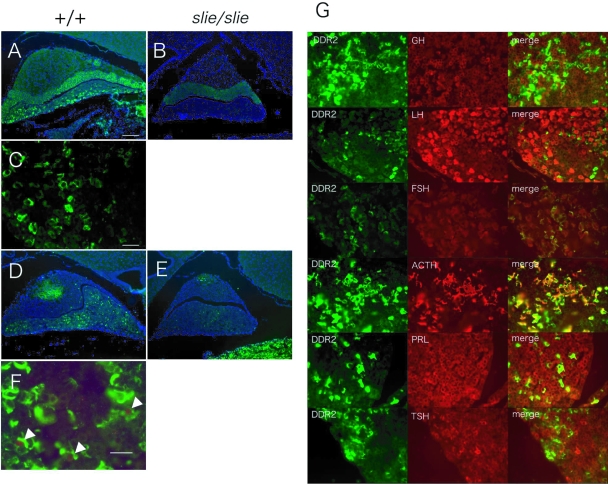
The localization of DDR2 in the mouse pituitary gland was detected by indirect immunofluorescence. Whereas DDR2 in wild-type mice was detected throughout the pituitary gland, staining was most abundant in the anterior lobe (Fig. 6A), and no staining was detected in the brain (data not shown). Within the anterior lobe of wild-type mice, DDR2 was expressed at the cell surface (Fig. 6C). As expected, DDR2 staining was absent in the pituitary gland of slie mutant mice (Fig. 6B). Furthermore, the number of cells staining positive for proliferating cell nuclear antigen (PCNA), a cell-proliferative marker, was decreased in the pituitary of slie mutant mice, compared with wild type, at 6 wk of age (Fig. 6, D and E). Notably, the PCNA-positive cells overlapped with DDR2-positive cells in the adenohypophysis (Fig. 6F). Colocalization for DDR2 immunostaining is absent in most pituitary cell types, except for adrenotropes (Fig. 6G).
Figure 6.
Pituitary Localization of DDR2 and PCNA Immunoreactivity and Cell-Proliferative Activity in slie Mutant Mice %Representative sections from 6-wk-old wild-type (n = 3/group; panels A, C, and D) and slie mutant mice (n = 3/group; panels B and E). DDR2 immunoreactivity was observed throughout the pituitary gland in wild-type mice (panels A and C) whereas it was absent in the pituitary gland of slie mutants (panel B). Numerous cells positive for PCNA immunoreactivity were detected in pituitary gland from wild-type mice (D and E), but few were detected in slie mutant mice. F, PCNA colocalized with DDR2 in some cells. G, Colocalization of DDR2 and specific anterior pituitary cell types in wild-type mice. Scale bar, 100 μm (A, B, D, and E) and 25 μm (C and G).
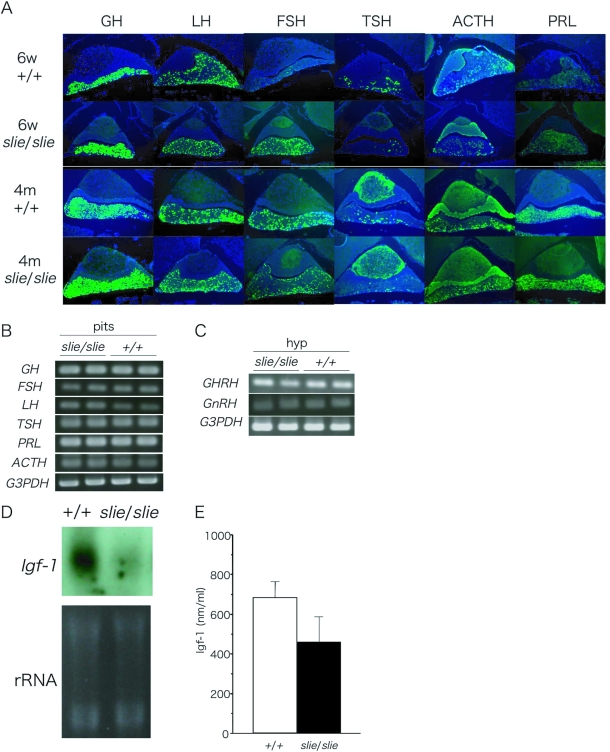
The cellular localization of the peptide hormones, LH, FSH, prolactin, ACTH, TSH, and GH, was detected in the adenohypophysis using indirect immunofluorescence. Each hormone was present in slie mutants, in sections of the pituitary from prepubertal and adult mice (Fig. 7A). Using semiquantitative RT-PCR, the expression level of mRNA for these pituitary hormones did not significantly differ between slie mutant and wild-type mice (Fig. 7B; P > 0.5). This complements the observation that no differences in basal circulating LH, FSH, and GH levels between smallie and wild-type mice were found (Table 2). To further understand the regulation of the hypothalamic-pituitary hormone axis, we used semiquantitative RT-PCR to analyze hypothalamic-releasing hormones, GHRH and GnRH, which regulate synthesis and release of pituitary hormones. Again, the expression levels of these two genes were not significantly different between slie mutant and homozygous wild-type mice (Fig. 7C; P > 0.3).
Figure 7.
Localization and Gene Expression of Peptide Hormones %A, Immunostaining of GH, LH, FSH, TSH, ACTH, and prolactin in pituitary glands from wild-type and slie mutant mice at 6 wk and 4 months of age. No significant differences between slie mutant and wild-type mice. Semiquantitative RT-PCR revealed no significant differences in gene expression levels for either pituitary hormones (B) or hypothalamic-releasing hormones (C) between wild-type and slie mutant adult mice. Northern blot analysis indicated that liver Igf-1 mRNA levels were reduced in slie mutant compared with wild-type mice (n = 3/genotype; panel D), but circulating levels of IGF-I were not found to be different (n = 12; P > 0.4; panel E).*, P < 0.01. PRL, Prolactin; G3PDH, glucose 3-phosphate dehydrogenase; hyp, hypothalamus; pit, pituitary; 6w, 6 wk; 4m, 4 months.
Table 2.
Plasma Levels of Pituitary Hormones in Slie and Control Mice
| Male | GH (ng/ml)
|
LH (ng/ml)
|
||||
|---|---|---|---|---|---|---|
| 4w | 20w | 4w | 20w | |||
| +/+ | 4.400 ± 1.030 | 7.000 ± 2.864 | 1.900 ± 0.367 | 1.000 ± 0.100 | ||
| slie/slie | 4.667 ± 1.202 | 5.750 ± 1.181 | 1.375 ± 0.165 | 1.150 ± 0.218 | ||
| P value | 0.437 | 0.724 | 0.272 | 0.521 | ||
| Female | GH (ng/ml)
|
LH (ng/ml)
|
FSH (ng/ml)
|
|||
|---|---|---|---|---|---|---|
| 4w | 20w | 4w | 20w | 4w | 20w | |
| +/+ | 3.600 ± 1.364 | 3.600 ± 0.678 | 1.020 ± 0.120 | 1.200 ± 0.184 | 15.800 ± 1.562 | 16.000 ± 1.225 |
| slie/slie | 3.400 ± 0.678 | 6.333 ± 2.140 | 1.260 ± 0.240 | 1.633 ± 0.338 | 14.800 ± 0.800 | 13.667 ± 0.333 |
| P value | 0.8988 | 0.2922 | 0.397 | 0.317 | 0.584 | 0.076 |
4w, 4 wk; 20w, 20 wk.
Longevity and Igf-1 Axis in slie Mutants
A maximal life span study has not been conducted in slie mutants, but our colony records indicate that slie mutants die prematurely compared with littermate controls (excluding mice used for experiments). For instance, 15% of slie mutants die before 3 months of age, and 47% were dead by 6 months of age. This was an unusual finding because dwarfism is associated with prolonged longevity, as seen in dwarf lit mice, presumably caused by deficiencies in IGF-I. Next, we measured Igf1 gene expression, a biomarker of GH activity and correlate of longevity. Despite remarkably low Igf1 mRNA in Northern blots of liver tissue from slie mutant mice (Fig. 7D; P < 0.05) circulating IGF-I levels did not significantly differ from their wild-type littermates, although the amount is somewhat variable (P > 0.4; Fig. 7E).
Gonadal Expression of DDR2 and Responsiveness to Hormones in slie Mutants
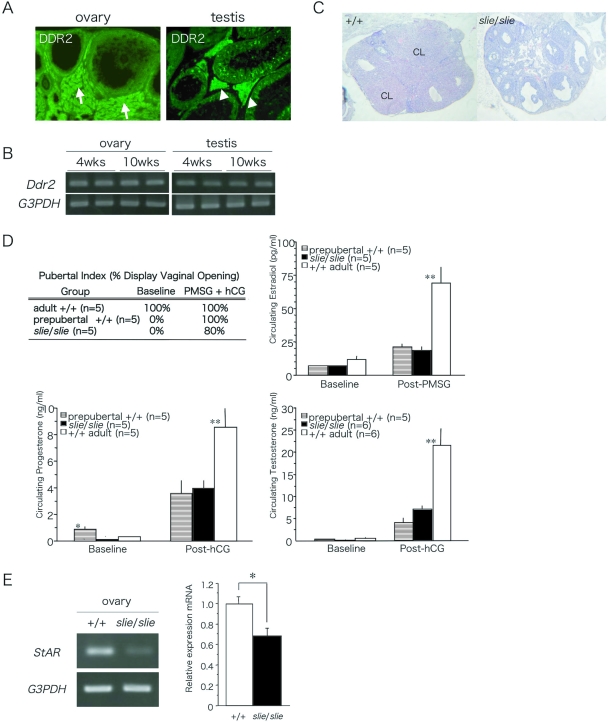
Using indirect immunofluorescence, we observed DDR2 staining in the ovaries of adult wild-type female mice in interstitial and thecal cells, but not in cumulus cells, inner layer of granulosa cells, or oocytes (Fig. 8A). In the testes of adult wild-type male mice, DDR2 was detected predominantly in the somatic Leydig cells (Fig. 8A). Using semiquantitative RT-PCR, Ddr2 expression in the gonads was not different between prepubertal and adult wild-type mice (Fig. 8B).
Figure 8.
Localization and Expression of DDR2 in Adult Gonad and Responsiveness to Exogenous Gonadotropin Administration %A, DDR2 immunoreactivity was localized throughout the somatic cells of the ovaries and testes. Arrows indicate interstitial cells between follicles (left panel), and arrowheads indicate Sertoli cells (right panel). B, Ddr2 was expressed in prepubertal and adult ovary and testis. C, Ovarian sections stained with hematoxylin and eosin after exogenous gonadotropin stimulation to 10-wk-old wild-type and slie mutant mice. D, Presence of vaginal orifice opening and serum estradiol levels 24 h after PMSG and serum progesterone levels 24 h after PMSG + hCG in female mice, and serum testosterone levels 24 h after 10 hCG in male mice (*, P < 0.05; **, P < 0.01). CL, Corpus lutea. E, Semiquantitative RT-PCR of StAR after gonadotropin administration in wild-type and slie mutants.
To investigate whether the absence of corpora lutea in slie mutant ovaries is caused by an inability to respond to gonadotrophic signals, pregnant mare serum gonadotropin (PMSG) and human chorionic gonadotropin (hCG) were injected into 6-wk-old female slie and wild-type mice to induce ovulation. Whereas the administration of exogenous gonadotropins induced follicular growth to the Graffian stage, it failed to alter the anovulatory phenotype of slie homozygotes, i.e. full luteinization of the thecal cells did not occur and no corpora lutea were formed (Fig. 8C).
To determine whether the endocrine gonadal responsiveness to pituitary peptide hormones was intact, exogenous gonadotropins were injected to adult (12–14 wk) and prepubertal (4 wk) wild-type and slie (12–14 wk) mutant mice. Each exogenous gonadotropin (10 IU) was used as the priming dose to elicit a robust, steroid hormone secretion from the gonads (Fig. 8D). Both female and male slie and wild-type mice secreted estradiol and progesterone and testosterone, respectively, in response to the exogenous gonadotropins. However, the circulating steroid levels in adult slie mutants were lower than in adult wild-type mice (Fig. 8D; estradiol: ANOVA, F2,14 = 11.96; P < 0.001; progesterone: ANOVA, F2,14 = 6.26; P < 0.01; testosterone: ANOVA, F2,16 = 14.6; P < 0.001). Notably, the amount of steroid hormones secreted by slie mutants was similar to that of prepubertal wild-type mice (Fig. 8D; NS). In addition, opening of the vaginal orifice, one of the initial signs of pubertal onset, was observed in both prepubertal wild-type and adult slie female mice upon gonadotropin treatment (Fig. 8D).
To confirm that blunted steroidogenesis contributes to infertility in slie mutants, we conducted semiquantitative RT-PCR experiments for the expression of StAR, a conclusive test for gonadotropin-induced steroidogenesis. Both wild-type and slie mutant mice expressed similar levels of StAR mRNA (data not shown); conversely, upon gonadotropin administration, slie mutants failed to increase StAR mRNA to levels seen in controls (Fig. 8E).
DISCUSSION
DDR2, a member of the nonintegrin tyrosine kinase receptors for fibrillar collagen, is expressed in numerous tissues (26,27,29). DDR2 is activated by collagen I, II, III, V, and X, with the notable exception of basement membrane collagen IV (26,27,30,31,32,33,34). DDR2 participates in remodeling of the extracellular matrix during morphogenesis and tissue repair, as well as differentiation and proliferation (26,28,35). DDR2 may contribute to disease because its expression is elevated in the bile duct of patients with primary biliary cirrhosis and in synovial fibroblasts of rheumatoid arthritis patients (35,36,37). Recent studies using nuclear magnetic resonance spectrometry, structural modeling, and mutational analysis confirm the collagen-binding site of DDR2 is contained within the discoidin domain, which binds with high affinity to the loop regions to form a furrow to accommodate the triple helix of the fibrillar collagen (30,31).
Mice homozygous for Ddr2 null allele resemble the observed phenotype in slie mutants, with shortening of the long bones and 30–40% reduction in body weight (28). It is not known whether either craniofacial or reproductive phenotypes observed in slie mutants are present in Ddr2 homozygote null mice because these studies were not conducted for the null mice. The dwarfism in slie mutant mice, as in Ddr2 null mutants, is caused by reduced proliferation of the long bones (28). In chondrocytes, collagen X is the main collagen present in the extracellular matrix, and mice lacking collagen X display reduced chrondrocyte proliferation resulting in stunted long bones (34), similar to slie homozygotes. Collagen X appears to be the primary signaling molecule in bones to induce proliferation, and DDR2 receptors may directly act upon chondrocytes through collagen X (28,34).
Initially, we interpreted the constellation of dwarfism, hyperglycemia, and sterility to imply a central mechanism mediating the slie mutant phenotypes. We hypothesized that the retarded growth and reproductive defects result from hypopituitarism, because it occurs in Snell and Ames dwarf mice (38,39). Despite the circumstantial data suggesting a central mechanism, our experimental work clearly supports a peripheral origin underlying the slie phenotypes. First of all, both the amount and distribution of cells secreting peptide hormones from the anterior pituitary and gene expression for ACTH, FSH, GH, LH, TSH, and prolactin did not differ in slie mutants. Second, GnRH and GHRH gene expression did not differ in slie mutants vs. wild type. Furthermore, serum concentrations of GH, FSH, LH, and T4 from slie mutants did not differ from wild-type. These data strongly argue against a primary role of pituitary in the manifestation of the slie phenotype. Our evidence clearly demonstrates that slie mutants contain gonadotropes and somatotropes that appropriately secrete the physiological baseline levels of circulating peptide hormones. Although it is possible that the hypothalamic-pituitary pulsatile system is inadequate, current methods cannot reliably detect its output in mice. In addition, further studies will reveal whether the neurohypophysis, which produces oxytocin and vasopressin, is intact and functional in slie mutant mice.
In the gonads, type I collagen appears to be an endogenous ligand for DDR2 because it is distributed throughout the ovary, particularly in the stroma, follicle, and epithelial layer (40). As the follicle matures, the antrum, oocytes, and granulosa cell layer express type I collagen (40,41), which overlaps with the distribution of DDR2 in the ovary. Furthermore, collagen type I appears to affect steroidogenesis within the follicle (38). Follicular growth in female slie mutants reaches the Graffian stage, but luteinization and steroid secretion from the thecal cells is deficient after exogenous gonadotropin stimulation. We have shown that DDR2 is expressed in the ovary and testis, including the interstitial cells, but notably, not in the germ cells. Upon further investigation of the gonadal endocrine system in slie mutants, it was found that exogenous gonadotropins might induce modest steroid hormone secretion in slie mice. However, the response is severely curtailed compared with adult wild-type mice and is similar to the response elicited in prepubertal wild-type mice. These data complement our observations in slie mutants and imply that collagen I and DDR2 receptors are in the same pathway that facilitates steroidogenesis. We hypothesize that slie mutant mice may not remodel gonadal tissue to permit appropriate steroid production that occurs in a fully mature system. Hence, the extracellular matrix may impact the prepubertal-to-adult developmental transition for the gonads via DDR2 signaling.
During diabetes, a lack of up-regulation of StAR gene expression after gonadotropin stimulation accounts for the low circulating levels of steroid hormones (42). The protein encoded by StAR gene enhances the conversion of cholesterol into pregnenolone to acutely regulate steroid hormone synthesis induced by gonadotropin stimulation and may possibly contribute to the aberrant steroid hormone secretion observed in slie mutants by retarding the transport of cholesterol from the outer mitochondrial membrane to the inner mitochondrial membrane and reducing pregnenolone synthesis. Our experimental results show that slie mutants are neither diabetic nor insulin resistant. However, despite normal baseline levels of StAR expression in slie mutants, they fail to surmount the appropriate increase in StAR expression after gonadotropin stimulation. This impaired response to exogenous hormone treatment suggests that DDR2 is an upstream pathway affecting gonadotropin-induced steroidogenesis via StAR gene expression.
Some authors suggest a relationship between infertility and dwarfism (43,44,45). Human DDR2 maps to chromosome 1q12-qter (46,47); however, to date, neither a mutation associated with human DDR2 nor any dwarfism nor sterility locus has yet been linked to this region in humans. Based on similarities in phenotype and mode of genetic inheritance, our data suggest that DDR2 may be a suitable candidate gene to investigate in cases of unidentified dwarfism and sterility in humans, such as Levi-type syndrome (25). However, to date, there is no molecular evidence supporting our speculation because the chromosomal position for Levi-type dwarfism has not been identified and its underlying genetic defect is unknown.
Before these observations, the effects of DDR2 upon fertility and its presence in the gonad were unknown. Our novel findings suggest that DDR2 conveys an important signal for endocrine regulation, perhaps via the interstitial cells, to enhance steroid hormone secretion, via StAR gene expression, after gonadotropin stimulation. The constellation of infertility and dwarfism phenotypes manifested by slie mutants may arise from inadequate signaling by collagen via its DDR2 receptors to remodel bone and gonadal tissue. Further analysis of slie mutant mice will provide new avenues of investigation by which to understand the contribution of the extracellular matrix as an upstream player intersecting with endocrine pathways that influence body size and reproduction.
MATERIALS AND METHODS
Experimental Animals
All mice used in this study were bred at the Jackson Laboratory, and all procedures were approved by the Institutional Animal Care and Use Committee according to the guidelines of both the United States Department of Agriculture and the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (1996). Mice were housed in groups of two to four, with autoclaved, white pine shavings as bedding, under 12-h light,12-h dark photoperiod (lights on at 0600 h), with ad libitum access to water and food (JL Mouse/Auto 6F, no. 5K52 PMI Feeds).
The mouse strain carrying the mutation are officially designated as BKS(HRS)-Ddr2slie/JngJ. Smallie mutant mice arose spontaneously in a BKSChpLt(HRS)Tg(Ins2-Cpe) 1LtCpefat/LtJng line, and an unaffected heterozygous sibling was crossed to C57BLKS/J to produce slie carriers because homozygote mutants were infertile. To briefly review the history of this colony, the fat mutation arose spontaneously in the Cpe gene on the HRS inbred mouse strain, and this mutation was transferred to the C57BLKS/J (BKS) strain through congenic backcrossing experiments in the laboratory of Drs. Dorothy Chapman (Chp) and Ed Leiter (Lt). In addition, these mice carry the transgene Cpefat under control of the Ins2 promoter, which was generated by Dr. Leiter. The slie mutation spontaneously arose in a colony of these mice in the laboratory of Dr. Jurgen K. Naggert (Jng) provided by Dr. Leiter. The naming of the mutation, alleles, transgene, strain, and investigator’s laboratory code conforms to the conventions of the International Committee on Standardized Genetic Nomenclature for Mice (http://www.informatics.jax.org/mgihome/nomen/inc.shtml) and was approved by Mouse Genomic Nomenclature Committee.
Selective breeding experiments demonstrated that the dwarfing phenotype was independent of the presence of the Cpefat mutation or the transgene, Ins2-Cpe. Both Cpefat and Ins2-Cpe were removed from the slie colony through selective breeding. To determine the chromosomal location of the slie gene, F1 progeny were generated by in vitro fertilization of BKS(HRS)-slie/Jng homozygotes to NOD.NON-H2nb1/LtJ mice. The heterozygous F1 mice, which do not exhibit dwarfism, were intercrossed for the initial linkage mapping study. The resultant F2 progeny mice were weighed at 10 wk of age to distinguish affected animals.
Microsatellite Marker Analysis
Tail DNA was isolated from intercross mice according to Buffone and Darlington (48). Genomic DNA from 798 F2 offspring was analyzed using microsatellite markers to construct a genetic map. To map the chromosomal location of the slie mutation, 25 genomic DNA samples from [BKS(HRS)-slie/Jng homozygote × NOD.NON-H2nb1]F1 intercross progeny were used to generate a genome-wide scan using microsatellite markers to detect genetic polymorphisms associated with dwarfism phenotype. The critical region was further reduced by identifying F2 homozygotes with nearby recombination. A minimum of 20 offspring from each F2 recombinant breeding pair were analyzed for genotype and phenotype. Microsatellite marker primer pairs for the genome scan were purchased from Research Genetics (Huntsville, AL). For genomic PCR amplification, 25 ng of tail DNA was used in a 10-μl volume containing 50 mm KCl; 10 mm Tris-Cl, pH 8.3; 2.5 mm MgCl2; 0.2 mm oligonucleotides; 200 μm deoxynucleotide triphosphate; and 0.02U AmpliTaq DNA polymerase (PerkinElmer, Norwalk, CT). The cycling conditions were 95 C for 2 min, followed by 49 cycles of 94 C, 20 sec; 50 C, 20 sec; 72 C, 30 sec; and a 7-min extension at 72 C. PCR products were separated by electrophoresis on a 4% MetaPhor agarose gel (FMC Bioproducts, Rockland, ME) and visualized under UV light after staining with ethidium bromide.
Isolation and Detection of mRNA
For quantitative real-time PCR (qPCR) and semiquantitative RT-PCR, total RNA was isolated from whole brain, hypothalamus, pituitary gland, testis, and ovary in wild-type (n = 3) and slie mutant (n = 3) mice using TRIzol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized from total RNA with Superscript First-Strand Synthesis System for RT-PCR (Invitrogen). The expression level [cycle time (Ct)] of each transcript was detected by counting the number of necessary cycles to yield equivalent expression level of each gene. Ct values were converted to fold differences in expression according to the equation [Ct1(slie/slie) − Ct1(18s rRNA)] − [Ct2(+/+) − Ct2(18s rRNA)], where Ct1(18s rRNA) and Ct2(18s rRNA) represent the Ct values for the 18s rRNA gene in the slie and wild-type samples, respectively. Primers used to amplify the genes for qPCR and semiquantative RT-PCR experiments are listed in Table 3. The amplification conditions used were 95 C, 2min followed by 25 cycles of 94 C, 15 sec; 58 C, 30 sec; 72 C, 30 sec. We performed Northern blot analysis using 20 μg liver total RNA, probed with mouse Igf-1 cDNA fragment labeled with [α32P] dCTP using Rediprime II DNA Labeling System (GE Healthcare, Piscataway, NJ) and hybridized using Rapid-Hyb Buffer (GE Healthcare) at 65 C overnight.
Table 3.
Primer Sequences
| Forward Primer | Reverse Primer | |
|---|---|---|
| ENSMUSG49405 | TGCTGGCACTGAGGATTATATCC | TGTGCTGGTCACCCTGTAGCT |
| 2410003C07Rik | CCGGGTGAATGACTTTGAGATT | AGCTTCCTGGTGCACATTGC |
| Rgs5 | TGAATTCAGTGAGGAAAACCTTGA | AGACGGTTCCACCAGGTTCTT |
| Rgs4 | TCCTGCGAACACAGTTCTTCA | GAAGTCAATGTTCTCCTCGCTGTA |
| ENSMUSG45537 | GACTGCAAAGGCTGGAGATCA | TGCGTGCCTCTGTCCTGTT |
| Hsd17b7 | CGCTCACTGTGACACCGTACA | ACGCTTTTCCAGCTCCAGTAAG |
| Ddr2 | CGGATCCTGATTGGTTGCTT | GTGATGAGGAGCGGTTGTTATTG |
| AA437972 | GGTGGCCGTGAAAATGCT | GCTCGTGGCGAGAAAGAAAC |
| Kist | TGGAGTGCGGAGAATGAGTG | CGAGGCTCCACAGATCAACA |
| Q9D590 | CCTCCCACCTACAGGACCAA | CAGTTGCAGGGTCACAGGAA |
| GH | AGATTCCAAACTGCTCAGAGTC | GCTCGAACTCTTTGTAGGTGTC |
| FSH | GATAGGGAGAAGGAACTCAGG | TTCCTTTCCTCCCTCTAGACTC |
| LH | FPTAGACCCCATAGTCTCCTTTCC | GAGGGAGGGATGATTAGAACAC |
| TSH | GTAGTGGGTGGAGAAGAGTGAG | CATACAATACCCAGCACAGATG |
| PRL | GCTACACCTGAAGACAAGGAAC | AGGCCTGGCTAATTATCTTCTC |
| ACTH | TAAGAGCAGTGACTAAGAGAGGC | CACACATCTATGGAGGTCTGAAG |
| GHRH | CACCACCAACTACAGGAAACTC | GCAGAAGTAACAGGGTACAGTTG |
| GnRH | GGGAAAGAGAAACACTGAACAC | CCTCTTCAATCAGACTTTCCAG |
| StAR | GAAAGGATTAAGGCACCAAGC | TGACATTTGGGTTCCACTCTC |
| G3PDH | TGAAGGTCGGTGTCAACGGATTTGGC | CATGTAGGCCATGAGGTCCACCAC |
Histology and Morphological Analysis
In histological experiments, 5 IU of PMSG (Sigma Chemical Co., St. Louis, MO) followed 48 h later by 5 IU hCG (Sigma) was injected ip into female 10-wk-old wild-type and slie mutant mice to induce ovulation. Testes and ovaries were collected and placed into Bouin’s fixative overnight, dehydrated, embedded in paraffin, cut at 5 μm sections, and stained with hematoxylin and eosin. Pituitary glands were collected and sectioned sagittally from 6-wk-old and 4-month-old wild-type and slie homozygotes for histological examination using hematoxylin and eosin and immunohistological experiments. Area measurements of pituitary gland and whole brain in sections stained with hematoxylin and eosin were performed on a Macintosh computer using the NIH Image program (http://rsb.info.nih.gov/nih-image/ ).
Phenotyping
Body composition was determined by dual x-ray absorptiometry on a Lunar PIXImus integrated densitometer (Inside Outside, Fitchburg, WI) (49,50). Serum albumin, calcium, glucose, high-density lipoprotein, low-density lipoprotein, alkaline phosphatase, cholesterol, total protein, bilirubin, triglycerides, blood urea nitrogen, and T4 were measured using a Beckman Coulter Synchron CX5 Delta chemistry analyzer (Beckman Coulter, Inc., Brea, CA).
Immunohistochemistry
To localize anterior pituitary hormones in slie and wild-type homozygotes, polyclonal antibodies to DDR2 (H-108), LH (R-16), prolactin (C-17), and TSHβ (C-16) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and antibodies for GH (antibody 8490), FSH (antibody 8746), and ACTH (antibody 11020) were purchased from Abcam, Inc. (Cambridge, MA). After deparaffinization, the sections of the pituitary glands and ovaries were boiled in sodium citrate buffer (pH 6.0) for 1 min in a microwave oven for antigen retrieval. They were preincubated with 5% normal rabbit or goat serum for 30 min, incubated for 1 h with the primary antibody (1:100; Vector Laboratories, Burlingame, CA), then with biotinylated secondary antibody (1:100; Vector Laboratories), and next with streptavidin-biotin-fluorescein isothiocyanate complex (Vector Laboratories). For a negative control, experiments without the primary antibody were performed. All the reactions were carried out at room temperature. Digital images to analyze sections were generated using a Spot Camera (Diagnostic Instruments, Sterling Heights, MI).
Pituitary and Steroid Hormone Measurements
The anterior pituitary hormones, FSH, LH, and GH, were measured by RIA. Whole blood (300 μl) from wild-type (n = 4–5) and slie (n = 5) mice was collected at 4 and 20 wk of age during the morning (0800–1000 h) from the retroorbital vein and placed immediately on ice. Sera were harvested from whole blood chilled at 4 C by centrifugation at 5000 rpm for 8 min and stored at −20 C until used in RIAs. Serum peptide hormone levels were measured in duplicate 50-μl samples by RIA. The sensitivity was determined to be the following for each RIA: FSH, 2.0 ng/ml; LH, 0.9 ng/ml; GH, 2.0 ng/ml. Intraassay variability was calculated as 4.7%, 9.2%, and 6.1% for LH, FSH, and GH, respectively. A standard curve was generated from known concentrations of hormones, and a logit-linear function equation was generated from these data with high correlations for the standard curve (r ≥ 0.9).
Exogenous gonadotropins were administrated to adult (12–14 wk) and prepubertal (4 wk) wild-type as well as adult slie (12–14 wk) mutant mice. To elicit a robust steroid hormone secretion from the gonads, 10 IU of PMSG and hCG (Sigma) were used. At 0900 h, females (n = 5/group; n = 15) were weighed, and vaginal opening, an index of puberty, was noted. In addition, before the start of gonadotropin injections, each adult female mouse was confirmed to be in the diestrous stage of estrus by inspection of a vaginal smear. Next, baseline blood samples (75 μl) were collected and followed immediately by ip injection of PMSG to each mouse. A second blood sample (75 μl) was collected 24 h after PMSG injection to assess the peak levels of circulating estradiol. hCG was injected 48 h after PMSG injection to all female mice, and a third blood sample was taken 24 h after hCG injection to measure circulating progesterone levels. Male mice (n = 5–6/group; n = 17) were weighed, and baseline blood samples (75 μl) were collected, followed by ip injection of 10 IU hCG to each male mouse to stimulate endogenous testosterone secretion. A second blood sample (60 μl) was taken 24 h after hCG injection to measure circulating levels of testosterone. To maintain consistent blood volume during the multiple blood collections, sterile physiological saline was administrated in a volume equal to whole blood removed. Estradiol, progesterone, and testosterone were measured using competitive enzyme immunoassay kits (Cayman Chemical Co., Ann Arbor, MI). We first verified that these kits do not require extraction or chromatography of plasma samples from mice and accurately detected hormone-spiked controls of mouse plasma. Mouse IGF-I was measured by competitive coated-well ELISA, following the guidelines provided by the manufacturer (Diagnostic Systems Laboratories, Webster, TX). A spectrophotometer (SpectraMax 190, Molecular Devices Corp., Sunnyvale, CA) was used to measure sample intensity at 407 nm. Samples from each mouse were measured in duplicate, using 5–30 μl of plasma. For the estradiol enzyme immunoassay (EIA), after PMSG injection the assay sensitivity was calculated at 5 pg/ml, and the intraassay coefficient was 2.4%. The sensitivity for the progesterone EIA was 0.01 ng/ml, and the intraassay coefficient was 3.5%. The sensitivity for the testosterone EIA was 0.04 ng/ml, and the intraassay coefficient was 2.8%. For the IGF-I ELISA, the assay sensitivity was calculated at 8 ng/ml, and the intraassay coefficient was 4.3%. For all assays, the correlation of the linear range from the standard curve was tight (r ≥ 0.95). For values that were below the limit of detection for the specific assay, the value of lowest sensitivity was assigned to that specific sample.
Statistical Analysis
Descriptive statistics were reported for body weight, body composition, 12 serum analytes, pituitary area, pituitary hormones, and gene expressions. To detect significant statistical differences, ANOVA (least-squares model) and Tukey’s multiple pairwise comparisons were used to compare among experimental groups, or between-group t tests were used within same-sex groups. Differences were considered significant at P < 0.05. For circulating steroid hormones, analysis included descriptive statistics, and significant differences among groups were detected by one-way ANOVA followed by multiple comparisons (hormone treatment) separately for female and males. Differences were considered significant at P < 0.05 (JMP 6.0; SAS Institute, Cary, NC).
Acknowledgments
We thank Dr. Wesley Beamer and Dr. Alexei Evsikov for their critiques of the manuscript.
Footnotes
The research was supported by National Institutes of Health Grant DK46977 (to J.K.N.).
Part of this research was presented in abstract form at the Annual Meeting of the Society for the Study of Reproduction, Omaha, Nebraska in 2006.
Disclosure Statement: The authors have nothing to declare.
First Published Online May 15, 2008
Abbreviations: BMC, Bone mineral content; Ct, cycle time; DDR2, discoidin domain receptor 2; EIA, enzyme immunoassay (nonradioactive RIA); hCG, human chorionic gonadotropin; PCNA, proliferating cell nuclear antigen; PMSG, pregnant mare serum gonadotropin; qPCR, quantative real-time PCR.
References
- Snell GD 1929 Dwarf, a new Mendelian recessive character of the house mouse. Proc Natl Acad Sci USA 15:733–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaiber RH, Gowen JW 1961 A new dwarf mouse. Genetics 46:896 [Google Scholar]
- Bartke A 1964 Histology of the anterior hypophysis, thyroid and gonads of two types of dwarf mice. Anat Rec 149:225–235 [DOI] [PubMed] [Google Scholar]
- Eicher EM, Beamer WG 1976 Inherited ateliotic dwarfism in mice: characteristics of the mutation, little, on chromosome 6. J Hered 67:87–91 [DOI] [PubMed] [Google Scholar]
- Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O'Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG 1996 Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 384:327–333 [DOI] [PubMed] [Google Scholar]
- Li S, Crenshaw III EB, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG 1990 Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature 347:528–533 [DOI] [PubMed] [Google Scholar]
- Cheng TC, Beamer WG, Phillips III JA, Bartke A, Mallonee RL, Dowling C 1983 Etiology of growth hormone deficiency in little, Ames, and Snell dwarf mice. Endocrinology 113:1669–1678 [DOI] [PubMed] [Google Scholar]
- Sinha YN, Salocks CB, Vanderlaan WP 1975 Pituitary and serum concentrations of prolactin and GH in Snell dwarf mice. Proc Soc Exp Biol Med 150:207–210 [DOI] [PubMed] [Google Scholar]
- Bartke A 1965 The response of two types of dwarf mice to growth hormone, thyrotropin, and thyroxine. Gen Comp Endocrinol 5:418–426 [DOI] [PubMed] [Google Scholar]
- van Buul-Offers S 1983 Hormonal and other inherited growth disturbances in mice with special reference to the Snell dwarf mouse. Acta Endocrinol (Copenh) 103:1–47 [PubMed] [Google Scholar]
- Bartke A 2000 Delayed aging in Ames dwarf mice. Relationships to endocrine function and body size. Berlin: Springer [DOI] [PubMed] [Google Scholar]
- Smith PE, MacDowell EC 1931 The differential effect of hereditary mouse dwarfism on the anterior pituitary hormones. Anat Rec 50:85–93 [Google Scholar]
- Bartke A, Lloyd CW 1970 Influence of prolactin and pituitary isografts on spermatogenesis in dwarf mice and hypophysectomized rats. J Endocrinol 46:321–329 [DOI] [PubMed] [Google Scholar]
- Kemp T 1938 Hereditary and endocrine function. An investigation of hereditary pituitary deficiency in the mouse. Acta Pathol Microbiol Scand Suppl 14:290–305 [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A 1996 Dwarf mice and the ageing process. Nature 384:33 [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg HM, Bode AM, Carlson J, Hunter WS, Bronson RT 1998 Does growth hormone prevent or accelerate aging? Exp Gerontol 33:675–687 [DOI] [PubMed] [Google Scholar]
- Flurkey K, Papaconstantinou J, Miller RA, Harrison DE 2001 Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci USA 98:6736–6741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flurkey K, Papaconstantinou J, Harrison DE 2002 The Snell dwarf mutation Pit1 (dw) can increase life span in mice. Mech Ageing Dev 123:121–130 [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H 2004 Life extension in the dwarf mouse. Curr Top Dev Biol 63:189–225 [DOI] [PubMed] [Google Scholar]
- Beamer WG, Eicher EM, Maltais LJ, Southard JL 1981 Inherited primary hypothyroidism in mice. Science 212:61–63 [DOI] [PubMed] [Google Scholar]
- Beamer WG, Maltais LJ, DeBeats MH, Eicher EM 1987 Inherited congenital goiter in mice. Endocrinology 120:838–840 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Yamanaka K, Atsumi S, Tsumura H, Sasaki R, Tomita K, Ishikawa E, Ozawa H, Watanabe K, Totsuka T 1994 A novel hypothyroid ‘growth-retarded’ mouse derived from Snell’s dwarf mouse. J Endocrinol 142:435–446 [DOI] [PubMed] [Google Scholar]
- Takabayashi S, Uneki K, Yamamoto E, Suzuki T, Okayama A, Katoh H 2006 A novel hypothyroid dwarfism due to missense mutation Arg479Cys of the thyroid peroxidase gene in the mouse. Mol Endocrinol 20:2584–2590 [DOI] [PubMed] [Google Scholar]
- Flamant F, Poguet A-L, Plateroti M, Chassande O, Gauthier K, Streichenberger N, Mansouri A, Samarut J 2002 Congential hypothyroid. Pax8(−/−) mutant mice can be rescued by inactivating the TRα gene. Mol Endocrinol 16:24–32 [DOI] [PubMed] [Google Scholar]
- Black J 1961 Low birth weight dwarfism. Arch Dis Child 36:633–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel W, Gish GD, Alves F, Pawson T 1997 The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell 1:13–23 [DOI] [PubMed] [Google Scholar]
- Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD 1997 An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell 1:25–34 [DOI] [PubMed] [Google Scholar]
- Labrador JP, Azcoitia V, Tuckermann J, Lin C, Olaso E, Manes S, Bruckner K, Goergen JL, Lemke G, Yancopoulos G, Angel P, Martinez C, Klein R 2001 The collagen receptor DDR2 regulates proliferation and its elimination leads to dwarfism. EMBO Rep 2:446–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel W 1999 Discoidin domain receptors: structural relations and functional implications. FASEB J 13:S77–S82 [DOI] [PubMed] [Google Scholar]
- Ichikawa O, Osawa M, Nishida N, Goshima N, Nomura N, Shimada I 2007 Structural basis of the collagen-binding mode of discoidin domain receptor 2. EMBO J 26:4168–4176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konitsiotis A, Raynal N, Bihan D, Hohenester E, Farndale RW, Leitinger B. Characterization of high affinity binding motifs for the discoidin domain receptotr DDR2 in collagen. J Biol Chem 283:6861–6868 [DOI] [PubMed] [Google Scholar]
- Leitinger B 2003 Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2: identification of collagen binding sites in DDR2. J Biol Chem 278:16761–16769 [DOI] [PubMed] [Google Scholar]
- Leitinger B, Steplewski A, Fertala A 2004 The D2 period of collagen II contains a specific binding site for the human discoidin domain receptor, DDR2. J Mol Biol 344:993–1003 [DOI] [PubMed] [Google Scholar]
- Leitinger B, Kwan APL 2006 The discoidin domain receptor DDR2 is a receptor for type X collagen. Matrix Biol 25:355–364 [DOI] [PubMed] [Google Scholar]
- Ferri N, Carragher NO, Raines EW 2004 Role of discoidin domain receptors 1 and 2 in human smooth muscle cell-mediated collagen remodeling: potential implications in atherosclerosis and lymphangioleiomyomatosis. Am J Pathol 164:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao TK, Kimura Y, Kenny TP, Branchi A, Gishi RG, Van de Water J, Kung HJ, Friedman SL, Gershwin ME 2002 Elevated expression of tyrosine kinase DDR2 in primary biliary cirrhosis. Autoimmunity 35:521–529 [DOI] [PubMed] [Google Scholar]
- Wang J, Lu H, Liu X, Deng Y, Sun T, Li F, Ji S, Nie X, Yao L 2002 Functional analysis of discoidin domain receptor 2 in synovial fibroblasts in rheumatoid arthritis. J Autoimmun 19:161–168 [DOI] [PubMed] [Google Scholar]
- Watkins-Chow DE, Camper SA 1998 How many homeobox genes does it take to make a pituitary gland? Trends Genet 14:284–290 [DOI] [PubMed] [Google Scholar]
- Ma X, Dong Y, Matzuk MM, Kumar TR 2004 Targeted disruption of luteinizing hormone β-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci USA 101:17294–17299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkholtz CB, Lai BE, Woodruff TK, Shea LD 2006 Distribution of extracellular matrix proteins type I collagen, type IV collagen, fibronectin, and laminin in mouse folliculogenesis. Histochem Cell Biol 126:583–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkholtz CB, Shea LD, Woodruff TK 2006 Extracellular matrix functions in follicle maturation. Semin Reprod Med 24:262–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna PR, Stocco DM 2005 Regulation of the steroidogenic acute regulatory protein expression: functional and physiological consequences. Curr Drug Targets Immune Endocrin Metab Disord 5:93–108 [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Kannan K, Givol D, Yoshida Y, Tajima K, Dantes A 2001 Apoptosis of granulosa cells and female infertility in achondroplastic mice expressing mutant fibroblast growth factor receptor 3G374R. Mol Endocrinol 15:1610–1623 [DOI] [PubMed] [Google Scholar]
- Chen L, Li C, Qiao W, Xu X, Deng C 2001 A Ser(365)→Cys mutation of fibroblast growth factor receptor 3 in mouse downregulates Ihh/PTHrP signals and causes severe achondroplasia. Hum Mol Genet 10:457–465 [DOI] [PubMed] [Google Scholar]
- Cha KB, Karolyi IJ, Hunt A, Wenglikowski AM, Wilkinson JE, Dolan DF, Dootz, G, Finnegan AA, Seasholtz AF, Hankenson KD, Siracusa LD, Camper SA 2004 Skeletal dysplasia and male infertility locus on mouse chromosome 9. Genomics 83:951–960 [DOI] [PubMed] [Google Scholar]
- Karn T, Holtrich U, Brauninger A, Bohme B, Wolf G, Rubsamen-Waigmann H, Strebhardt K 1993 Structure, expression and chromosomal mapping of TKT from man and mouse: a new subclass of receptor tyrosine kinases with a factor VIII-like domain. Oncogene 8:3433–3440 [PubMed] [Google Scholar]
- Miozzo M, Pierotti MA, Sozzi G, Radice P, Bongarzone I, Spurr NK, Della Porta G 1990 Human TRK proto-oncogene maps to chromosome 1q32–q41. Oncogene 5: 1411–141449 [PubMed] [Google Scholar]
- Buffone GJ, Darlington GJ 1985 Isolation of DNA from biological specimens without extraction with phenol. Clin Chem 31:164–165 [PubMed] [Google Scholar]
- Nagy TR, Clair A-L 2000 Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obesity Res 8:392–398 [DOI] [PubMed] [Google Scholar]
- Hunter HL, Nagy TR 2002 Body composition in a seasonal model of obesity: longitudinal measures and validation of DXA. Obes Res 10:1180–1187 [DOI] [PubMed] [Google Scholar]