Abstract
The importance of the adopted metabolite receptors, such as peroxisome proliferator-activated receptor, liver X receptor, and farnesoid X receptor, in transcriptional control of metabolic pathways has been appreciated for many years. However, it is becoming increasingly clear that the number of nuclear receptors with roles in metabolism is much larger than initially suspected. Recent years have brought an intense effort to define the biological functions of the most enigmatic group of the nuclear receptor superfamily, the true orphan receptors, including nuclear receptor 4As, estrogen-related receptors, retinoid-related orphan receptors, and Rev-erbs. Unexpectedly, several of these receptors also turn out to have important functions in various aspects of metabolic control.
PROPER METABOLIC regulation is fundamental to the survival of multicellular organisms. As such, sophisticated regulatory pathways exist to ensure appropriate metabolic responses to changing cellular and environmental conditions. Complex mechanisms are in place to deal with tissue-specific metabolic demands and fluctuating energy levels. Nutritional deprivation and excess can both lead to pathological consequences for the organism. Under starvation conditions, glucose and lipid stores are eventually depleted and the body turns to protein catabolism to meet metabolic demands. Excess caloric intake, on the other hand, can lead to the development of obesity and metabolic disease.
Consequences associated with nutritional overabundance are slow to develop, typically requiring years before associated metabolic diseases become apparent. Unfortunately, the current lifestyle trends in Western society, characterized by diets rich in fat and cholesterol, accompanied by lack of exercise, are resulting in increased incidence and earlier onset of obesity, diabetes, and atherosclerosis (1,2). Despite extensive research efforts and advances in treatment, these conditions remain among the leading causes of mortality and morbidity in Western society (3). Current trends indicate that the scope of the problem is only likely to grow, highlighting the increasing importance of understanding the pathways that regulate metabolism and how they are disrupted in metabolic disease.
The identification of members of the nuclear receptor superfamily as intracellular receptors for both dietary lipids and their metabolic derivatives has focused attention on them as key regulators of metabolism (4,5). As ligand-activated transcription factors, nuclear receptors have the capacity to regulate genes involved in lipid and energy metabolism directly in response to varying nutrient availability. For example, the peroxisome proliferator-activated receptor (PPAR) family responds to fatty acid derivatives to regulate aspects of fatty acid metabolism and storage. Targets of PPARs include genes involved in adipogenesis and lipogenesis in white adipose tissue, energy uncoupling and thermogenesis in brown fat, fatty acid oxidation in muscle, and fasting responses in the liver (reviewed in Refs. 6 and 7). The liver X receptors (LXRs) serve as cholesterol sensors and play important roles in the regulation of cholesterol homeostasis. LXR target genes are involved in various facets of cholesterol metabolism, including bile acid synthesis, cholesterol absorption, and reverse cholesterol transport (8,9). Other adopted orphan receptors with prominent roles in metabolism include farnesoid X receptor, which regulates bile acid metabolism in response to bile acid levels, and the xenobiotic receptors pregnane X receptor and constitutive androstane receptor, which are involved in metabolism of a range of endogenous and exogenous compounds (10,11).
A number of nuclear receptors, initially identified on the basis of sequence homology to classic steroid receptors, still do not have known ligands and thus remain classified as true orphan receptors. For some of these, the search for a natural ligand continues. Others are believed to be unliganded receptors the activity of which is regulated by receptor abundance, posttranslational modification, and/or cofactor recruitment. The inability to readily modulate the activity of these receptors by ligand treatment has caused the characterization of orphan nuclear receptors to lag behind the adopted or nutritional sensors described above. Ongoing research employing a variety of strategies (i.e. examination of expression profiles, analysis of cofactor interactions, and manipulation of receptor levels) is pointing to unexpected roles for a number of these in metabolic control, including the estrogen-receptor-related receptors, the AsNR4As, the retinoic acid receptor-related orphan receptors, and the Rev-erbs.
ESTROGEN-RECEPTOR-RELATED RECEPTORS (ERRs)
ERRα was the first orphan nuclear receptor to be identified and was cloned based on sequence similarity to the estrogen receptor (ER) (12). Subsequently, two closely related family members, ERRβ and ERRγ, were discovered (12,13). All three ERR isotypes bind consensus ER-binding sites (estrogen response elements) as monomers or homodimers, consistent with the high degree of similarity of ERRs to ERs in the DNA-binding domain (14). Recently it has been suggested that dimerization is required for coactivator recruitment and transactivation (15,16). In addition, heterodimerization has been reported between ERR isoforms as an additional modulator of receptor activity17). The ERRs also bind to extended half-sites (ERR response elements) in the promoters of some target genes (18). Unlike the classical ERs, ERRs are not activated by estrogen. To date, no endogenous ligand has been identified, and it remains open to debate whether a natural ligand or ligands exist for these receptors (19). Increasing evidence suggests the ERRs are constitutive receptors with activity dependent on expression levels and cofactor interactions rather than ligand binding (20,21,22,23). Several synthetic modulators of ERR activity have been reported, however. These include estrogen-like low-affinity antagonists of ERR function, including the organic pesticides, toxaphene and chlordane, and the synthetic estrogen diethylstilbesterol (19). Recently, high-throughput screening strategies have also identified ERR antagonists with submicromolar activity including indole, pyrazole, and thiazolidinedione (24).
Initial research on ERRs examined their ability to modulate estrogen signaling and explored possible roles in bone formation and breast cancer (14). The identification of medium chain acyl-coenzyme A dehydrogenase, a rate-limiting enzyme in mitochondrial β-oxidation, as a target of ERRα has focused attention on the ERRs as regulators of energy homeostasis (25,26). Subsequently, various profiling strategies using ERRα-overexpressing cell lines, ERRα null mouse tissues, and chromatin immunoprecipitation-on-chip assays examining ERRα and ERRγ promoter occupancy have shown regulation of a number of other targets involved in fatty acid transport, fatty acid oxidation, and mitochondrial respiration by ERRs (27,28,29,30,31). Consistent with a regulatory role in these processes, ERRα and ERRγ are highly expressed in tissues dependent on fatty acid oxidation for energy, such as heart, brown fat, and slow-twitch skeletal muscle (12,26). Moreover, ERRα expression is induced upon exposure to energy stresses such as cold, fasting, or exercise (23,32,33). ERRβ has more limited expression in adult tissues, and its function to date is less well characterized (12,34,35).
At least some of the metabolic activities attributed to ERRα and ERRγ involve interaction with the PGC-1 coactivators. Like ERRs, PGC-1α is expressed primarily in tissues with high-energy demands and plays an important role in energy metabolism via regulation of fatty acid oxidation, mitochondrial biogenesis, gluconeogenesis, and thermogenesis (36,37). Both ERRα and ERRγ have been shown to recruit PGC-1α to target gene promoters, such as pyruvate dehydrogenase kinase 4 (38). In addition, a functional ERRα-binding site is required for regulation of some PGC-1-regulated genes such as the mitochondrial membrane protein mitofusion 2 (39). Induction of a number of other PGC-1α-induced genes involved in mitochondrial biogenesis is reduced in cells in which ERRα is knocked down, indicating that PGC-1α regulation of these targets requires ERRα (30).
The PGC-1 and ERR pathways do not completely overlap, however. In brown adipose tissue, ERRα is not required for PGC-1-mediated regulation of uncoupling protein 1 or the acute response to cold despite its involvement in mitochondrial biogenesis and lipid oxidation (31). Moreover, ERRα has been shown to reduce the expression of the gluconeogenic regulator, phosphoenolpyruvate carboxykinase (PEPCK) whereas PGC-1 strongly induces PEPCK expression and gluconeogenesis (40,41,42). Indeed, ERRs have been linked to cofactors other than PGC-1α that exert distinct effects on lipid metabolism. Specifically, both ERRα and ERRγ have been shown to interact with receptor-interacting protein 140 (RIP140), a corepressor that is important for adipocyte function and triglyceride storage in adipocytes (43). ERRα also induces the expression of RIP140 during adipogenesis (44). Cofactor preference by ERRs may reflect tissue- or situation-dependent differences in cofactor availability and/or selective recruitment of distinct cofactors to specific target gene response elements. In support of the latter, ERRγ has been shown to differentially recruit PGC-1 or RIP140 depending on the sequence element in the promoters of target genes (45).
In contrast to the growing amount of in vitro data linking ERRs with oxidative metabolism, initial observations in vivo showed that ERRα knockout mice did not have altered energy expenditure (46). In fact, mice lacking ERRα were slightly less fat and resistant to diet-induced obesity. Microarray analysis of white adipose tissue from these mice showed changes in genes involved in lipid synthesis and, accordingly, triglyceride levels were reduced in knockout mice. Conversely, examination of other tissues in ERRα knockout mice confirmed a role for ERRα in energy expenditure. When challenged with cold, ERRα knockout mice were unable to maintain body temperature as well as wild-type controls (31). This was not due to a defect in the acute response to cold, because β-adrenergic signaling and induction of energy uncoupling targets were unaffected. Rather, knockout mice exhibited diminished energy production due to decreased number of mitochondria and defects in fatty acid oxidation and the tricarboxylic acid cycle. Reduced expression of energy metabolism genes has also been reported in intestine, liver, and muscle of ERRα−/− mice (41,47). Interactions with different cofactors, such as the prolipogenic RIP140 vs. the fat catabolizing PGC-1, in different tissues or at different target gene promoters, may contribute to tissue-specific ERR effects (Fig. 1). Indeed ERRs have been linked to cofactors other than PGC-1α that exert distinct effects on lipid metabolism. Specifically, both ERRα and ERRγ have been shown to interact with RIP140, a corepressor that is important for adipocyte function and triglyceride storage in adipocytes . ERRα also induces the expression of RIP140 during adipogenesis. Cofactor preference by ERRs may reflect tissue- or situation-dependent differences in cofactor availability and/or selective recruitment of distinct cofactors to specific target gene response elements. In support of the latter, ERRγ has been shown to differentially recruit PGC-1 or RIP140 depending on the sequence element in the promoters of target genes . Conversely, interactions with different cofactors, such as the prolipogenic RIP140 vs. the fat-catabolizing PGC-1, in different tissues or at different target gene promoters may contribute to tissue-specific ERR effects (Fig. 1). Moreover, the lack of an effect on total energy expenditure in ERRα knockout mice is likely due to tissue-specific actions of ERRs, such as seen in adipose tissue, as well as compensatory up-regulation of ERRγ and PGC-1 observed in some tissues of these mice (31).
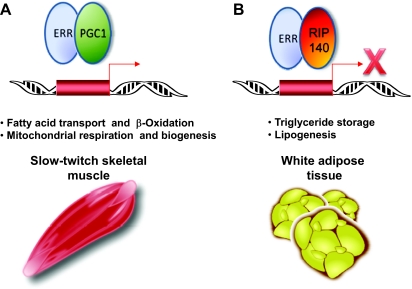
Figure 1.
Differential Cofactor Recruitment by ERRs %Members of the estrogen receptor-related family of nuclear receptors regulate metabolism in a tissue-specific manner, likely due to specific cofactor interactions. A, ERRα and ERRγ recruit PGC-1α to target gene promoters in slow-twitch skeletal muscle to induce genes involved in fatty acid oxidation and mitochondrial function. B, ERRα and ERRγ interact with RIP140, a corepressor that reduces expression of factors with negative affects on triglyceride storage. This interaction potentially explains the reduced body fat and decreased lipogenesis seen in ERRα −/− mice.
Ongoing studies of ERRs are revealing an important role for these orphan receptors in metabolism. ERRα and ERRγ are now known to serve as regulators of β-oxidation and mitochondrial biogenesis in energy-dependent tissues such as slow-twitch skeletal muscle and brown fat. Tissue- and cofactor-specific functions are also observed for the ERRs as seen in white adipose tissue. Future work should continue to define the role of ERRs in specific tissues and how this affects whole-body metabolism and metabolic disease.
THE NR4A FAMILY
The NR4A family of nuclear receptors consists of three highly conserved members, Nur77 or NGF-IB (NR4A1), Nurr 1 (NR4A2), and NOR-1 (NR4A3). These receptors bind as monomers to NGF-IB response elements in target gene promoters (48,49). Nur77 and Nurr1 have also been reported to form heterodimers with retinoid X receptor and thereby modulate retinoid signaling (50,51). The NR4As themselves are ligand-independent nuclear receptors as evidenced by crystallography studies showing bulky hydrophobic groups in the ligand-binding pocket and a constitutively active conformation (52,53). Activity of this family of nuclear receptors is controlled primarily by modulation of receptor levels and/or posttranslational modification. Consistent with expression levels influencing receptor activity, the NR4As were first characterized as early response genes that are rapidly and transiently induced in response to changes in the extracellular environment. NR4A protein levels are now known to be regulated by a variety of stimuli, including fatty acids, growth factors, cytokines, peptide hormones, neurotransmitters, and physical stress. In response to these signals, the NR4As modulate a wide range of important biological functions, such as cell cycle progression, apoptosis, neurological diseases, carcinogenesis, and inflammation (reviewed in Ref. 54).
Regulation of energy metabolism in response to sympathetic nervous system signaling has recently been added to the list of NR4A-mediated activities (Fig. 2). In the liver, all three NR4As are induced by cAMP in vitro, and glucagon and fasting in vivo, suggestive of a role as downstream effectors of sympathetic and cAMP signaling (55). Consistent with the established role for cAMP in hepatic glucose production, adenoviral mediated expression of Nur77 in liver was shown to induce the expression of a number of genes involved in glucose transport and gluconeogenesis, including fructose bis-phosphatase 1 and glucose transporter 2. Conversely, introduction of a dominant-negative Nur77 that antagonizes the function of all three NR4As inhibits hepatic glucose metabolism (55). NR4A-mediated regulation of glucose metabolism in the liver is important in the context of metabolic disease, as evidenced by increased levels of all three family members in both streptozotoxin-induced diabetic and db/db mice. Introduction of a dominant-negative Nur77 into these mice by adenoviral delivery reduces blood glucose levels, suggesting that NR4A activity may contribute to the hyperglycemia associated with diabetes (55).
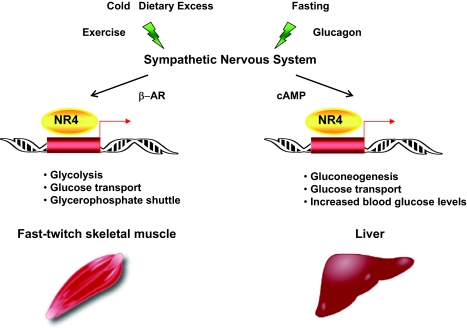
Figure 2.
Extracellular Signals Regulate NR4 Receptor Levels and Activity %Expression of members of the NR4 family of nuclear receptors is induced by a variety of stimuli that activate sympathetic signaling to regulate glucose metabolism in liver and fast-twitch skeletal muscle. β-AR, β-Andrenergic receptor.
Skeletal muscle is also emerging as an important site of NR4A-mediated regulation of glucose metabolism (Fig. 2). Recently, Nur77 was shown to directly regulate expression of glucose transporter 4, and ectopic expression of Nur77 increased glucose uptake in muscle cell lines (56,57). Expression of Nurr77 in vitro and in vivo was also shown to drive expression of a number of other genes involved in various aspects of glucose metabolism, including glucose transport, glycolysis, and the glycerophosphate shuttle. Conversely, inhibition of Nur77 activity using small interfering RNA (siRNA)-mediated knockdown and genetic deletion approaches results in reduced expression of a battery of genes involved in skeletal muscle glucose utilization (56).
Consistent with a role in glucose metabolism, NR4As are predominantly expressed in glycolytic fast-twitch muscle rather than slow-twitch skeletal muscle, which is dependent on fatty acid oxidation for energy (56). Fast-twitch muscle takes up and utilizes glucose to meet energy demands under stress conditions in response to signals from the sympathetic nervous system, suggesting that NR4A regulation of glucose metabolism in muscle may also be under sympathetic control. In support of this idea, denervation of rodent muscle was found to result in decreased expression of Nur77 as well as a number of genes involved in glucose metabolism (56). Although these data strongly suggest that Nur77 mediates glucose metabolism in response to neuromuscular signaling, they do not distinguish whether the effect is mediated by motor neurons, sympathetic neurons, or both. Further evidence of sympathetic involvement comes from several studies showing induction of Nur77 mRNA and protein levels in skeletal muscle by β-adrenogenic agonists (56,57). Dietary excess and cold exposure activate sympathetic nervous system induction of β-adrenogenic signaling to regulate energy expenditure and adaptive thermogenesis. Thus, increasing evidence points to Nur77 as an important regulator of glucose metabolism downstream of sympathetic nervous system signaling. It is interesting to note the complementary expression and functions of NR4A and ERR nuclear receptors in muscle metabolism. ERRs are most highly expressed in oxidative fibers and control oxidative metabolism, whereas NR4As are highly expressed in glycolytic fibers and promote glucose utilization.
In addition to modulation of glucose homeostasis, NR4As have been implicated in lipid metabolism, energy expenditure, thermogenesis, and differentiation in several tissues. siRNA-mediated knockdown of Nur77 has been reported to alter expression of genes associated with fatty acid mobilization and utilization in a skeletal muscle cell line (57). Consistent with the gene expression data, muscle cells expressing siRNA to Nur77 exhibited reduced lipolysis compared with wild-type cells. Some of the genes altered by the Nur77 siRNA in this study were not observed to be altered in cells overexpressing Nur77 or in muscles of Nur77 null mice, indicating the need for more research into the role of this receptor in lipid metabolism (56). Recently, the Nur77 family member, NOR-1, has also been suggested to play a role in control of fatty acid utilization and muscle mass in vitro (58).
Nur77 has also been shown to be responsive to β-adrenergic signaling and cold exposure in brown adipose tissue, suggesting Nur77 may be involved in the sympathetic control of adaptive thermogenesis. siRNA-mediated ablation of Nur77 in brown fat identified energy uncoupling protein 1 as a Nur77 target, supporting a role in adaptive heat production and energy expenditure (59). Regulation of uncoupling proteins by Nur77 and NOR1 has also been reported in skeletal muscle, consistent with a possible role for NR4As in energy expenditure and adaptive thermogenesis in other tissues (57,58). However, studies using knockout mouse models have cast doubt on whether UCP2 and UCP3 are truly involved in energy uncoupling (60,61,62).
In addition to brown fat, the NR4A family members are also expressed in white adipose tissue. Moreover, expression of all three is induced early in the differentiation of 3T3-L1 cells, indicating a possible role in white adipose tissue formation or function (51). Early studies have reported that siRNA ablation of Nur77 expression was suggested to inhibit adipogenesis, whereas transient expression of Nur77 induced the differentiation process (63). The role of Nur77 in white adipose tissue differentiation and function in vivo, however, has not been addressed. Whether Nurr1 and Nor-1 also play a role in lipid or glucose metabolism in white adipose tissue remains to be determined.
REV-ERB AND ROR
The retinoic acid receptor-related orphan receptors (RORs) and Rev-erbs are two closely related families of nuclear receptors. The ROR family consists of three members, RORα, RORβ, and RORγ, and the Rev-erb family contains two members, Rev-erbα and Rev-erbβ. Alternatively spliced variants of RORα, RORγ, and Rev-erbβ have also been described (64,65,66). The RORs and Rev-erbs both bind as monomers to asymmetric (A/T) 6 RGGTCA motifs in target gene promoters (65,67,68,69), or as heterodimers to two tandem core motifs separated by two nucleotides (70,71,72). No consensus ligand has been identified for RORs, although some evidence exists for cholesterol sulfates as agonists for RORα and retinoids as partial agonists for RORβ (73,74). Crystallography studies support the idea that RORs are ligand-activated transcription factors that shift between an active and a repressive conformation upon ligand binding. Rev-erbs, on the other hand, were initially believed to be constitutive transcriptional repressors, because they lack an activation function 2 transactivation domain and contain bulky amino acid side changes in the ligand-binding pocket (75). However, the Drosophila homolog of Rev-erb, E75, has been reported to bind a heme prosthetic group that affects receptor heterodimerization (76). Recently, both Rev-erbα and Rev-erbβ were also shown to reversibly bind heme, an iron-containing porphyrin, and to require heme for recruitment of corepressors to target gene promoters (77,78).
Like other members of the nuclear receptor superfamily, the RORs and Rev-erbs have been shown to play a role in metabolic regulation. Both share similar expression patterns with high expression in metabolically important tissues including liver, muscle, and fat (64,65,68,79,80,81). They compete for binding to the same sequence in target gene promoters and generally appear to have opposing effects on target gene regulation and associated metabolic processes. Examination of mice harboring genetic deletions of ROR or Rev-erb family members confirms opposite roles for these receptors in lipoprotein metabolism. Staggerer mice, which harbor a natural mutation in the RORα gene, exhibit dyslipidemia including decreased levels of apolipoprotein (Apo)AI, ApoCIII, high-density lipoprotein, and plasma triglycerides and have increased susceptibility to atherosclerosis (82). Conversely Rev-Erb knockout mice have increased ApoCIII, very low density lipoprotein, and plasma triglyceride levels (83). Subsequent cell culture studies showed that ROR induces, whereas Rev-erb represses, expression of ApoAI and ApoCIII, confirming a role for both in lipoprotein metabolism (82,83,84).
Members of both the Rev-erb and ROR families are highly expressed in skeletal muscle and also show opposing affects on myocyte differentiation and lipid metabolism. Specifically, RORs have been reported to enhance muscle cell differentiation, whereas some studies suggest Rev-erbs inhibit muscle differentiation (85,86,87). The high expression of Rev-erbs in muscle, particularly fast-twitch muscle, is not consistent with a negative role in the differentiation process however. Rev-erbs do appear to influence muscle fiber type, because Rev-erb-deficient mice have increased levels of type I slow-twitch muscle, suggesting a role in muscle type switching (88). Both RORs and Rev-erbs have also been implicated in lipid metabolism in the muscle. Introduction of a dominant-negative ROR into muscle cells results in reduced expression of a number of genes involved in lipid metabolism, including the lipogenic gene SREBP1-c and steroyl-coenzyme A desaturase-1 (89). By contrast, dominant-negative expression of Rev-erbs in muscle leads to increased expression of SREBP-1c and steroyl-coenzyme A desaturase-1 as well as fatty acid transport genes such as CD36 and aP2 (90).
In addition to muscle, Rev-erbs have also been shown to have important metabolic functions in liver and adipose tissue. Depletion of Rev-erbα by siRNA in HepG2 hepatoma cell lines was recently shown to induce the expression of the gluconeogenic genes PEPCK and glucose 6-phospatase. Conversely overexpression of Rev-erbα resulted in reduced levels of PEPCK and glucose 6-phospatase (78). Expression of genes involved in fatty acid metabolism was not affected, suggesting that Rev-erbα specifically regulates glucose metabolism in the liver. In adipose tissue, Rev-erbα expression increases during the differentiation process and upon PPARγ ligand activation, suggesting an important role for Rev-erbs in this tissue (91,92). Indeed, ectopic expression of Rev-erbα has been reported to induce adipocyte differentiation in 3T3-L1 preadipocyte cells, possibly through repression of antiadipogenic factors (93). RORα is also highly expressed in adipose tissue and induced during adipogenesis; however, the role of this receptor in fat has not been determined (94).
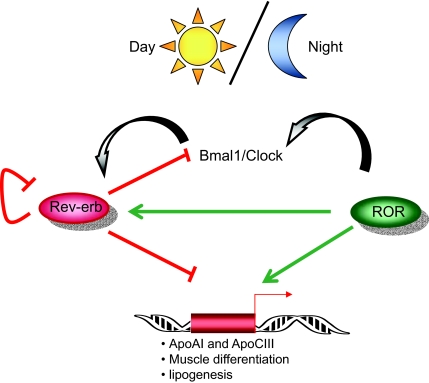
Along with roles in lipid metabolism, Rev-erbs and RORs have also regulated expression of clock genes in a reciprocal manner, and Rev-erbα knockout mice have defects in circadian rhythmicity (95,96,97). Circadian rhythms are generated by autoregulatory translational and transcriptional feedback loops of clock genes. Rev-erbα represses the expression of two key clock transcription factors, maBmal1 and, to a lesser extent, Clock (96,98,99). In turn, Bmal1-Clock heterodimers induce Rev-erbα expression, thereby inhibiting their own transcription (96). Conversely, RORα competes with Rev-erbα for binding to an ROR response element in the promoter of maBmal1and induces its expression (97,98,100). Like Rev-erbα, RORα is itself regulated by circadian rhythms. Interestingly RORα induces expression of Rev-erbα, whereas Rev-erbα represses its own expression (70,101). Moreover, expression of heme, a ligand for Rev-erbs, is also affected by circadian cycles, and heme serves as a cofactor for the clock genes Npas2 and Per2, illustrating the complexity of circadian circuitry (102,103). Recently, the other Rev-erb and ROR family members have also been shown to regulate expression of Bmal1 (98).
A number of physiological processes, including metabolism, are under the control of circadian clocks. It is becoming increasingly evident that perturbations of day/night cycles have implications for proper metabolic function and metabolic disease. Animal models with defects in clock genes develop obesity and features of metabolic disease (104,105). Moreover, shift workers exhibit an increased incidence of metabolic syndrome, and circadian rhythms are known to be altered in type 2 diabetic patients (106,107,108). The identification of RORs and Rev-erbs as regulators of clock genes provides a direct link between regulation of metabolism and circadian rhythms (Fig. 3).
Figure 3.
Rev-erbs and RORs Link Circadean Rhythms and Metabolisms %The Ror and Rev-erb families of nuclear receptors regulate clock genes and metabolic targets in a reciprocal manner.
CONCLUSIONS
Cellular and systemic metabolism is tightly controlled to ensure the appropriate response to metabolic stresses and the specific demands of each tissue. Nuclear receptors have emerged as crucial players in orchestration of the correct metabolic outcomes in response to a range of environmental and extracellular signals. Initial characterization of the orphan nuclear receptors focused on ligand-activated transcription factors such as LXR and PPAR, which respond to nutrient-derived and circulating lipids to modulate a range of metabolically important pathways. As described in this review, characterization of orphan nuclear receptors is revealing unexpected roles for these proteins in metabolic control.
Different nuclear receptor families have distinct tissue expression profiles, and many exhibit differential expression of individual isoforms. The result is a unique nuclear receptor composition for every tissue. Moreover, within each tissue the activity of nuclear receptors is regulated by different mechanisms. Some respond to nutrient-derived ligands, whereas the activity of others depends on changes in receptor expression levels in response to various physiological stimuli, such as sympathetic induction of the NR4 receptors (Fig. 2) (55,57). In many cases, a number of different ligands and/or signaling events work in combination to control receptor expression and activity. This allows for a fine-tuned metabolic response in which tissues respond in an appropriate manner to distinct stimuli, based on the specific nuclear receptors expressed in that tissue. Cofactor composition can further influence cell-specific metabolism, as seen in the differential lipogenic vs. lipid catabolizing effect of ERRs in the presence of RIP140 and PGC-1, respectively (Fig. 1) (30,38,39,43,44,45). Future work should continue to shed light on how nuclear receptors work in concert to ensure proper regulation of a broad range of metabolic processes.
Nuclear receptors have also been implicated in a range of other important biological processes, suggesting cross talk between metabolic and other regulatory pathways. One example is the regulation of lipid metabolism and circadian rhythms by the ROR and Rev-erb receptor families (Fig. 3). In addition, a number of nuclear receptors, including LXRs, PPARs, NR4s, RORs, and Rev-erbs, have been implicated in modulation of inflammatory responses (109,110). It is becoming increasingly apparent that inflammatory and metabolic pathways are closely linked. Many pathologies associated with metabolic disease, including atherosclerosis, diabetes, and obesity, are believed to have an inflammatory component. Exactly how nuclear receptors modulate cross talk between biological pathways in the context of both normal physiology and disease is a promising area for future research.
Footnotes
Disclosure Statement: P.T. consults for Wyeth, Daiichi Sankyo, and Takeda. S.H. has nothing to disclose.
First Published Online February 7, 2008
Abbreviations: Apo, Apolipoprotein; ER, estrogen receptor; ERR, estrogen-related receptor; LXR, liver X receptor; PEPCK, phosphoenolpyruvate carboxykinase; PGC-1, PPARγ coactivator 1; PPAR, peroxisome proliferator-activated receptor; RIP140, receptor-interacting protein 140; ROR, retinoic acid receptor-related orphan receptor; siRNA, small interfering RNA.
References
- Flegal KM, Carroll MD, Ogden CL, Johnson CL 2002 Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288:1723–1727 [DOI] [PubMed] [Google Scholar]
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM 2004 Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 291:2847–2850 [DOI] [PubMed] [Google Scholar]
- Ford ES, Mokdad AH, Giles WH, Galuska DA, Serdula MK 2005 Geographic variation in the prevalence of obesity, diabetes, and obesity-related behaviors. Obes Res 13:118–122 [DOI] [PubMed] [Google Scholar]
- Chambon P 2005 The nuclear receptor superfamily: a personal retrospect on the first two decades. Mol Endocrinol 19:1418–1428 [DOI] [PubMed] [Google Scholar]
- Evans RM 2005 The nuclear receptor superfamily: a rosetta stone for physiology. Mol Endocrinol 19:1429–1438 [DOI] [PubMed] [Google Scholar]
- Beaven SW, Tontonoz P 2006 Nuclear receptors in lipid metabolism: targeting the heart of dyslipidemia. Annu Rev Med 57:313–329 [DOI] [PubMed] [Google Scholar]
- Lee CH, Olson P, Evans RM 2003 Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology 144:2201–2207 [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Mangelsdorf DJ 2003 Liver X receptor signaling pathways in cardiovascular disease. Mol Endocrinol 17:985–993 [DOI] [PubMed] [Google Scholar]
- Zelcer N, Tontonoz P 2006 Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest 116:607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y 2006 FXR, a multipurpose nuclear receptor. Trends Biochem Sci 31:572–580 [DOI] [PubMed] [Google Scholar]
- Timsit YE, Negishi M 2007 CAR and PXR: the xenobiotic-sensing receptors. Steroids 72:231–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V, Yang N, Segui P, Evans RM 1988 Identification of a new class of steroid hormone receptors. Nature 331:91–94 [DOI] [PubMed] [Google Scholar]
- Hong H, Yang L, Stallcup MR 1999 Hormone-independent transcriptional activation and coactivator binding by novel orphan nuclear receptor ERR3. J Biol Chem 274:22618–22626 [DOI] [PubMed] [Google Scholar]
- Giguere V 2002 To ERR in the estrogen pathway. Trends Endocrinol Metab 13:220–225 [DOI] [PubMed] [Google Scholar]
- Barry JB, Laganiere J, Giguere V 2006 A single nucleotide in an estrogen-related receptor α site can dictate mode of binding and peroxisome proliferator-activated receptor γ coactivator 1α activation of target promoters. Mol Endocrinol 20:302–310 [DOI] [PubMed] [Google Scholar]
- Horard B, Castet A, Bardet PL, Laudet V, Cavailles V, Vanacker JM 2004 Dimerization is required for transactivation by estrogen-receptor-related (ERR) orphan receptors: evidence from amphioxus ERR. J Mol Endocrinol 33:493–509 [DOI] [PubMed] [Google Scholar]
- Huppunen J, Aarnisalo P 2004 Dimerization modulates the activity of the orphan nuclear receptor ERRγ. Biochem Biophys Res Commun 314:964–970 [DOI] [PubMed] [Google Scholar]
- Johnston SD, Liu X, Zuo F, Eisenbraun TL, Wiley SR, Kraus RJ, Mertz JE 1997 Estrogen-related receptor α 1 functionally binds as a monomer to extended half-site sequences including ones contained within estrogen-response elements. Mol Endocrinol 11:342–352 [DOI] [PubMed] [Google Scholar]
- Horard B, Vanacker JM 2003 Estrogen receptor-related receptors: orphan receptors desperately seeking a ligand. J Mol Endocrinol 31:349–357 [DOI] [PubMed] [Google Scholar]
- Greschik H, Wurtz JM, Sanglier S, Bourguet W, van Dorsselaer A, Moras D, Renaud JP 2002 Structural and functional evidence for ligand-independent transcriptional activation by the estrogen-related receptor 3. Mol Cell 9:303–313 [DOI] [PubMed] [Google Scholar]
- Kallen J, Schlaeppi JM, Bitsch F, Filipuzzi I, Schilb A, Riou V, Graham A, Strauss A, Geiser M, Fournier B 2004 Evidence for ligand-independent transcriptional activation of the human estrogen-related receptor α (ERRα): crystal structure of ERRα ligand binding domain in complex with peroxisome proliferator-activated receptor coactivator-1α. J Biol Chem 279:49330–49337 [DOI] [PubMed] [Google Scholar]
- Kamei Y, Ohizumi H, Fujitani Y, Nemoto T, Tanaka T, Takahashi N, Kawada T, Miyoshi M, Ezaki O, Kakizuka A 2003 PPARγ coactivator 1β/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity. Proc Natl Acad Sci USA 100:12378–12383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A 2003 The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor α (ERRα). J Biol Chem 278:9013–9018 [DOI] [PubMed] [Google Scholar]
- Stein RA, McDonnell DP 2006 Estrogen-related receptor α as a therapeutic target in cancer. Endocr Relat Cancer 13(Suppl 1):S25–S32 [DOI] [PubMed] [Google Scholar]
- Sladek R, Bader JA, Giguere V 1997 The orphan nuclear receptor estrogen-related receptor α is a transcriptional regulator of the human medium-chain acyl coenzyme A dehydrogenase gene. Mol Cell Biol 17:5400–5409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega RB, Kelly DP 1997 A role for estrogen-related receptor α in the control of mitochondrial fatty acid β-oxidation during brown adipocyte differentiation. J Biol Chem 272:31693–31699 [DOI] [PubMed] [Google Scholar]
- Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguere V, Evans RM 2007 ERRγ directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab 6:13–24 [DOI] [PubMed] [Google Scholar]
- Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, Evans RM, Blanchette M, Giguere V 2007 Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRα and γ. Cell Metab 5:345–356 [DOI] [PubMed] [Google Scholar]
- Huss JM, Torra IP, Staels B, Giguere V, Kelly DP 2004 Estrogen-related receptor α directs peroxisome proliferator-activated receptor α signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol 24:9079–9091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber SN, Emter R, Hock MB, Knutti D, Cardenas J, Podvinec M, Oakeley EJ, Kralli A 2004 The estrogen-related receptor α (ERRα) functions in PPARγ coactivator 1α (PGC-1α)-induced mitochondrial biogenesis. Proc Natl Acad Sci USA 101:6472–6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena JA, Hock MB, Chang WY, Barcas JE, Giguere V, Kralli A 2007 Orphan nuclear receptor estrogen-related receptor α is essential for adaptive thermogenesis. Proc Natl Acad Sci USA 104:1418–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartoni R, Leger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener JL, Luthi F, Deriaz O, Zorzano A, Gobelet C, Kralli A, Russell AP 2005 Mitofusins 1/2 and ERRα expression are increased in human skeletal muscle after physical exercise. J Physiol 567:349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida M, Nemoto S, Finkel T 2002 Identification of a specific molecular repressor of the peroxisome proliferator-activated receptor γ coactivator-1 α (PGC-1α). J Biol Chem 277:50991–50995 [DOI] [PubMed] [Google Scholar]
- Luo J, Sladek R, Bader JA, Matthyssen A, Rossant J, Giguere V 1997 Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-β. Nature 388:778–782 [DOI] [PubMed] [Google Scholar]
- Pettersson K, Svensson K, Mattsson R, Carlsson B, Ohlsson R, Berkenstam A 1996 Expression of a novel member of estrogen response element-binding nuclear receptors is restricted to the early stages of chorion formation during mouse embryogenesis. Mech Dev 54:211–223 [DOI] [PubMed] [Google Scholar]
- Finck BN, Kelly DP 2006 PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest 116:615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM 2005 Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1:361–370 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ma K, Sadana P, Chowdhury F, Gaillard S, Wang F, McDonnell DP, Unterman TG, Elam MB, Park EA 2006 Estrogen-related receptors stimulate pyruvate dehydrogenase kinase isoform 4 gene expression. J Biol Chem 281:39897–39906 [DOI] [PubMed] [Google Scholar]
- Soriano FX, Liesa M, Bach D, Chan DC, Palacin M, Zorzano A 2006 Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-γ coactivator-1 α, estrogen-related receptor-α, and mitofusin 2. Diabetes 55:1783–1791 [DOI] [PubMed] [Google Scholar]
- Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M 2001 CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413:179–183 [DOI] [PubMed] [Google Scholar]
- Herzog B, Cardenas J, Hall RK, Villena JA, Budge PJ, Giguere V, Granner DK, Kralli A 2006 Estrogen-related receptor α is a repressor of phosphoenolpyruvate carboxykinase gene transcription. J Biol Chem 281:99–106 [DOI] [PubMed] [Google Scholar]
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM 2001 Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413:131–138 [DOI] [PubMed] [Google Scholar]
- Castet A, Herledan A, Bonnet S, Jalaguier S, Vanacker JM, Cavailles V 2006 Receptor-interacting protein 140 differentially regulates estrogen receptor-related receptor transactivation depending on target genes. Mol Endocrinol 20:1035–1047 [DOI] [PubMed] [Google Scholar]
- Nichol D, Christian M, Steel JH, White R, Parker MG 2006 RIP140 expression is stimulated by estrogen-related receptor α during adipogenesis. J Biol Chem 281:32140–32147 [DOI] [PubMed] [Google Scholar]
- Sanyal S, Matthews J, Bouton D, Kim HJ, Choi HS, Treuter E, Gustafsson JA 2004 Deoxyribonucleic acid response element-dependent regulation of transcription by orphan nuclear receptor estrogen receptor-related receptor γ. Mol Endocrinol 18:312–325 [DOI] [PubMed] [Google Scholar]
- Luo J, Sladek R, Carrier J, Bader JA, Richard D, Giguere V 2003 Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor α. Mol Cell Biol 23:7947–7956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier JC, Deblois G, Champigny C, Levy E, Giguere V 2004 Estrogen-related receptor α (ERRα) is a transcriptional regulator of apolipoprotein A-IV and controls lipid handling in the intestine. J Biol Chem 279:52052–52058 [DOI] [PubMed] [Google Scholar]
- Philips A, Lesage S, Gingras R, Maira MH, Gauthier Y, Hugo P, Drouin J 1997 Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells. Mol Cell Biol 17:5946–59451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Fahrner TJ, Johnston M, Milbrandt J 1991 Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science 252:1296–1300 [DOI] [PubMed] [Google Scholar]
- Perlmann T, Jansson L 1995 A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev 9:769–782 [DOI] [PubMed] [Google Scholar]
- Zetterstrom RH, Solomin L, Mitsiadis T, Olson L, Perlmann T 1996 Retinoid X receptor heterodimerization and developmental expression distinguish the orphan nuclear receptors NGFI-B, Nurr1, and Nor1. Mol Endocrinol 10:1656–1666 [DOI] [PubMed] [Google Scholar]
- Baker KD, Shewchuk LM, Kozlova T, Makishima M, Hassell A, Wisely B, Caravella JA, Lambert MH, Reinking JL, Krause H, Thummel CS, Willson TM, Mangelsdorf DJ 2003 The Drosophila orphan nuclear receptor DHR38 mediates an atypical ecdysteroid signaling pathway. Cell 113:731–742 [DOI] [PubMed] [Google Scholar]
- Wang Z, Benoit G, Liu J, Prasad S, Aarnisalo P, Liu X, Xu H, Walker NP, Perlmann T 2003 Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature 423:555–560 [DOI] [PubMed] [Google Scholar]
- Maxwell MA, Muscat GE 2006 The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal 4:e002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei L, Waki H, Vaitheesvaran B, Wilpitz DC, Kurland IJ, Tontonoz P 2006 NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat Med 12:1048–1055 [DOI] [PubMed] [Google Scholar]
- Chao LC, Zhang Z, Pei L, Saito T, Tontonoz P, Pilch PF 2007 Nur77 coordinately regulates expression of genes linked to glucose metabolism in skeletal muscle. Mol Endocrinol 21:2152–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell MA, Cleasby ME, Harding A, Stark A, Cooney GJ, Muscat GE 2005 Nur77 regulates lipolysis in skeletal muscle cells. Evidence for cross-talk between the β-adrenergic and an orphan nuclear hormone receptor pathway. J Biol Chem 280:12573–12584 [DOI] [PubMed] [Google Scholar]
- Pearen MA, Ryall JG, Maxwell MA, Ohkura N, Lynch GS, Muscat GE 2006 The orphan nuclear receptor, NOR-1, is a target of β-adrenergic signaling in skeletal muscle. Endocrinology 147:5217–5227 [DOI] [PubMed] [Google Scholar]
- Kanzleiter T, Schneider T, Walter I, Bolze F, Eickhorst C, Heldmaier G, Klaus S, Klingenspor M 2005 Evidence for Nr4a1 as a cold-induced effector of brown fat thermogenesis. Physiol Genomics 24:37–44 [DOI] [PubMed] [Google Scholar]
- Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D 2000 Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet 26:435–439 [DOI] [PubMed] [Google Scholar]
- Gong DW, Monemdjou S, Gavrilova O, Leon LR, Marcus-Samuels B, Chou CJ, Everett C, Kozak LP, Li C, Deng C, Harper ME, Reitman ML 2000 Lack of obesity and normal response to fasting and thyroid hormone in mice lacking uncoupling protein-3. J Biol Chem 275:16251–16257 [DOI] [PubMed] [Google Scholar]
- Vidal-Puig AJ, Grujic D, Zhang CY, Hagen T, Boss O, Ido Y, Szczepanik A, Wade J, Mootha V, Cortright R, Muoio DM, Lowell BB 2000 Energy metabolism in uncoupling protein 3 gene knockout mice. J Biol Chem 275:16258–16266 [DOI] [PubMed] [Google Scholar]
- Fumoto T, Yamaguchi T, Hirose F, Osumi T 2007 Orphan nuclear receptor Nur77 accelerates the initial phase of adipocyte differentiation in 3T3-L1 cells by promoting mitotic clonal expansion. J Biochem (Tokyo) 141:181–192 [DOI] [PubMed] [Google Scholar]
- Becker-Andre M, Andre E, DeLamarter JF 1993 Identification of nuclear receptor mRNAs by RT-PCR amplification of conserved zinc-finger motif sequences. Biochem Biophys Res Commun 194:1371–1379 [DOI] [PubMed] [Google Scholar]
- Giguere V, Tini M, Flock G, Ong E, Evans RM, Otulakowski G 1994 Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR α, a novel family of orphan hormone nuclear receptors. Genes Dev 8:538–553 [DOI] [PubMed] [Google Scholar]
- Triqueneaux G, Thenot S, Kakizawa T, Antoch MP, Safi R, Takahashi JS, Delaunay F, Laudet V 2004 The orphan receptor Rev-erbα gene is a target of the circadian clock pacemaker. J Mol Endocrinol 33:585–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg C, Hooft van Huijsduijnen R, Staple JK, DeLamarter JF, Becker-Andre M 1994 RZRs, a new family of retinoid-related orphan receptors that function as both monomers and homodimers. Mol Endocrinol 8:757–770 [DOI] [PubMed] [Google Scholar]
- Forman BM, Chen J, Blumberg B, Kliewer SA, Henshaw R, Ong ES, Evans RM 1994 Cross-talk among ROR α 1 and the Rev-erb family of orphan nuclear receptors. Mol Endocrinol 8:1253–1261 [DOI] [PubMed] [Google Scholar]
- Harding HP, Lazar MA 1993 The orphan receptor Rev-ErbA α activates transcription via a novel response element. Mol Cell Biol 13:3113–3121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelmant G, Begue A, Stehelin D, Laudet V 1996 A functional Rev-erb α responsive element located in the human Rev-erb α promoter mediates a repressing activity. Proc Natl Acad Sci USA 93:3553–3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Atkins GB, Jaffe AB, Seo WJ, Lazar MA 1997 Transcriptional activation and repression by RORα, an orphan nuclear receptor required for cerebellar development. Mol Endocrinol 11:1737–1746 [DOI] [PubMed] [Google Scholar]
- Harding HP, Lazar MA 1995 The monomer-binding orphan receptor Rev-Erb represses transcription as a dimer on a novel direct repeat. Mol Cell Biol 15:4791–4802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen J, Schlaeppi JM, Bitsch F, Delhon I, Fournier B 2004 Crystal structure of the human RORα ligand binding domain in complex with cholesterol sulfate at 2.2 A. J Biol Chem 279:14033–14038 [DOI] [PubMed] [Google Scholar]
- Stehlin C, Wurtz JM, Steinmetz A, Greiner E, Schule R, Moras D, Renaud JP 2001 X-ray structure of the orphan nuclear receptor RORβ ligand-binding domain in the active conformation. EMBO J 20:5822–5831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud JP, Harris JM, Downes M, Burke LJ, Muscat GE 2000 Structure-function analysis of the Rev-erbA and RVR ligand-binding domains reveals a large hydrophobic surface that mediates corepressor binding and a ligand cavity occupied by side chains. Mol Endocrinol 14:700–717 [DOI] [PubMed] [Google Scholar]
- Reinking J, Lam MM, Pardee K, Sampson HM, Liu S, Yang P, Williams S, White W, Lajoie G, Edwards A, Krause HM 2005 The Drosophila nuclear receptor e75 contains heme and is gas responsive. Cell 122:195–207 [DOI] [PubMed] [Google Scholar]
- Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F 2007 Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ. Nat Struct Mol Biol 14:1207–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA 2007 Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways. Science 318:1786–1789 [DOI] [PubMed] [Google Scholar]
- Dumas B, Harding HP, Choi HS, Lehmann KA, Chung M, Lazar MA, Moore DD 1994 A new orphan member of the nuclear hormone receptor superfamily closely related to Rev-Erb. Mol Endocrinol 8:996–1005 [DOI] [PubMed] [Google Scholar]
- Hirose T, Smith RJ, Jetten AM 1994 ROR γ: the third member of ROR/RZR orphan receptor subfamily that is highly expressed in skeletal muscle. Biochem Biophys Res Commun 205:1976–1983 [DOI] [PubMed] [Google Scholar]
- Lazar MA, Hodin RA, Darling DS, Chin WW 1989 A novel member of the thyroid/steroid hormone receptor family is encoded by the opposite strand of the rat c-erbA α transcriptional unit. Mol Cell Biol 9:1128–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raspe E, Duez H, Gervois P, Fievet C, Fruchart JC, Besnard S, Mariani J, Tedgui A, Staels B 2001 Transcriptional regulation of apolipoprotein C-III gene expression by the orphan nuclear receptor RORα. J Biol Chem 276:2865–2871 [DOI] [PubMed] [Google Scholar]
- Raspe E, Duez H, Mansen A, Fontaine C, Fievet C, Fruchart JC, Vennstrom B, Staels B 2002 Identification of Rev-erbα as a physiological repressor of apoC-III gene transcription. J Lipid Res 43:2172–2179 [DOI] [PubMed] [Google Scholar]
- Vu-Dac N, Gervois P, Grotzinger T, De Vos P, Schoonjans K, Fruchart JC, Auwerx J, Mariani J, Tedgui A, Staels B 1997 Transcriptional regulation of apolipoprotein A-I gene expression by the nuclear receptor RORα. J Biol Chem 272:22401–22404 [DOI] [PubMed] [Google Scholar]
- Downes M, Carozzi AJ, Muscat GE 1995 Constitutive expression of the orphan receptor, Rev-erbA α, inhibits muscle differentiation and abrogates the expression of the myoD gene family. Mol Endocrinol 9:1666–1678 [DOI] [PubMed] [Google Scholar]
- Lau P, Bailey P, Dowhan DH, Muscat GE 1999 Exogenous expression of a dominant negative RORα1 vector in muscle cells impairs differentiation: RORα1 directly interacts with p300 and myoD. Nucleic Acids Res 27:411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmayr M, Andre E, Conquet F, Rondi-Reig L, Delhaye-Bouchaud N, Auclair N, Daniel H, Crepel F, Mariani J, Sotelo C, Becker-Andre M 1998 Staggerer phenotype in retinoid-related orphan receptor α-deficient mice. Proc Natl Acad Sci USA 95:3960–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher P, Chomez P, Yu F, Vennstrom B, Larsson L 2005 Aberrant expression of myosin isoforms in skeletal muscles from mice lacking the rev-erbAα orphan receptor gene. Am J Physiol Regul Integr Comp Physiol 288:R482–R490 [DOI] [PubMed] [Google Scholar]
- Lau P, Nixon SJ, Parton RG, Muscat GE 2004 RORα regulates the expression of genes involved in lipid homeostasis in skeletal muscle cells: caveolin-3 and CPT-1 are direct targets of ROR. J Biol Chem 279:36828–36840 [DOI] [PubMed] [Google Scholar]
- Ramakrishnan SN, Lau P, Burke LJ, Muscat GE 2005 Rev-erbβ regulates the expression of genes involved in lipid absorption in skeletal muscle cells: evidence for cross-talk between orphan nuclear receptors and myokines. J Biol Chem 280:8651–8659 [DOI] [PubMed] [Google Scholar]
- Chawla A, Lazar MA 1993 Induction of Rev-ErbA α, an orphan receptor encoded on the opposite strand of the α-thyroid hormone receptor gene, during adipocyte differentiation. J Biol Chem 268:16265–16269 [PubMed] [Google Scholar]
- Fontaine C, Dubois G, Duguay Y, Helledie T, Vu-Dac N, Gervois P, Soncin F, Mandrup S, Fruchart JC, Fruchart-Najib J, Staels B 2003 The orphan nuclear receptor Rev-Erbα is a peroxisome proliferator-activated receptor (PPAR) γ target gene and promotes PPARγ-induced adipocyte differentiation. J Biol Chem 278:37672– 37680 [DOI] [PubMed] [Google Scholar]
- Laitinen S, Fontaine C, Fruchart JC, Staels B 2005 The role of the orphan nuclear receptor Rev-Erb α in adipocyte differentiation and function. Biochimie 87:21–25 [DOI] [PubMed] [Google Scholar]
- Austin S, Medvedev A, Yan ZH, Adachi H, Hirose T, Jetten AM 1998 Induction of the nuclear orphan receptor RORγ during adipocyte differentiation of D1 and 3T3-L1 cells. Cell Growth Differ 9:267–276 [PubMed] [Google Scholar]
- Akashi M, Takumi T 2005 The orphan nuclear receptor RORα regulates circadian transcription of the mammalian core-clock Bmal1. Nat Struct Mol Biol 12:441–448 [DOI] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U 2002 The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110:251–260 [DOI] [PubMed] [Google Scholar]
- Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB 2004 A functional genomics strategy reveals RORa as a component of the mammalian circadian clock. Neuron 43:527–537 [DOI] [PubMed] [Google Scholar]
- Guillaumond F, Dardente H, Giguere V, Cermakian N 2005 Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms 20:391–403 [DOI] [PubMed] [Google Scholar]
- Ripperger JA 2006 Mapping of binding regions for the circadian regulators BMAL1 and CLOCK within the mouse Rev-erbα gene. Chronobiol Int 23:135–142 [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Ikeda M, Kimura T, Honma S, Ohmiya Y, Honma K 2004 Bidirectional role of orphan nuclear receptor RORα in clock gene transcriptions demonstrated by a novel reporter assay system. FEBS Lett 565:122–126 [DOI] [PubMed] [Google Scholar]
- Raspe E, Mautino G, Duval C, Fontaine C, Duez H, Barbier O, Monte D, Fruchart J, Fruchart JC, Staels B 2002 Transcriptional regulation of human Rev-erbα gene expression by the orphan nuclear receptor retinoic acid-related orphan receptor α. J Biol Chem 277:49275–49281 [DOI] [PubMed] [Google Scholar]
- Dioum EM, Rutter J, Tuckerman JR, Gonzalez G, Gilles-Gonzalez MA, McKnight SL 2002 NPAS2: a gas-responsive transcription factor. Science 298:2385–2387 [DOI] [PubMed] [Google Scholar]
- Kaasik K, Lee CC 2004 Reciprocal regulation of haem biosynthesis and the circadian clock in mammals. Nature 430:467–471 [DOI] [PubMed] [Google Scholar]
- Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA 2004 BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2:e377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J 2005 Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308:1043–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, Chen X, Polansky M 1999 Disruption of circadian insulin secretion is associated with reduced glucose uptake in first-degree relatives of patients with type 2 diabetes. Diabetes 48:2182–2188 [DOI] [PubMed] [Google Scholar]
- Chaput JP, Brunet M, Tremblay A 2006 Relationship between short sleeping hours and childhood overweight/obesity: results from the ‘Quebec en Forme’ Project. Int J Obes Relat Metab Disord 30:1080–1085 [DOI] [PubMed] [Google Scholar]
- Karlsson B, Knutsson A, Lindahl B 2001 Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med 58:747–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual G, Glass CK 2006 Nuclear receptors versus inflammation: mechanisms of transrepression. Trends Endocrinol Metab 17:321–327 [DOI] [PubMed] [Google Scholar]
- Rizzo G, Fiorucci S 2006 PPARs and other nuclear receptors in inflammation. Curr Opin Pharmacol 6: 421–427 [DOI] [PubMed] [Google Scholar]