Abstract
17β-Estradiol (E2) acts through the estrogen receptor α (ERα) to stimulate breast cancer proliferation. Here, we investigated the functional relationship between ERα and signal transducer and activator of transcription (STAT)5b activity in ER+ MCF-7 and T47D human breast cancer cells after specific knockdown of STAT5b. STAT5b small interfering RNA (siRNA) inhibited E2-induced bromodeoxyuridine (BrdU) incorporation in both cell lines, as well as the E2-induced increase in MCF-7 cell number, cyclin D1 and c-myc mRNA, and cyclin D1 protein expression, indicating that STAT5b is required for E2-stimulated breast cancer proliferation. E2 treatment stimulated STAT5b tyrosine phosphorylation at the activating tyrosine Y699, resulting in increased STAT5-mediated transcriptional activity, which was inhibited by a Y669F STAT5b mutant. E2-induced STAT5-mediated transcriptional activity was inhibited by overexpressing a kinase-defective epidermal growth factor receptor (EGFR), or the EGFR tyrosine kinase inhibitor tyrphostin AG1478, indicating a requirement for EGFR kinase activity. Both E2-induced STAT5b tyrosine phosphorylation and STAT5-mediated transcription were also inhibited by the ER antagonist ICI 182,780 and the c-Src inhibitor PP2, indicating additional requirements for the ER and c-Src kinase activity. EGFR and c-Src kinase activities were also required for E2-induced cyclin D1 and c-myc mRNA. Together, these studies demonstrate positive cross talk between ER, c-Src, EGFR, and STAT5b in ER+ breast cancer cells. Increased EGFR and c-Src signaling is associated with tamoxifen resistance in ER+ breast cancer cells. Here we show that constitutively active STAT5b not only increased basal DNA synthesis, but also conferred tamoxifen resistance. Because STAT5b plays an integral role in E2-stimulated proliferation and tamoxifen resistance, it may be an effective therapeutic target in ER+ breast tumors.
THE STEROID HORMONE 17β-estradiol (E2) plays a critical role in the development and progression of breast cancer. Biological effects of E2 are mediated through the estrogen receptors (ERα or ERβ), which are members of the nuclear receptor family of transcription factors. Although ERα and ERβ are present in normal mammary cells, ERα predominates in breast tumors (1). In fact, 40–70% of breast tumors express ERα, thus allowing treatment with antiestrogen adjuvant therapy and aromatase inhibitors (2,3). E2 induces breast cancer proliferation both in vitro and in vivo, at least in part, by stimulating progression through the G1 phase of the cell cycle (4) and regulating the transcription of genes that control growth and cell survival (4,5). ER proteins act in the nucleus as ligand-activated transcription factors that either bind as dimers to estrogen response elements (EREs) in the promoters of target genes, or associate with other transcription factors bound to the DNA (6). In addition to its role in the nucleus, ER interacts with and activates cytoplasmic signaling molecules including c-Src, Shc, and phosphatidylinositol 3-kinase (7). Both the nuclear and cytoplasmic actions of ER may play a role in E2-dependent breast cancer. ER transcription can be modulated by selective ER modulators, which have tissue-specific agonist or antagonist functions. As a result, ER-positive (ER+) breast tumors, which are E2 dependent, can be treated with selective ER modulators such as tamoxifen (a tissue-specific antagonist) or full ER antagonists such as ICI 182,780 (ICI). However, about 30–50% of women with ER+ tumors are intrinsically resistant to tamoxifen, and others who initially respond acquire resistance over time (8).
More advanced, aggressive breast tumors are often ER negative and overexpress members of the human epidermal growth factor receptor (EGFR) family, including the EGFR, as well as the nonreceptor tyrosine kinase c-Src (9,10). When c-Src and the EGFR are cooverexpressed in model systems of breast cancer, c-Src mediates the EGF-induced phosphorylation of tyrosine 845 (Y845) of the EGFR (11). This is not an autophosphorylation site and is not required for EGFR kinase activity but has been shown to be required for EGF- and E2-stimulated breast cancer cell proliferation (11,12,13). The signal transducer and activator of transcription 5b (STAT5b) was the first identified downstream effector of phosphorylated Y845 in response to EGF (14). Phosphorylation of Y845 of the EGFR is required for EGF stimulation of STAT5b tyrosine phosphorylation and transcriptional activity (14).
STATs, of which seven have been identified (1–4, 5a, 5b, and 6), are transcription factors involved in cellular proliferation, differentiation, apoptosis, and oncogenesis (15). They were originally identified in the interferon-signaling pathway (16) but are also activated by numerous cytokines and growth factors, including epidermal growth factor (EGF), as well as the Src-family tyrosine kinases (17). STAT5a and STAT5b, which are encoded by separate genes, share greater than 94% amino acid sequence identity and differ mainly in their C terminus (18,19). They are activated by phosphorylation at a conserved C-terminal tyrosine residue, which is tyrosine 694 (Y694) for STAT5a and Y699 for STAT5b (20,21). Activated STAT proteins dimerize and translocate from the cytoplasm into the nucleus, where they bind STAT5 regulatory elements in the promoters of target genes to regulate transcription (15). In addition to Y699, EGF stimulates the phosphorylation of tyrosines 725, 740, and 743 in the C terminus of STAT5b (22,23). Deletion of the C-terminal transcriptional activation domain of STAT5a or STAT5b results in a dominant-negative molecule that dimerizes with either wild-type (wt)STAT5a or STAT5b and inhibits their transcriptional activation (24,25). Mutation of Y740 and Y743 increases STAT5b activity and renders it constitutively active (22,23). However, the effect of ER agonists and antagonists on STAT5b activity has not been fully elucidated.
The STAT proteins are downstream of numerous oncogenic signaling pathways, and persistent STAT signaling is often present in cancers. STAT1, STAT3, and STAT5 are activated in cells transformed by v-Src (26,27,28), and STAT3 signaling is required for oncogenic transformation by v-Src (29,30). Increased activation of STAT5a and STAT5b often results from the overexpression or constitutive activation of EGFR or c-Src (15,17,23). Furthermore, STAT3, STAT5a, and STAT5b are overexpressed or constitutively activated in breast cancer (31,32,33). STAT5b, but not STAT5a, is proproliferative in squamous cell carcinoma of the head and neck and breast cancer (23,34,35), and STAT5b, but not STAT5a, increases EGF-induced DNA-synthesis in ER-negative SKBr3 breast cancer cells (23). STAT5b that has been activated by c-Src translocates to the nucleus, whereas STAT5a does not (36). In addition, Yamashita et al. (33) have detected low levels of constitutive STAT5b tyrosine phosphorylation, but not that of STAT5a, in MCF-7 and T47D breast cancer cells. Both ERα and STAT5 have been shown to transcriptionally regulate E2-sensitive proliferative genes such as cyclin D1 and c-Myc (4,5,15,17), suggesting that STAT5 may play a role in E2-stimulated breast cancer growth. Furthermore, ER has been shown to physically interact with STAT3 and STAT5 in model cell systems (37,38,39,40).
To investigate the relationship between ERα and STAT activity in ER+ breast cancer, we used the ER+ human breast cancer MCF-7 and T47D cell lines that overexpress c-Src and express basal levels of EGFR. We found that STAT5b is required for E2-induced DNA synthesis and the subsequent increase in MCF-7 cell number, as well as E2-induced cyclin D1 protein and mRNA expression. E2 stimulates STAT5b Y699 phosphorylation as well as STAT5-mediated transcriptional activity, and both of these events require c-Src and the ER. EGFR kinase activity is also required for the E2-induced transcriptional activation of STAT5b. Furthermore, constitutively active STAT5b eliminates tamoxifen suppression of proliferation, highlighting the importance of STAT5b in ER-regulated breast cancer growth.
RESULTS
STAT5b Is Required for E2-induced Breast Cancer Proliferation
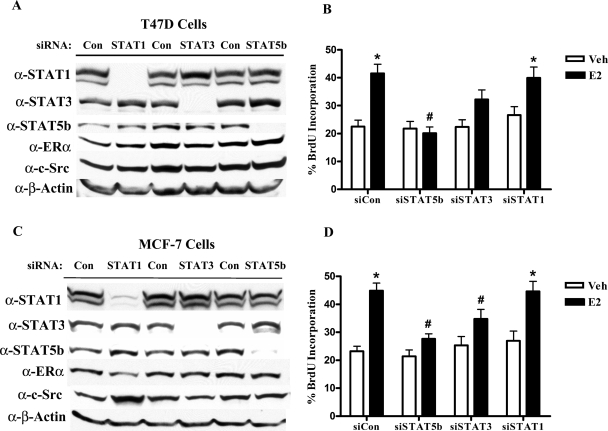
Our previous studies have shown that STAT5b transcriptional activation is required for EGF-induced proliferation of ER-negative SKBr3 breast cancer cells (14). However, the role of STAT5b in the proliferation of ER+ breast cancer is less well known. STAT5b is the predominant form of STAT5 expressed in ER+ MCF-7 and T47D human breast cancer cells, whereas the highly homologous STAT5a protein is minimally expressed and not detectable by immunoblotting (23). Consequently, the studies presented here focus on the STAT5b-signaling pathway. Using siRNA technology, we were able to significantly reduce the expression of STAT5b in two ER+ breast cancer cell lines. For comparison, we used the same methodology to knock down STAT1 and STAT3. In T47D cells, each STAT siRNA specifically knocked down its corresponding STAT protein to undetectable levels without affecting expression of the other STAT proteins, whereas control siRNA had no effect (Fig. 1A). Two bands were visible with the STAT5b antibody; the upper band, which we do not detect consistently, appears to be nonspecific or an artifact of the antibody, whereas the bottom band represents STAT5b. None of the siRNAs significantly affected the protein expression levels of ERα or c-Src. BrdU incorporation assays were then performed to determine the role of STAT proteins in DNA synthesis. As shown in Fig. 1B, E2 treatment resulted in a significant 1.8-fold stimulation in BrdU incorporation (a measure of DNA synthesis) in control cells (Fig. 1B; % incorporation, Veh: 22.5 ± 2.3, E2: 41.6 ± 3.3). Knockdown of STAT5b completely inhibited E2-stimulated BrdU incorporation, demonstrating that STAT5b is required for E2-stimulated DNA synthesis (% incorporation, Veh: 21.8 ± 2.6, E2: 20.1 ± 2.3). Knockdown of STAT3, which is also activated in some breast cancers (31,32), resulted in a partial, but not statistically significant, suppression of BrdU incorporation after E2 treatment (1.4-fold; % incorporation, Veh: 22.3 ± 2.7, E2: 32.3 ± 3.4). In contrast, knockdown of STAT1 caused a slight increase in basal BrdU incorporation, correlating with the tumor suppressor properties of STAT1 (15,17), but had no effect on E2-induced BrdU incorporation (Fig. 1B; Veh: 26.6% ± 3.1, E2: 40.0% ± 3.9). Effective and specific knockdown of the STATs was verified by Western blot analysis in each BrdU incorporation experiment, similar to immunoblot shown.
Figure 1.
STAT5b Is Required for E2-induced Proliferation in ER+ Breast Cancer Cells
A, T47D cells were nucleofected with 1.5 μg of 20 μm control, STAT1, or STAT5b siRNA or 2.5 μg of STAT3 SMARTpool siRNA per reaction. The cells were lysed after 72 h, and proteins were separated by 8% SDS-PAGE, transferred to nitrocellulose, and immunoblotted with specific antibodies. B, T47D cells were transfected with control, STAT1, STAT5b, or STAT3 siRNA as above along with 2 μg GFP. After 32 h, cells were serum starved for 16 h and then treated with either control media containing ethanol (Veh) or 10 nm E2 for 24 h. BrdU was added for the last 4 h of treatment. The cells were then fixed and stained with a fluorescently labeled anti-BrdU antibody, and GFP-positive cells were scored for BrdU incorporation by immunofluorescence microscopy. The data represent the mean ± sem of two experiments with at least 10 field counts per experiment. C, MCF-7 cells were transfected and analyzed as in panel A. D, MCF-7 cells were transfected with 1.5 μg control, STAT1, STAT3, or STAT5b siRNA along with 2 μg GFP. The cells were serum starved 32 h later, treated, stained, and scored for BrdU incorporation as in panel B. The data represent the mean ± sem of three experiments. *, P < 0.01 vs. Veh; #, P < 0.05 vs. E2-stimulated siCon cells. Con, Control.
To confirm that the involvement of STAT5b in E2-stimulated DNA synthesis is not restricted to T47D cells, similar studies were also performed in MCF-7 cells. Western blot analysis demonstrated that siRNA treatment resulted in efficient and specific knockdown of its corresponding STAT protein without significantly altering ERα or c-Src protein levels (Fig. 1C). In these cells, E2 induced a 1.9-fold stimulation in BrdU incorporation [small interfering control (siCon), Fig. 1D; % incorporation, Veh: 23.2 ± 1.8, E2: 44.9 ± 2.7). Knockdown of either STAT5b or STAT3 significantly inhibited E2-stimulated BrdU incorporation [% incorporation: small interfering STAT (siSTAT)5b, vehicle (Veh): 21.5 ± 2.2, E2: 27.7 ± 1.8; siSTAT3, Veh: 25.4 ± 3.2, E2: 34.8 ± 3.4). These results agree with data from our laboratory demonstrating that dominant-negative STAT5b inhibited E2-induced BrdU incorporation in MCF-7 cells (data not shown). In addition, these data suggest that STAT3 may also play an important role in E2-stimulated proliferation. As in T47D cells, siSTAT1 had no effect on E2-stimulated BrdU incorporation in MCF-7 cells (Fig. 1D; % incorporation, Veh: 26.9 ± 3.5, E2: 44.7 ± 3.6). Western blot analysis also confirmed effective knockdown of the STATs in these BrdU incorporation experiments, similar to the immunoblot shown. Together, these results demonstrate that STAT5b signaling plays an integral role in E2-stimulated DNA synthesis in two different ER+ breast cancer cell lines.
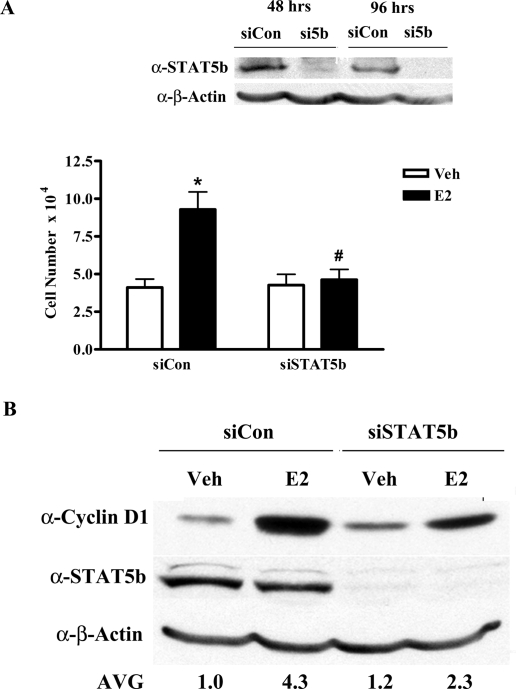
To support the results of the BrdU incorporation assays, cell counting assays were used as a second measure of cell proliferation. We examined whether the knockdown of STAT5b resulted in an effect on E2-induced increases in MCF-7 cell number. Western blot analysis confirmed that STAT5b siRNA treatment resulted in efficient knockdown of STAT5b protein both before and after E2 treatment (Fig. 2A, inset). E2 treatment for 48 h significantly stimulated cell growth as reflected by a 2.3-fold increase in cell number (siCon, Fig. 2A), and this increase was significantly inhibited when STAT5b protein was decreased by siRNA knock-down (Fig. 2A). Thus, STAT5b knockdown inhibits both E2-induced DNA synthesis and cell proliferation, supporting a crucial role for STAT5b in E2-induced breast cancer growth.
Figure 2.
STAT5b Is Required for E2-Stimulated Cell Number and Cyclin D1 Protein Expression
A, MCF-7 cells were nucleofected with 1.5 μg of 20 μm control or STAT5b siRNA per reaction. Twenty-four hours later, the cells were serum-starved for 16 h, treated with control media containing ethanol (Veh) or 10 nm E2 for 48 h, stained with Trypan blue, and counted using a hemacytometer. The data represent the mean ± sem of three experiments with triplicate samples per group. *, P < 0.001 vs. Veh; #, P < 0.001 vs. E2-stimulated siCon cells. Inset, Western blot of STAT5b knockdown before and after E2 treatment. B, MCF-7 cells were nucleofected as in panel A, serum starved for 24 h, treated with either control media containing ethanol (Veh) or 10 nm E2 for 8 h, and lysed. Proteins were then separated by 10% SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antibodies specific for cyclin D1, STAT5, and β-actin. Numbers below the representative blot are densitometric values for cyclin D1 normalized to actin for three experiments. AVG, Average; si5B, siSTAT5b.
E2 induces breast cancer proliferation, in part, by stimulating the expression of endogenous proliferative genes such as cyclin D1 (4,5). Because cyclin D1 is a downstream target of both ERα and STAT5b, we investigated the role of STAT5b in the E2-induced expression of cyclin D1 protein. MCF-7 cells were nucleofected with control or STAT5b siRNA, serum-starved for 24 h, and then treated with control media or E2 for 8 h. Western blot analysis confirmed efficient knockdown of STAT5b in this experiment (Fig. 2B). E2 treatment induced a significant 4.3-fold increase in cyclin D1 protein expression (4.3 ± 0.2). However, knockdown of STAT5b significantly decreased E2 induction of cyclin D1 protein expression, resulting in only a 1.9-fold increase in cyclin D1 protein levels (Fig. 2B; Veh: 1.2 ± 0.1, E2: 2.3 ± 0.1 vs. siCon cells with the same treatment). These results demonstrate that STAT5b plays an important role in E2-induced increases in cyclin D1 expression and may be one mechanism by which STAT5b contributes to E2-induced breast cancer proliferation.
E2 Stimulates STAT5-Mediated Transcription and Phosphorylation of Y699
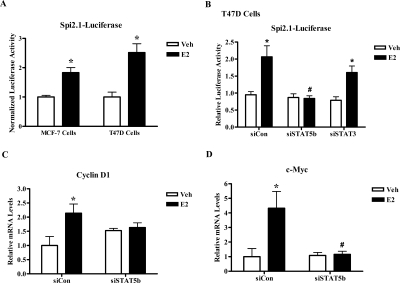
The inhibitory effect of STAT5b knockdown on E2-mediated DNA synthesis and cell proliferation as well as cyclin D1 protein expression suggests that E2 functionally activates STAT5b. Because STAT5b is a transcription factor, we first investigated whether the STAT5 pathway is activated by E2 in ER+ breast cancer cells by examining the E2-mediated activation of the STAT5-specific reporter Spi2.1-luciferase. In Fig. 3A, MCF-7 and T47D cells transfected with Spi2.1-luciferase containing a STAT5 response element were treated for 24 h with control media or E2. E2 treatment induced a statistically significant 1.8-fold ± 0.2 and 2.5-fold ± 0.3 increase in STAT5-mediated transcription in both ER+ breast cancer cell lines (MCF-7 and T47D). In Fig. 3B, E2 induced a significant 1.9-fold stimulation in Spi2.1-luciferase activity in T47D cells, which was significantly inhibited by knockdown of STAT5b. In contrast, knockdown of STAT3 did not inhibit E2-induced stimulation of Spi2.1-luciferase, indicating that E2-induced activation of Spi2.1-luciferase activity is specific to STAT5b in ER+ breast cancer cell lines (Fig. 3B).
Figure 3.
Estrogen Stimulates STAT5-Mediated Transcription of Endogenous Proliferative Genes
A, MCF-7 or T47D cells were transfected with 1 μg/well of the STAT5 luciferase reporter pGL2-Spi2.1. Transfected cells were serum starved for 24 h, treated with control media containing ethanol (Veh) or 10 nm E2 for 24 h, and then collected and assayed for luciferase activity. The data are shown as luciferase activity normalized for total protein and represents the mean ± sem for three experiments in MCF-7 or T47D cells with triplicate samples per group. Student’s t test was used to determine statistical significance. *, P < 0.01 vs. Veh. B, T47D cells were nucleofected with 3.5 μg of pGL2-Spi2.1 along with 1.5 μg of 20 μm control, STAT5b, or STAT3 siRNA per reaction. Transfected cells were serum starved, treated, and assayed for luciferase activity as in panel A. The data are shown as luciferase activity normalized for luciferase plasmid and represent the mean ± sem for four experiments with triplicate samples per group. *, P < 0.05 vs. Veh; #, P < 0.05 vs. E2-stimulated siCon and siSTAT3 cells. C and D, T47D cells were nucleofected with 2.5 μg of 20 μm control or STAT5b siRNA per reaction. After approximately 44 h, the cells were serum starved for 24 h and then treated with either control media containing ethanol (Veh) or 10 nm E2 for 4 h. The cells were then collected and the RNA was isolated, reverse transcribed into cDNA, and analyzed by real-time PCR using primers for cyclin D1 (C) or c-Myc (D). Threshold cycle values were normalized for actin, and the data represent the mean ± sem for seven (C) or six (D) experiments done in triplicate. Two-way ANOVA was used to determine statistical significance. *, P < 0.01 vs. Veh; #, P < 0.01 vs. E2-stimulated siCon cells.
To confirm that results from these luciferase reporter assays are reflected in physiological E2 transcriptional responses, we also examined the effect of STAT5b knockdown on the E2-induced mRNA expression levels of the endogenous proliferative genes cyclin D1 and c-myc. T47D cells were nucleofected with control or STAT5b siRNA, serum-starved for 24 h, and then treated with control media or E2 for 4 h. Western blot analysis confirmed effective knockdown of STAT5b in these experiments (data not shown). Total RNA was isolated, reverse transcribed, and analyzed by real-time RT-PCR using primers for cyclin D1 and c-Myc, which are transcriptionally regulated by ERα and STAT5b (4,17). Relative to actin, which serves as a housekeeping gene control, E2 significantly increased the mRNA expression levels of cyclin D1 and c-Myc (2.1- and 4.3-fold, respectively) in cells expressing control siRNA. Knockdown of STAT5b completely inhibited the E2-induced increase in cyclin D1 and c-Myc mRNA levels (Fig. 3, C and D), thus indicating that STAT5b is required for the E2-induced transcriptional regulation of these proliferative genes.
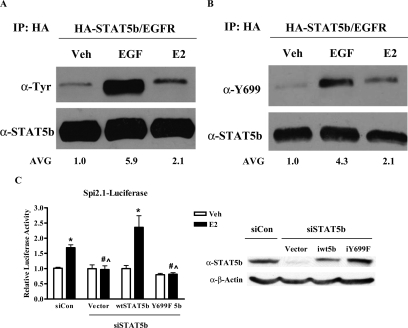
Because phosphorylation of STAT5b Y699 is required for STAT5b transcriptional activation, we next analyzed the ability of E2 to activate STAT5b tyrosine phosphorylation. In Fig. 4A, MCF-7 cells were transfected with HA-wtSTAT5b and EGFR by previously defined methods (14). After treatment with control media, 100 ng/ml EGF, or 10 nm E2, STAT5b immunoprecipitates were analyzed by immunoblotting with antibodies directed against total phosphotyrosine or STAT5b. As previously observed, exogenous expression of the EGFR resulted in detectable EGF-induced STAT5b tyrosine phosphorylation (Ref. 14 and shown in Fig. 4A, 5.9-fold ± 1.9). Under these conditions, E2 treatment also stimulated STAT5b tyrosine phosphorylation (Fig. 4A, 2.1-fold ± 0.1), supporting the transcriptional role of STAT5b in E2-induced cyclin D1 and c-myc mRNA expression as well as DNA synthesis and proliferation (Figs. 1–3).
Figure 4.
Estrogen Activates STAT5b Y699 Phosphorylation
A, MCF-7 cells transfected with 1.2 μg of HA-wtSTAT5b and 8.4 ng of EGFR were treated with control media (Veh), 100 ng/ml EGF for 5 min, or 10 nm E2 for 10 min. Lysates were immunoprecipitated with an anti-HA antibody and immunoblotted with antibodies directed against total phosphotyrosine (pTyr) (top) or STAT5b (bottom). Numbers below the representative blot are phosphorylated STAT5b densitometric values normalized to total STAT5b levels for the mean of three experiments. B, MCF-7 cells were transfected, treated, and immunoprecipitated as in panel A, followed by immunoblotting with antibodies directed against phospho-Y699 (top) or STAT5b (bottom). A representative blot is shown for each panel. Numbers below the blot are phosphorylated STAT5b Y699 densitometric values normalized to total STAT5b levels for the mean of three experiments. C, MCF-7 cells were nucleofected with 3.5 μg of pGL2-Spi2.1 per reaction along with 2 μg of 20 μm control siRNA, or 2 μg of STAT5b G3 siRNA plus 60 ng/well of the HA-CMV vector, immune wtSTAT5b, or immune iY699F STAT5b. After 24 h the cells were serum starved for 24 h, treated with either control media containing ethanol (Veh) or 10 nm E2 for 24 h, collected, and assayed for luciferase activity. The data are shown as normalized luciferase activity and represent the mean ± sem for four experiments with triplicate samples per group. *, P < 0.01 vs. Veh; #, P < 0.01 vs. E2-stimulated siCon cells; ^, P < 0.001 vs. E2-stimulated iwtSTAT5b-transfected cells. Inset, Western blot depicting knockdown of STAT5b and reintroduction of CMV-HA vector, iwtSTAT5b, and iY699F STAT5b after 72 h. IP, Immunoprecipitation.
We next examined the ability of E2 to specifically phosphorylate the activating tyrosine Y699 of STAT5b. To facilitate this analysis, we have developed an antiphospho-Y699 STAT5b/Y694 STAT5a antibody. To test the sensitivity and specificity of this antibody, MEF5−/− cells were transfected with EGFR along with STAT5b constructs. A total antiphosphotyrosine antibody recognized the immunoprecipitated forms of EGF-treated wtSTAT5b as well as the tyrosine mutant Y699F (22). However, the antiphospho-Y699 antibody specifically recognized the EGF-treated wtSTAT5b, and not the Y699F mutant (supplemental Fig. 1published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend. endojournals.org).
Using the antiphospho-Y699 STAT5b-specific antibody, we investigated the effect of E2 treatment on STAT5b Y699 phosphorylation specifically. Both EGF (4.3-fold ± 1.2) and E2 (2.1-fold ± 0.4) significantly stimulated the phosphorylation of Y699, the activating tyrosine of STAT5b (Fig. 4B). We investigated whether this tyrosine is required for E2-induced transcriptional activation of STAT5b. STAT5b expression was knocked down in MCF-7 cells, and either an empty vector or one of two STAT5b constructs immune to siRNA knockdown (wt or iY699F STAT5b) was simultaneously reexpressed, as confirmed by Western blot analysis (Fig. 4C, inset). As shown in Fig. 3, E2 induced a significant 1.7-fold ± 0.1 increase in STAT5-mediated transcription (siCon, Fig. 4C). This transcriptional response to E2 was lost when STAT5b was knocked down but was rescued and significantly increased (2.4-fold ± 0.4) after reexpression of immune wtSTAT5b (iwtSTAT5b). However, expression of iY699F STAT5b was unable to rescue the E2 stimulation of STAT5-mediated transcription, supporting the integral role of Y699 phosphorylation in E2-induced activation of STAT5b (Fig. 4C). Taken together, Figs. 1–4 demonstrate that E2 stimulation of STAT5b Y699 phosphorylation has functional consequences on STAT5 transcriptional activity and biological consequences on E2-induced proliferation.
E2-Induced STAT5 Transcriptional Activity Requires EGFR Kinase Activity
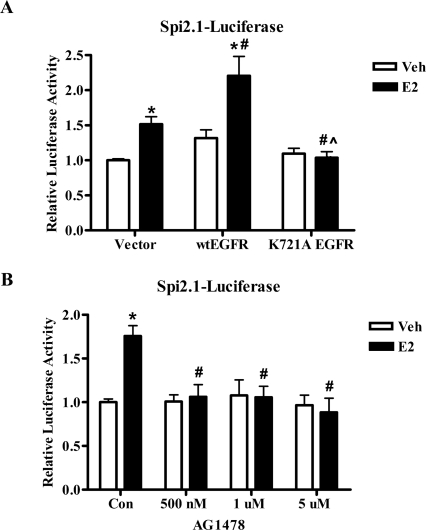
Our next studies focused on the potential mechanism by which E2 activates STAT5b signaling in ER+ breast cancer cells. Because ER is not a tyrosine kinase, phosphorylation and subsequent transcriptional activation of STAT5b must occur indirectly through another molecule that interacts with or is activated by ER. Two such candidate molecules include the EGFR and c-Src tyrosine kinases. Cross talk between the ER, EGFR, and c-Src has been documented in various cell systems (reviewed in Ref. 7), and the kinase activities of EGFR and c-Src have been shown to play an important role in EGF-induced STAT5b activation in ER-negative breast cancer cell models (14,23). We first investigated the role of EGFR kinase activity in the E2 stimulation of STAT5 transcriptional activity. T47D cells were transiently transfected with an empty vector, wtEGFR, or a K721A mutation that renders the EGFR kinase inactive (14), along with the Spi2.1-luciferase reporter construct. Figure 5A demonstrates that E2 induced a significant 1.5-fold increase in STAT5-mediated transcription when only the empty vector was exogenously expressed. Transfection of wtEGFR significantly increased the transcriptional response to E2 as compared with the vector-transfected cells, resulting in a 1.7-fold increase in STAT5-mediated transcription after E2 treatment. In contrast, expression of the kinase-defective K721A EGFR mutant completely inhibited E2 stimulation of STAT5-mediated transcription (Fig. 5A), indicating that EGFR kinase activity is required for the E2-induced transcriptional activation of STAT5.
Figure 5.
E2-Induced STAT5 Transcriptional Activity Requires EGFR Kinase Activity
A, T47D cells were transfected with 1 μg/well of the STAT5 luciferase reporter pGL2-Spi2.1 along with 50 ng/well of the empty vector pcDNA3.1, wtEGFR, or kinase-defective (K721A) EGFR. Transfected cells were serum starved for 24 h, treated with either control media containing ethanol (Veh) or 10 nm E2 for 24 h, collected, and assayed for luciferase activity. The data are shown as normalized luciferase activity and represent the mean ± sem for four experiments with triplicate samples per group. *, P < 0.05 vs. Veh; #, P < 0.05 vs. E2-stimulated vector-transfected cells; ^, P < 0.001 vs. E2-stimulated wtEGFR-transfected cells. B, T47D cells were transfected with 1 μg/well of pGL2-Spi2.1. After 24 h of serum starvation, the cells were pretreated for 1 h with 500 nm, 1 μm, or 5 μm AG1478 or its vehicle DMSO, treated with either control media containing ethanol (Veh) or 10 nm E2 for 24 h, collected, and assayed for luciferase activity. The data are shown as normalized luciferase activity and represent the mean ± sem for six experiments with triplicate samples per group. Student’s t test was used to determine statistical significance. *, P < 0.0001 vs. Veh; #, P < 0.01 vs. E2-stimulated Con. Con, Control.
To determine the role of endogenous EGFR kinase activity in the E2-induced activation of STAT5b, we used the highly selective EGFR kinase inhibitor tyrphostin AG1478. AG1478 specifically inhibits EGFR tyrosine kinase activity in the nanomolar range, whereas concentrations of greater than 100 μm are required to inhibit other tyrosine kinases such as human EGFR2-Neu or platelet-derived growth factor receptor (41). T47D cells transfected with the Spi2.1-luciferase reporter construct were pretreated with increasing concentrations of AG1478 (500 nm to 5 μm or vehicle before treatment with control media or E2 for 24 h. These AG1478 concentrations have previously been used to inhibit EGFR kinase activity (42,43,44) but should not inhibit the activity of other tyrosine kinases (41). Importantly, these same concentrations of AG1478 do not inhibit GH-induced STAT5b signaling that requires Janus kinase 2 activity (Ref. 45 and data not shown). As shown in Fig. 5B, AG1478 significantly inhibits E2 stimulation of STAT5-mediated transcription, even at the lowest concentration of 500 nm, further verifying that EGFR kinase activity is required for E2-induced STAT5 transcriptional activity.
E2-Induced STAT5b Activation Is Mediated By c-Src and the ER
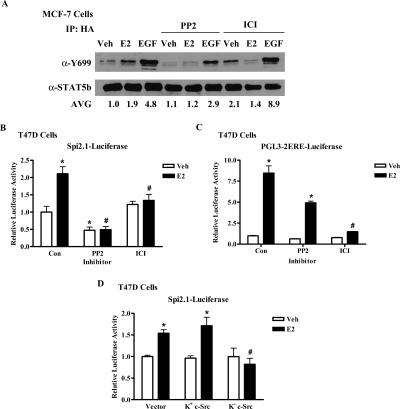
A number of published studies demonstrate that cross talk between the ER, EGFR, and STAT5b may also involve c-Src. For example, c-Src can increase EGFR activity by phosphorylating specific tyrosine residues of the EGFR (11,12); c-Src and EGFR can both phosphorylate STAT5b Y699 (14,22,23); and E2 activates c-Src in ER+ breast cancer cells (46). Thus, we investigated whether c-Src is involved in E2-stimulated STAT5b activation by inhibiting c-Src kinase activity with the pharmacological inhibitor PP2. PP2 competitively inhibits c-Src tyrosine kinase activity and is selective for the Src family over other tyrosine kinase families such as EGFR and Janus kinase 2 (47). The complete ER antagonist ICI was also used to directly assess the role of the ER in this pathway. MCF-7 cells transfected with HA-wtSTAT5b and the EGFR were pretreated with either vehicle, 10 μm PP2, or 1 μm ICI, followed by stimulation with media alone, E2, or EGF. This concentration of PP2 has previously been shown to inhibit c-Src phosphorylation in breast cancer cells (48). As shown in Fig. 6A, PP2 partially inhibited EGF-induced STAT5b tyrosine phosphorylation, whereas ICI did not. Most importantly, the E2-induced phosphorylation of STAT5b Y699 was inhibited by either PP2 or ICI, demonstrating that both c-Src and the ER are required for E2-induced stimulation of STAT5b Y699 phosphorylation (Fig. 6A).
Figure 6.
E2-Induced STAT5b Activation Is Mediated by c-Src and ERα
A, MCF-7 cells transfected with HA-wtSTAT5b and EGFR were pretreated with 1 μm ICI or 10 μm PP2 for 1 h, and then with control media (Veh), 100 ng/ml EGF for 5 min, or 10 nm E2 for 10 min. Lysates were immunoprecipitated with an anti-HA antibody and immunoblotted with antibodies directed against phospho-Y699 (top) or STAT5b (bottom). A representative blot is shown, and numbers below the blot are phosphorylated STAT5b Y699 densitometric values normalized to total STAT5b levels for the mean of three experiments. Fold induction ± sem: E2 (1.9 ± 0.1); EGF (4.8 ± 1.5); PP2 + Veh (1.1 ± 0.2), E2 (1.2 ± 0.3), EGF (2.9 ± 0.3); ICI + Veh (2.1 ± 0.6), E2 (1.4 ± 0.3), EGF (8.8 ± 4.7). B and C, T47D cells were transfected with 1 μg/well of the STAT5 luciferase reporter pGL2-Spi2.1 (B) or 0.5 μg/well of the ERE-containing luciferase reporter pGL3-2ERE (C). After serum starvation the cells were pretreated for 1 h with 10 μm PP2 or its vehicle DMSO, treated with either control media (Veh), E2, ICI, or E2 + ICI for 24 h, collected, and assayed for luciferase activity. The data are shown as normalized luciferase activity and represent the mean ± sem for six (B) or three (C) experiments with triplicate samples per group. *, P < 0.05 vs. Veh-stimulated Con; #, P < 0.001 vs. E2-stimulated Con. Some error bars in panel C are too small to be detected. D, T47D cells were transfected with 1 μg/well of pGL2-Spi2.1 along with 0.5 μg/well of the empty vector pcDNA3.1, K+ c-Src, or K− c-Src. Transfected cells were serum starved for 24 h, treated with control media (Veh) or 10 nm E2 for 24 h, collected, and assayed for luciferase activity. The data are shown as normalized luciferase activity and represent the mean ± sem for three experiments with triplicate samples per group. *, P < 0.05 vs. Veh; #, P < 0.01 vs. E2-stimulated vector- and K+ c-Src-transfected cells. Con, Control; IP, immunoprecipitation.
To determine whether c-Src and ER are also required for STAT5 transcriptional activity in the context of endogenous EGFR, T47D cells transfected with the Spi2.1-luciferase reporter construct were pretreated with PP2 or vehicle before treatment with control media, E2, ICI, or E2 + ICI. Figure 6B demonstrates that E2 stimulation of STAT5-mediated transcription was significantly inhibited by ICI, confirming that ER mediates E2-induced STAT5 transcriptional activity. Basal STAT5-mediated transcription was also significantly decreased by PP2, implying a role for c-Src in basal STAT5 transcriptional activity in these cells. Most importantly, E2 stimulation of STAT5-mediated transcription was significantly inhibited by PP2, thus demonstrating that c-Src, in addition to ER, is required for E2-induced STAT5 transcriptional activity. As a control to test the specificity of our inhibitors, we also assessed the effects of ICI and PP2 on ER-mediated transcription of an estrogen response element (ERE) reporter. E2 induced an 8.5-fold stimulation of a luciferase reporter containing E2 response elements (pGL3-2ERE-luciferase), which was significantly inhibited by ICI (Fig. 6C). In contrast, despite a decrease in basal transcription, E2 still elicited a 7.8-fold stimulation of ERE-dependent transcription in the presence of PP2. This result verifies that inhibition of c-Src tyrosine kinase activity does not affect the transcriptional activity of ER on an ERE, supporting the specificity and selectivity of our inhibitors.
Because PP2 may potentially inhibit other kinases of the Src family, we used a second experimental approach to verify the specific involvement of c-Src in E2-stimulated STAT5-mediated transcription. T47D cells were transfected with an empty vector, kinase-active (K+) c-Src, or dominant-negative kinase-defective (K−) c-Src, along with the Spi2.1-luciferase reporter construct. Figure 6D demonstrates that E2 significantly increased STAT5-mediated transcription when the empty vector or K+ c-Src was exogenously expressed (1.5- and 1.8-fold, respectively). However, expression of the kinase-defective form of c-Src (K− c-Src) completely inhibited E2 stimulation of STAT5-mediated transcription (Fig. 6D), verifying that c-Src kinase activity is required for the E2-induced transcriptional activation of STAT5. Taken together, Fig. 6 illustrates that E2-stimulated STAT5b activation is mediated by c-Src and ER.
c-Src and EGFR Are Required for the E2-Induced Transcriptional Regulation of Proliferative Genes
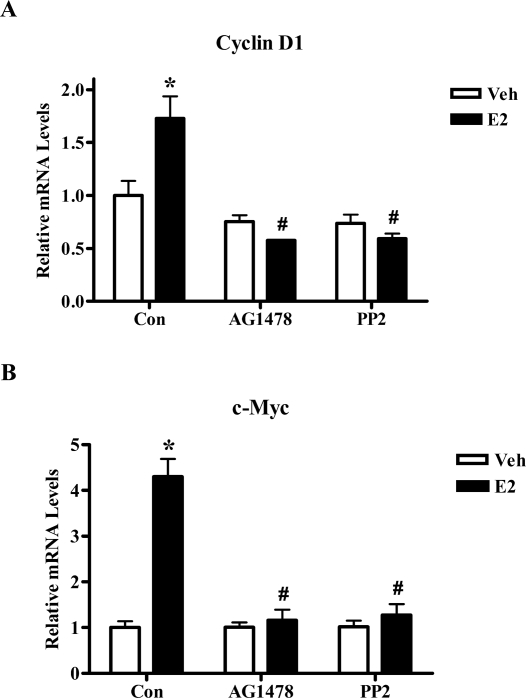
We next verified that EGFR and c-Src kinases are also involved in the E2-induced activation of endogenous STAT5b target genes. T47D cells were serum starved, pretreated with either vehicle, 10 μm PP2, or 1 μm AG1478 and then treated with control media or E2 for 4 h. E2 treatment significantly increased the mRNA expression levels of cyclin D1 and c-Myc (1.7- and 4.3-fold, respectively), but this induction was significantly inhibited by pretreatment with PP2 or AG1478 (Fig. 7). Thus, the kinase activities of EGFR and c-Src are required for the E2-induced activation of these two endogenous STAT5b target genes, providing additional proof that STAT5b plays an important role in endogenous E2 signaling in breast cancer cells by a mechanism dependent on c-Src and EGFR tyrosine kinases.
Figure 7.
c-Src and EGFR Kinase Activities Are Required for the E2-induced Transcriptional Regulation of STAT5b Target Genes
T47D cells were mock-transfected as in Fig. 3, C and D. After 24 h of serum starvation the cells were pretreated for 1 h with 10 μm PP2, 1 μm AG1478, or their vehicle DMSO, followed by treatment with either control media containing ethanol (Veh) or 10 nm E2 for 4 h. RNA was isolated, reverse transcribed to cDNA, and analyzed by real-time PCR using primers for cyclin D1 (A) or c-Myc (B). Threshold cycle values were normalized for actin, and the data represent the mean ± sem for three (A) or four (B) experiments done in triplicate. Two-way ANOVA was used to determine statistical significance. *, P < 0.01 vs. Veh; #, P < 0.001. vs. E2-stimulated Con. Con, Control.
Increased STAT5b Activity Confers Tamoxifen Resistance
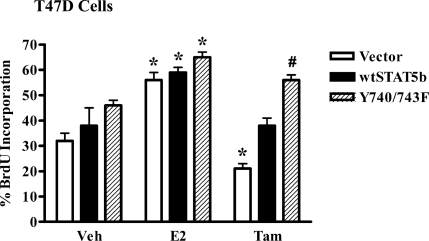
ER+ breast tumors are routinely treated with antiestrogens such as tamoxifen; however, resistance to this treatment frequently occurs (8). One mechanism of tamoxifen resistance involves increased EGFR and c-Src activity and/or expression (8,49), both of which have recently been shown to stimulate STAT5b activity (13). Because our data demonstrate that EGFR, c-Src, and STAT5b also play an important role in E2 signaling, we explored the potential role of E2-stimulated STAT5b activity in tamoxifen resistance. We tested whether a constitutively active STAT5b construct (Y740/743F STAT5b) could influence the tamoxifen suppression of breast cancer cell proliferation. The c-Src-mediated basal tyrosine phosphorylation and activity of this mutant has been described previously (23). To explore the effects of the Y740/743F STAT5b mutant on DNA synthesis, T47D cells were transfected with an empty vector, wtSTAT5b, or Y740/743F STAT5b (Fig. 8). After overnight serum starvation, the cells were pulsed with BrdU in the presence of control media, E2, or tamoxifen, and BrdU incorporation was measured. In the cells expressing empty vector, wtSTAT5b, or Y740/743F STAT5b, BrdU incorporation was significantly stimulated by E2 (1.8-, 1.6-, and 1.4-fold, respectively). In contrast, tamoxifen suppressed BrdU incorporation to below basal levels in vector-expressing cells. BrdU incorporation remained just above basal levels after tamoxifen treatment for cells expressing wtSTAT5b, but it was significantly higher than that seen in the tamoxifen-treated vector cells. Strikingly, the Y740/743F STAT5b mutant not only significantly increased basal BrdU incorporation in vehicle-treated cells, but also eliminated the suppression of DNA synthesis by tamoxifen. In fact, tamoxifen-treated cells expressing the Y740/743F mutant showed a level of BrdU incorporation comparable to that seen in E2-treated vector cells [Fig. 8, striped bar (Tam) vs. white bar (E2)]. Together, these results demonstrate that constitutively active STAT5b can overcome sensitivity to tamoxifen and confer tamoxifen resistance in T47D cells.
Figure 8.
Constitutively Active STAT5b Confers Tamoxifen Resistance
T47D cells were nucleofected with 1 μg of either the HA-CMV vector, HA-wtSTAT5b, or HA-Y740/743F STAT5b, along with 1 μg of GFP per reaction. Thirty two hours later the cells were serum starved for 16 h and then treated with control media containing ethanol (Veh), 10 nm E2, or 1 μm tamoxifen for 24 h. BrdU was added for the last 4 h of treatment. The cells were then fixed and stained with a fluorescently labeled anti-BrdU antibody, and GFP-positive cells were scored for BrdU incorporation by immunofluorescence microscopy. The data represent the mean ± sem of three experiments. *, P < 0.05 vs. Veh; #, P < 0.01 vs. tamoxifen-treated vector cells; ^, P < 0.01 vs. tamoxifen-treated wtSTAT5b cells.
DISCUSSION
STATs, especially STAT5a, STAT5b, and STAT3, have been shown to play important roles in breast cancer (15,17), and cross talk between the ER and these STATs has been documented in ER+ breast cancer (33,37,40). Using a knockdown strategy, we explored the role of STAT5b in E2-stimulated breast cancer proliferation. In the ER+ breast cancer cell lines MCF-7 and T47D, knockdown of STAT5b significantly inhibited E2-induced incorporation of BrdU into DNA (Fig. 1, B and D) as well as the E2-induced increase in MCF-7 cell number (Fig. 2A), supporting an important role for STAT5b in E2-induced breast cancer proliferation and cell growth. Furthermore, our results are the first to show that endogenous STAT5b is required for E2-mediated increases in cyclin D1 and c-myc mRNA, DNA synthesis, and proliferation (Figs. 1–3). We demonstrate an integral link between ER, c-Src, and EGFR in E2-induced STAT5b signaling in ER+ breast cancer cells.
STAT5a/5b and STAT3 have been shown to functionally interact with steroid receptors, including the progesterone, glucocorticoid, and estrogen receptors (33,37,38,39,40,50,51,52,53,54). Our studies also support a role for STAT3 in E2-induced proliferation; however, knockdown studies (Fig. 1, B and D) suggest that these two transcription factors cannot compensate for each other in this context. Our present studies have focused on the role of the STAT5b signaling pathway. ERα and ERβ interact in vitro with STAT5a and STAT5b through the ER DNA-binding domain/hinge region (38,39), and endogenous ERα and STAT5a coimmunoprecipitate from MCF-7 and T47D cells (40). Our results show that E2 stimulates phosphorylation of STAT5b Y699 as well as STAT5-mediated transcription in ER+ breast cancer cells (Figs. 4B and 3A). Phosphorylation of Y699, the activating tyrosine, is required for E2-induced STAT5b transcriptional activation (Fig. 4C). Our data support and extend a previous study demonstrating that dominant-negative STAT5a inhibits ER transcriptional activation in ER+ breast cancer cells (33), and that introduction of dominant-negative STAT5a into T47D cells inhibits E2-stimulated proliferation, suppresses xenograft tumor growth in nude mice, and induces apoptosis both in vitro and in vivo (33,55).
Several ER+ cell studies have shown positive interactions between ER and STAT5. ER-E2 activates STAT5 in ER+ porcine aortic endothelial cells (50) and enhances prolactin-stimulated STAT5 transcriptional activity in HC11 cells (38). Importantly, we demonstrate that E2 induces the activation of endogenous STAT5b target genes, cyclin D1 and c-myc, in addition to STAT5 reporter activity (Figs. 2B and 3). In fact, STAT5b signaling plays an important role in the E2 stimulation of endogenous cyclin D1 mRNA and protein (Figs. 2B and 3C), and may be one mechanism by which STAT5b contributes to E2-induced breast cancer proliferation. Of note, we consistently see a slight (but not significant) increase in the basal level of cyclin D1 mRNA upon knockdown of STAT5b (Fig. 3C). Because cyclin D1 has a complex promoter, this increase could be due to any number of transcription factors, the activity of which may be enhanced upon loss of STAT5b. However, despite this increase in basal transcription, knockdown of STAT5b still inhibits the E2-induced increase in cyclin D1 mRNA (Fig. 3C). Nevertheless, there remains a 1.9-fold induction of cyclin D1 protein after STAT5b knockdown (Fig. 2B), which may be explained by the complex transcriptional and posttranscriptional regulation of cyclin D1. Knockdown of STAT5b completely inhibits E2-induced cyclin D1 mRNA (Fig. 3C) but only partially inhibits E2-induced cyclin D1 protein (Fig. 2B). These results suggest that E2-induced signaling also impacts on the nontranscriptional regulation of cyclin D1. However, the 1.9-fold induction in cyclin D1 protein was not reflected in cell number (Fig. 2A), possibly because STAT5b regulates the transcription of multiple downstream proliferative genes, such as c-myc, in addition to cyclin D1 (Fig. 3, C and D). Hence, depleting STAT5b expression hinders the transcription of multiple downstream proliferative genes and results in a more profound effect on proliferation. These data, together with the observed requirement for endogenous STAT5b expression in E2-induced proliferation (Figs. 1 and 2A), demonstrate the importance of endogenous STAT5b signaling in E2-stimulated ER+ breast cancer.
Our studies show that E2 activation of STAT5b signaling in ER+ breast cancer cells includes EGFR and c-Src. Effects of E2 on STAT5 activation are cell context specific and may depend on the cellular stoichiometry of important signaling molecules such as ER, c-Src, and EGFR. For example, in ER-negative breast cancer cells expressing high levels of EGFR and c-Src, introduction of ER suppresses EGF- and E2-stimulated STAT5 activity (54). The present studies with ER+ cells expressing basal levels of EGFR provide evidence that E2-induced STAT5 transcriptional activation requires EGFR and c-Src kinase activity as well as the ER (Figs. 5 and 6, B and D). E2-induced activation of the endogenous STAT5b target genes cyclin D1 and c-myc also requires EGFR and c-Src kinase activity (Fig. 7). Furthermore, E2 stimulation of STAT5b Y699 phosphorylation is also mediated by c-Src and ER (Fig. 6A). A recent study has shown that ER, Src, and EGFR form a complex in an E2-dependent manner in MCF-7 cells, and that activation of ER and EGFR is required for E2-dependent Src activation and MCF-7 cell growth (56). Furthermore, E2-induced breast cancer proliferation requires c-Src kinase activity as well as the c-Src target Y845 of the EGFR (13,57). Our finding that STAT5b is also required for E2-induced proliferation (Figs. 1 and 2A) demonstrates the importance of the cross talk between ER, c-Src, EGFR, and STAT5b in ER+ breast cancer growth. Because c-Src and the EGFR are found at the membrane and E2-induced effects on STAT5b signaling are rapid, these results further support a nonnuclear role for ER in proliferative responses.
Our demonstration that ER, c-Src, and EGFR impinge on the STAT5b-signaling pathway is critical, especially since the dysregulation of these pathways can have therapeutic significance (58,59,60,61). Although c-Src signaling clearly plays a fundamental role in E2 signaling in ER+ breast cancer cells, c-Src overexpression and/or increased c-Src signaling, as well as other signaling pathways, may decrease the importance of or requirement for ER signaling in these cells, thus leading to tamoxifen resistance (58). Increased expression and/or signaling of the EGFR family have been implicated in acquired tamoxifen resistance (59), and an enhanced interaction between EGFR, ERα, and c-Src has recently been shown in tamoxifen-resistant MCF-7 cells (43). Furthermore, high expression of the adaptor molecule Cas/BCAR1, which binds to and activates c-Src, has been associated with intrinsic tamoxifen resistance (60,61). Recent work demonstrated that STAT5b signaling plays an important role in tamoxifen resistance induced by c-Src hyperactivation (13). In these studies, overexpression of the adaptor molecule Cas enhanced c-Src activation, which in turn increased STAT5b activity and ultimately led to tamoxifen resistance (13). Inhibiting STAT5b activity in this model prevented tamoxifen resistance (13). Our work extends these observations and demonstrates that constitutive activation of STAT5b activity eliminated tamoxifen suppression of breast cancer proliferation in the ER+ cell line T47D (Fig. 8), thus suggesting that increased STAT5b activity can contribute to the development of tamoxifen resistance.
High levels of c-Src expression are noted in more than 70% of breast tumors, including both ER+ and ER-negative breast cancers and human breast cancer cells (9,10,58). Thus, c-Src may play a complex and integral role in modulating the signaling from the ER, as well as from growth factor receptors such as the EGFR family members also known to be overexpressed in breast tumors. Recently, Chu et al. (62) have found that c-Src promotes E2-stimulated proteolysis, ubiquitylation, and degradation in MCF-7 cells, which is associated with increased E2-dependent ERα transcriptional activity. However, Src and ERα protein levels are inversely correlated in primary human breast tumors, indicating that overexpression of Src may result in lower ERα protein levels and ultimately less signaling through and dependence on ER (62). In ER-negative tumors, c-Src contributes to signaling through the growth factor receptors, which also includes signaling through STAT5 (14). Together, these findings suggest that the role of c-Src signaling may change with breast cancer progression or the expression of other signaling molecules in the tumor, in which either steroid or growth factor signaling is predominant.
The results in this manuscript support an integral role of STAT5b in E2-induced proliferative responses and the requirement for c-Src in this signaling. Together these studies provide a functional link between ER, c-Src, STAT5b, and E2-induced proliferative responses. Our studies also provide evidence of the important role of STAT5b activity in tamoxifen-resistant cells. Given our results demonstrating the critical role of STAT5b signaling in E2-induced breast cancer proliferation as well as tamoxifen resistance, we propose that STAT5b may be an important therapeutic target for ER+ breast cancer.
MATERIALS AND METHODS
Cell Lines and Transient Transfections
The MCF-7 and T47D human breast cancer cell lines were passaged twice per week and maintained in Cellgro DMEM (Fisher, Herndon, VA) with 10% fetal bovine serum (Invitrogen, Carlsbad, CA). For immunoprecipitation studies, cells were transiently transfected with HA-tagged STAT5b constructs (22,23) using Lipofectamine Plus per manufacturer’s directions (Invitrogen). For the luciferase assays, cells were transiently transfected with the indicated constructs using 2.5 μl of Lipofectamine 2000 per μg of DNA according to the manufacturer’s instructions (Invitrogen).
Reagents
Recombinant human EGF was obtained from Invitrogen and 17β-estradiol (E2) and tamoxifen (Tam) were purchased from Sigma-Aldrich Corp. (St. Louis, MO). The complete ER antagonist ICI was obtained from Tocris (Ellisville, MO), and the c-Src inhibitor PP2 as well as the EGFR tyrosine kinase inhibitor AG1478 were obtained from Calbiochem (La Jolla, CA). The polyclonal STAT5b-specific antibody was developed in our laboratory, as previously described (22). The monoclonal antibody specific for the STAT5 SH2 domain was obtained from BD Transduction Laboratories (BD Biosciences, Palo Alto, CA), and the monoclonal anti-HA antibody was obtained from the University of Virginia hybridoma facility (Charlottesville, VA). The monoclonal antiphosphotyrosine antibody (PY-99) and the polyclonal antibody specific for STAT1 p84/p91 (M-22) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The monoclonal c-Src 2–17 antibody was provided by Dr. Sarah J. Parsons (University of Virginia). Monoclonal antibodies specific for ERα (1D5; DakoCytomation, Glostrup, Denmark), β-actin (Sigma-Aldrich Corp.), and Cyclin D1 (Cell Signaling Technology, Danvers, MA) as well as the polyclonal antibody specific for STAT3 (Cell Signaling Technology), were obtained from the indicated sources. We generated the antiphospho-Y699 STAT5b/Y694 STAT5a (H-285) antibody in conjunction with Aves Laboratory (Tigard, OR). The phosphopeptide CZKAVDG(p)YVKPQIKQ, corresponding to amino acid residues 690/4–701/6, was used as an immunogen in chickens. For immunoblotting, we used a 1:100,000 dilution of the 0.4 mg/ml purified antibody and an antichicken secondary antibody.
siRNA Transfection and Immunoblotting
To knock down STAT protein expression, siGENOME SMARTpool siRNA reagents (Dharmacon RNA Technologies, Lafayette, CO) targeting human STAT5b, STAT3, or STAT1 were used unless otherwise specified. siCon nontargeting siRNA no. 1 (Dharmacon) was used as a negative control. A STAT5b custom G3 siRNA oligo (siGENOME SMARTpool duplex no. 3, Dharmacon) targeting the STAT5b DNA-binding domain was used only in the STAT5b iY699F luciferase studies. MCF-7 and T47D cells were transfected by nucleofection with 20 μm siRNA according to the manufacturer’s instructions (Amaxa Corp., Gaithersburg, MD) using program P20 and Solution T (4 × 106). Cells were used per nucleofection reaction, and one reaction was added to three wells of a six-well plate filled with phenol red-free DMEM 5% stripped newborn calf serum (SNCS) media with 2 mm l-glutamine warmed to 37 C. To validate efficient and specific knockdown, MCF-7 and T47D cells were nucleofected with the indicated amounts of control siRNA, STAT1 siRNA, STAT3 siRNA, or STAT5b siRNA. MCF-7 cells were lysed after 72 h for STAT1, STAT3, and STAT5b G3 or 48 h for SMARTpool STAT5b, and T47D cells were lysed after 72 h for all siRNA constructs. The cells were washed two times with E2-free PBS and then lysed in 2× gel loading buffer containing 100 mm Tris (pH 6.8), 2% sodium dodecyl sulfate, 20% glycerol, and a cocktail of protease inhibitors. Total protein was determined using the bicinchoninic acid protein assay (Pierce Chemical Co., Rockford, IL), after which lysate containing 60 μg of protein per sample was separated by 8% polyacrylamide-sodium dodecyl sulfate gels and transferred to nitrocellulose membranes. Membranes were then blotted with the indicated primary antibodies, followed by probing with either horseradish peroxidase-conjugated sheep antimouse (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) or donkey antirabbit (Amersham Biosciences, Piscataway, NJ) secondary antibodies. The primary antibodies were then detected with SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical Co.)
BrdU Incorporation Assays
For the siRNA BrdU experiments, MCF-7 and T47D cells were nucleofected with the indicated amounts of 20 μm control, STAT1, STAT3, or STAT5b SMARTpool siRNA per reaction, along with 2 μg of green fluorescent protein (GFP) as a marker of transfection. To investigate the effect of the Y740/743F STAT5b mutant on BrdU incorporation, T47D cells were nucleofected with 1 μg of either the hemagglutinin (HA)-cytomegalovirus (CMV) vector, HA-wtSTAT5b, or HA-Y740/743F STAT5b (23), along with 1 μg of GFP per reaction. Cells (4 × 106) were used per nucleofection reaction, and one reaction was added to three wells of a six-well plate. For both experiments, the transfected cells were plated on coverslips coated with poly-l-lysine (Sigma-Aldrich) in six-well plates. The cells were serum-starved 32 h later in phenol red-free DMEM + 0.1% BSA for 16 h, and then treated with ethanol (Veh), 10 nm E2, or 1 μm tamoxifen for 24 h in phenol red-free DMEM 5% SNCS media. 100 BrdU (μm) (Sigma) was added to the media for the last 4 h of the treatment. Cells on the coverslips were then fixed, permeabilized, and neutralized as described previously (23), and then incubated with anti-BrdU-Alexa Fluor 594 (Molecular Probes, Eugene, OR) for 1 h at 37 C. After the cells had been washed with PBS and water the coverslips were mounted on glass slides using Aqueous Mounting Medium with Anti-Fading Agents (Biomeda Corp., Foster City, CA) and Cytoseal (Richard-Allan Scientific, Kalamazoo, MI), dried overnight, and visualized using a Leica DM RBE Fluorescence microscope (model RS232C; Leica Corp., Deerfield, IL). GFP-positive cells were counted and scored for BrdU incorporation so that BrdU incorporation was measured only for the cells transfected with the siRNA or HA-tagged constructs. Approximately 150–300 cells were counted for each treatment group. The percent of BrdU incorporation was then calculated, and each experiment was repeated at least three times. Two-way ANOVA was used to determine statistical significance for all experiments in the manuscript unless otherwise stated.
Cell Counting Assays
MCF-7 cells were nucleofected with 1.5 μg of 20 μm control or STAT5b siRNA per reaction. Cells (4 × 106) were used per nucleofection reaction, and approximately 1.2 × 106 cells were plated in each well of a six-well plate (to compensate for cell death after nucleofection). The cells were serum-starved 24 h later in phenol red-free DMEM + 0.1% BSA for 16 h, and then treated with ethanol (Veh) or 10 nm E2 for 48 h in phenol red-free DMEM 5% SNCS media. Cells were then washed twice with PBS, collected in PBS, and stained with 0.4% Trypan blue (MP Biomedicals, Inc., Aurora, OH). The number of cells in each well was counted using a hemacytometer. Each treatment was performed in triplicate, and each experiment was repeated at least three times.
Luciferase Assays
MCF-7 and T47D cells were plated in six-well plates (Corning) in phenol red-free DMEM (Fisher) containing 5% SNCS plus 2 mm l-glutamine (Invitrogen) at a density of 2.5 × 105 or 3 × 105 cells per well, respectively. To monitor ER transcriptional activity, 0.5 μg/well of the pGL3-2ERE reporter was transfected the following day along with 0.5 μg/well of the empty vector pcDNA3.1. pGL3-2ERE contains two consensus EREs upstream of a prolactin TATA box and the firefly luciferase gene (63). To assess STAT5 transcriptional activity, 1 μg/well of pGL2-Spi2.1 reporter, which contains six copies of the STAT5-dependent γ-interferon-activated sequence-like element of the Spi2.1 gene (64), was transfected. To study the role of EGFR kinase activity, T47D cells were also transfected with either 50 ng/well of pcDNA3.1, wtEGFR, or the kinase-defective EGFR mutant K721A (previously described in Ref. 14). To study the role of c-Src kinase activity, T47D cells were transfected with either 0.5 μg/well of pcDNA3.1, kinase active (K+) c-Src, or kinase-defective (K−) c-Src (14). To investigate the effect of STAT5b siRNA on Spi2.1-luciferase activity, T47D cells were nucleofected with 3.5 μg of pGL2-Spi2.1 and 1.5 μg of 20 μm of control, STAT5b, or STAT3 siRNA per reaction. Cells (4 × 106 per nucleofection reaction) were used, and one reaction was added to five wells of a six-well plate. After overnight incubation the cells were washed with E2-free PBS and serum starved for 24 h in phenol red-free DMEM + 0.1% BSA, and then treated with phenol red-free DMEM 5% SNCS media containing either 10 nm E2 or ethanol, which served as its vehicle, for 24 h. For the Y699F studies, MCF-7 cells were nucleofected with pGL2-Spi2.1 and 20 μm of the control or STAT5b custom G3 siRNA oligo per reaction. Cells receiving STAT5b siRNA were simultaneously nucleofected with either the CMV-HA vector or one of two STAT5b constructs (iwtSTAT5b or iY669F) rendered immune to knockdown by siRNA. The immune constructs were made by introducing four silent point mutations in the STAT5b sequence within the G3 siRNA target sequence. For the inhibitor studies, T47D cells were pretreated for 1 h with 500 nm, 1 μm, or 5 μm AG1478 or its vehicle dimethylsulfoxide (DMSO), followed by treatment with ethanol or 10 nm E2 for 24 h. To determine the role of c-Src and ER, the cells were pretreated for 1 h with either 10 μm of the c-Src inhibitor PP2 or its vehicle DMSO. After a 24-h treatment with ethanol, 10 nm E2, 1 μm ICI or E2 + ICI, the cells were washed with PBS and collected in 250 μl of 1× Cell Culture Lysis Reagent (Promega Corp., Madison, WI). The lysates were then measured for luciferase activity using a Turner TD-20e luminometer (Molecular Dynamics, Inc., Sunnyvale, CA), and the luciferase values were normalized for total protein as determined by the Bio-Rad Protein Assay (Bio-Rad Laboratories, Inc., Hercules, CA). Each treatment was performed in triplicate, and each experiment was repeated at least three times. To validate the use of protein normalization and account for variances in transfection efficiency, real-time PCRs were conducted in 96-well plates using the iCycler iQ (Bio-Rad) as described previously (13) using primers specific for the luciferase gene and cell lysates from representative experiments. Luciferase values were normalized for the amount of transfected luciferase plasmid, and in all cases the results agreed with those obtained with protein normalization (Figs. 3B, 4D, 5, and 6D). All figures except Fig. 3B depict luciferase values normalized for total protein.
Immunoprecipitations and Immunoblotting
MCF-7 or MEF5−/− cells were plated in 6- or 10-cm dishes in DMEM 10% FBS media and then transiently transfected in DMEM with the indicated constructs. If applicable, the cells were serum starved overnight in phenol red-free DMEM containing 5% SNCS and 0.1% BSA and then treated at 37 C either with media alone (Veh), 100 ng/ml EGF for 5 or 15 min, or 10 nm E2 for 10 min. For the inhibitor studies, the cells were pretreated for 1 h at 37 C with either media alone (Veh), 1 μm ICI, or 10 μm PP2, followed by stimulation with 100 ng/ml EGF for 5 min or 10 nm E2 for 10 min. After treatment the cells were washed twice in cold PBS and lysed in radioimmune precipitation assay buffer plus a protease inhibitor cocktail (Calbiochem) and sodium orthovanadate. Lysates were incubated with the indicated antibody overnight at 4 C, and protein A agarose or protein G agarose (Santa Cruz) was added for an additional 1 h at 4 C. Agarose pellets were washed three times in the detergent lysis buffer, and the bound proteins were removed by boiling in 1× Laemmli buffer. Eluted proteins were then separated on a 7.5% polyacrylamide gel and electrophoretically transferred to nitrocellulose (Pall Corp., Pensacola, FL). Blocking buffers were made in TBS-T [0.15 m NaCl, 0.1% Tween 20, 50 mm Tris (pH 8.0)] and contained 3% BSA for the antiphosphotyrosine antibody or 5% nonfat dry milk for all other antibodies. Secondary antibodies were either donkey antirabbit (Amersham), sheep antimouse (Amersham), or goat antichicken (Chemicon) conjugated to horseradish peroxidase, and antibody binding was detected using the enhanced chemiluminescence detection kit (Amersham).
Real-Time RT-PCR
T47D cells were nucleofected with 2.5 μg of 20 μm control or STAT5b SMARTpool siRNA per reaction. Cells (5 × 106 per nucleofection reaction) were used, and one reaction was added to two wells of a four-well plate. The cells were serum-starved 44 h later in phenol red-free DMEM + 0.1% BSA for 24 h and then treated with ethanol or 10 nm E2 for 4 h in phenol red-free DMEM 5% SNCS media. For the inhibitor studies, T47D cells were pretreated for 1 h with 10 μm PP2, 1 μm AG1478, or their vehicle DMSO, followed by treatment with ethanol or 10 nm E2 for 4 h. The cells were then collected, the RNA was extracted using the RNeasy Mini kit (QIAGEN, Chatsworth, CA), 5 μl of RNA was reverse transcribed to cDNA using the iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA), and real-time PCRs were conducted in 96-well plates using the iCycler iQ (Bio-Rad) as described previously (13). Each cDNA was assayed in triplicate, and each experiment was repeated at least three times. iCycler iQ Optical System Software version 1.0 was used to obtain the threshold cycle values, which were normalized for actin mRNA. Variables for cyclin D1, c-Myc, and actin mRNA were the following: CD1 (95 C, 59 C, and 72 C for 30 sec) × 40 cycles; c-Myc (95 C, 67.5 C, and 72 C for 30 sec) × 40 cycles; actin (95 C for 30 sec and 60 C for 60 sec) × 40 cycles. The forward and reverse primers were as follows: Cyclin D1 forward (CTACACCGACAACTCCATCC) and cyclin D1 reverse (TGTTCTCCTCCGCCTCTG); c-Myc forward (GGCGCCCAGCGAGGATATCT) and c-Myc reverse (AAGCTGGAGGTGGAGCAGACG); actin forward (AGGTCATCACTATTGGCAACGA) and actin reverse (CACTTCATGATGGAATTGAATGTAGTT).
Supplementary Material
Acknowledgments
We thank Dr. Ian Macara for technical advice and helpful discussions, and Dr. J. Ihle for providing us with STAT5a/5b knockout mouse embryonic fibroblasts. We thank Elise Branch for technical support, Dr. George Amorino for the EGFR tyrosine kinase inhibitor AG1478, and Dr. Sarah J. Parsons for the c-Src 2–17 antibody. E.M.F. is a doctoral student in the laboratory of M.A.S.
Footnotes
This work was supported by the National Institutes of Health Grant DK57082 (to M.A.S.), grants from the Susan G. Komen Breast Cancer Foundation (BCTR0503476 to C.M.S.) and the Women’s Oncology Research Program of the University of Virginia Cancer Center (to M.A.S. and C.M.S.) and the University of Virginia Cancer Center Support Grant NCI-P30 CA44579.
Disclosure Summary: M.A.S. receives a stipend as an Endocrine Society officer and antibody royalties from Millipore Corp. C.M.S. receives antibody royalties from BD BioSciences. E.M.F., T.M.B., J.W., and A.M.W. have nothing to declare.
First Published Online June 11, 2008
Abbreviations: BrdU, Bromodeoxyuridine; CMV, cytomegalovirus; DMSO, dimethylsulfoxide; E2, 17β-estradiol; EGF, epidermal growth factor; EGFR, EGF receptor; ER, estrogen receptor; ERE, estrogen response element; GFP, green fluorescent protein; HA, hemagglutinin; ICI, ICI 182,780; iwt, immune wt; siCon, small interfering control; siRNA, small interfering RNA; siSTAT, small interfering STAT; SNCS, stripped newborn calf serum; STAT, signal transducer and activator of transcription; Veh, vehicle; wt, wild type.
References
- Herynk MH, Fuqua SA 2004 Estrogen receptor mutations in human disease. Endocr Rev 25:869–898 [DOI] [PubMed] [Google Scholar]
- McGuire WL 1975 Endocrine therapy of breast cancer. Annu Rev Med 26:353–363 [DOI] [PubMed] [Google Scholar]
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA 2001 Mechanisms of estrogen action. Physiol Rev 81:1535–1565 [DOI] [PubMed] [Google Scholar]
- Foster JS, Henley DC, Ahamed S, Wimalasena J 2001 Estrogens and cell-cycle regulation in breast cancer. Trends Endocrinol Metab 12:320–327 [DOI] [PubMed] [Google Scholar]
- Basu A, Rowan BG 2005 Genes related to estrogen action in reproduction and breast cancer. Front Biosci 10:2346–2372 [DOI] [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS 2001 The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 276:36869–36872 [DOI] [PubMed] [Google Scholar]
- Levin ER 2003 Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol 17:309–317 [DOI] [PubMed] [Google Scholar]
- Clarke R, Liu MC, Bouker KB, Gu Z, Lee RY, Zhu Y, Skaar TC, Gomez B, O'Brien K, Wang Y, Hilakivi-Clarke LA 2003 Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene 22:7316–7339 [DOI] [PubMed] [Google Scholar]
- Biscardi JS, Belsches AP, Parsons SJ 1998 Characterization of human epidermal growth factor receptor and c-Src interactions in human breast tumor cells. Mol Carcinog 21:261–272 [DOI] [PubMed] [Google Scholar]
- Belsches-Jablonski AP, Biscardi JS, Peavy DR, Tice DA, Romney DA, Parsons SJ 2001 Src family kinases and HER2 interactions in human breast cancer cell growth and survival. Oncogene 20:1465–1475 [DOI] [PubMed] [Google Scholar]
- Tice DA, Biscardi JS, Nickles AL, Parsons SJ 1999 Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci USA 96:1415–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ 1999 c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem 274:8335–8343 [DOI] [PubMed] [Google Scholar]
- Riggins RB, Thomas KS, Ta HQ, Wen J, Davis RJ, Schuh NR, Donelan SS, Owen KA, Gibson MA, Shupnik MA, Silva CM, Parsons SJ, Clarke R, Bouton AH 2006 Physical and functional interactions between Cas and c-Src induce tamoxifen resistance of breast cancer cells through pathways involving epidermal growth factor receptor and signal transducer and activator of transcription 5b. Cancer Res 66:7007–7015 [DOI] [PubMed] [Google Scholar]
- Kloth MT, Laughlin KK, Biscardi JS, Boerner JL, Parsons SJ, Silva CM 2003 STAT5b, a mediator of synergism between c-Src and the epidermal growth factor receptor. J Biol Chem 278:1671–1679 [DOI] [PubMed] [Google Scholar]
- Yu H, Jove R 2004 The STATs of cancer—new molecular targets come of age. Nat Rev Cancer 4:97–105 [DOI] [PubMed] [Google Scholar]
- Darnell Jr JE 1998 Studies of IFN-induced transcriptional activation uncover the Jak-Stat pathway. J Interferon Cytokine Res 18:549–554 [DOI] [PubMed] [Google Scholar]
- Bowman T, Garcia R, Turkson J, Jove R 2000 STATs in oncogenesis. Oncogene 19:2474–2488 [DOI] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Gouilleux F, Groner B, Henninghausen L 1995 Cloning and expression of STAT5 and an additional homologue (STAT5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci USA 92:8831–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva CM, Lu H, Day RN 1996 Characterization and cloning of STAT5 from IM-9 cells and its activation by growth hormone. Mol Endocrinol 10:508–518 [DOI] [PubMed] [Google Scholar]
- Lin JX, Mietz J, Modi WS, John S, Leonard WJ 1996 Cloning of human Stat5B. Reconstitution of interleukin-2-induced Stat5A and Stat5B DNA binding activity in COS-7 cells. J Biol Chem 271:10738–10744 [PubMed] [Google Scholar]
- Gouilleux F, Wakao H, Mundt M, Groner B 1994 Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J 13:4361–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloth MT, Catling AD, Silva CM 2002 Novel activation of STAT5b in response to epidermal growth factor. J Biol Chem 277:8693–8701 [DOI] [PubMed] [Google Scholar]
- Weaver AM, Silva CM 2006 Modulation of signal transducer and activator of transcription 5b activity in breast cancer cells by mutation of tyrosines within the transactivation domain. Mol Endocrinol 20:2392–2405 [DOI] [PubMed] [Google Scholar]
- Moriggl R, Gouilleux-Gruart V, Jahne R, Berchtold S, Gartmann C, Liu X, Hennighausen L, Sotiropoulos A, Groner B, Gouilleux F 1996 Deletion of the carboxyl-terminal transactivation domain of MGF-Stat5 results in sustained DNA binding and a dominant negative phenotype. Mol Cell Biol 16:5691–5700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Stravopodis D, Teglund S, Kitazawa J, Ihle JN 1996 Naturally occurring dominant negative variants of Stat5. Mol Cell Biol 16:6141–6148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CL, Meyer DJ, Campbell GS, Larner AC, Carter-Su C, Schwartz J, Jove R 1995 Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science 269:81–83 [DOI] [PubMed] [Google Scholar]
- Cao X, Tay A, Guy GR, Tan YH 1996 Activation and association of Stat3 with Src in v-Src-transformed cell lines. Mol Cell Biol 16:1595–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi P, Sharma S, Reddy EP 1997 Abrogation of interleukin-3 dependence of myeloid cells by the v-src oncogene requires SH2 and SH3 domains which specify activation of STATs. Mol Cell Biol 17:3295–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg JF, Horvath CM, Besser D, Lathem WW, Darnell Jr JE 1998 Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol 18:2553–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot RP, Jove R 1998 Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol 18:2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, Yu CL, Hudnall A, Catlett R, Nelson KL, Smithgall T, Fujita DJ, Ethier SP, Jove R 1997 Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ 8:1267–1276 [PubMed] [Google Scholar]
- Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, Cox CE, Falcone R, Fairclough R, Parsons S, Laudano A, Gazit A, Levitzki A, Kraker A, Jove R 2001 Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene 20:2499–2513 [DOI] [PubMed] [Google Scholar]
- Yamashita H, Iwase H, Toyama T, Fujii Y 2003 Naturally occurring dominant-negative Stat5 suppresses transcriptional activity of estrogen receptors and induces apoptosis in T47D breast cancer cells. Oncogene 22:1638–1652 [DOI] [PubMed] [Google Scholar]
- Xi S, Zhang Q, Gooding WE, Smithgall TE, Grandis JR 2003 Constitutive activation of Stat5b contributes to carcinogenesis in vivo. Cancer Res 63:6763–6771 [PubMed] [Google Scholar]
- Sultan AS, Xie J, LeBaron MJ, Ealley EL, Nevalainen MT, Rui H 2005 STAT5 promotes homotypic adhesion and inhibits invasive characteristics of human breast cancer cells. Oncogene 24:746–760 [DOI] [PubMed] [Google Scholar]
- Kazansky AV, Kabotyanski EB, Wyszomierski SL, Mancini MA, Rosen JM 1999 Differential effects of prolactin and src/abl kinases on the nuclear translocation of STAT5B and STAT5A. J Biol Chem 274:22484–22492 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Matsuda T, Junicho A, Kishi H, Saatcioglu F, Muraguchi A 2000 Cross-talk between signal transducer and activator of transcription 3 and estrogen receptor signaling. FEBS Lett 486:143–148 [DOI] [PubMed] [Google Scholar]
- Bjornstrom L, Kilic E, Norman M, Parker MG, Sjoberg M 2001 Cross-talk between Stat5b and estrogen receptor-α and -β in mammary epithelial cells. J Mol Endocrinol 27:93–106 [DOI] [PubMed] [Google Scholar]
- Faulds MH, Pettersson K, Gustafsson JA, Haldosen LA 2001 Cross-talk between ERs and signal transducer and activator of transcription 5 is E2 dependent and involves two functionally separate mechanisms. Mol Endocrinol 15:1929–1940 [DOI] [PubMed] [Google Scholar]
- Wang Y, Cheng CH 2004 ERα and STAT5a cross-talk: interaction through C-terminal portions of the proteins decreases STAT5a phosphorylation, nuclear translocation and DNA-binding. FEBS Lett 572:238–244 [DOI] [PubMed] [Google Scholar]
- Levitzki A, Gazit A 1995 Tyrosine kinase inhibition: an approach to drug development. Science 267:1782–1788 [DOI] [PubMed] [Google Scholar]
- Amorino GP, Deeble PD, Parsons SJ 2007 Neurotensin stimulates mitogenesis of prostate cancer cells through a novel c-Src/Stat5b pathway. Oncogene 26:745–756 [DOI] [PubMed] [Google Scholar]
- Fan P, Wang J, Santen RJ, Yue W 2007 Long-term treatment with tamoxifen facilitates translocation of estrogen receptor α out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res 67:1352–1360 [DOI] [PubMed] [Google Scholar]
- Golubovskaya V, Beviglia L, Xu LH, Earp III HS, Craven R, Cance W 2002 Dual inhibition of focal adhesion kinase and epidermal growth factor receptor pathways cooperatively induces death receptor-mediated apoptosis in human breast cancer cells. J Biol Chem 277:38978–38987 [DOI] [PubMed] [Google Scholar]
- Smit LS, Meyer DJ, Billestrup N, Norstedt G, Schwartz J, Carter-Su C 1996 The role of the growth hormone (GH) receptor and JAK1 and JAK2 kinases in the activation of Stats 1, 3, and 5 by GH. Mol Endocrinol 10:519–533 [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Pagano M, Auricchio F 1993 Immediate and transient stimulation of protein tyrosine phosphorylation by estradiol in MCF-7 cells. Oncogene 8:2183–2191 [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA 1996 Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem 271:695–701 [DOI] [PubMed] [Google Scholar]
- Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, Hortobagyi GN, Hung MC, Yu D 2004 PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6:117–127 [DOI] [PubMed] [Google Scholar]
- Silva CM, Shupnik MA 2007 Integration of steroid and growth factor pathways in breast cancer: focus on signal transducers and activators of transcription and their potential role in resistance. Mol Endocrinol 21:1499–1512 [DOI] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M 2002 Signal transducers and activators of transcription as downstream targets of nongenomic estrogen receptor actions. Mol Endocrinol 16:2202–2214 [DOI] [PubMed] [Google Scholar]
- Lange CA, Richer JK, Shen T, Horwitz KB 1998 Convergence of progesterone and epidermal growth factor signaling in breast cancer. Potentiation of mitogen-activated protein kinase pathways. J Biol Chem 273:31308–31316 [DOI] [PubMed] [Google Scholar]
- Richer JK, Lange CA, Manning NG, Owen G, Powell R, Horwitz KB 1998 Convergence of progesterone with growth factor and cytokine signaling in breast cancer. Progesterone receptors regulate signal transducers and activators of transcription expression and activity. J Biol Chem 273:31317–31326 [DOI] [PubMed] [Google Scholar]
- Stocklin E, Wissler M, Gouilleux F, Groner B 1996 Functional interactions between Stat5 and the glucocorticoid receptor. Nature 383:726–728 [DOI] [PubMed] [Google Scholar]
- Boerner JL, Gibson MA, Fox EM, Posner ED, Parsons SJ, Silva CM, Shupnik MA 2005 Estrogen negatively regulates epidermal growth factor (EGF)-mediated signal transducer and activator of transcription 5 signaling in human EGF family receptor-overexpressing breast cancer cells. Mol Endocrinol 19:2660–2670 [DOI] [PubMed] [Google Scholar]
- Yamashita H, Nishio M, Fujii Y, Iwase H 2004 Dominant-negative Stat5 inhibits growth and induces apoptosis in T47D-derived tumors in nude mice. Cancer Sci 95:662–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitosugi T, Sasaki K, Sato M, Suzuki Y, Umezawa Y 2007 Epidermal growth factor directs sex-specific steroid signaling through Src activation. J Biol Chem 282:10697–10706 [DOI] [PubMed] [Google Scholar]
- Castoria G, Barone MV, Di Domenico MD, Bilancio A, Ametrano D, Migliaccio A, Auricchio F 1999 Non-transcriptional action of oestradiol and progestin triggers DNA synthesis. EMBO J 18:2500–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shupnik MA 2004 Crosstalk between steroid receptors and the c-Src-receptor tyrosine kinase pathways: implications for cell proliferation. Oncogene 23:7979–7989 [DOI] [PubMed] [Google Scholar]
- Knowlden JM, Hutcheson IR, Jones HE, Madden T, Gee JM, Harper ME, Barrow D, Wakeling AE, Nicholson RI 2003 Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology 144:1032–1044 [DOI] [PubMed] [Google Scholar]
- Brinkman A, van der Flier S, Kok EM, Dorssers LC 2000 BCAR1, a human homologue of the adapter protein p130Cas, and antiestrogen resistance in breast cancer cells. J Natl Cancer Inst 92:112–120 [DOI] [PubMed] [Google Scholar]
- van der Flier S, Brinkman A, Look MP, Kok EM, Meijer-van Gelder ME, Klijn JG, Dorssers LC, Foekens JA 2000 Bcar1/p130Cas protein and primary breast cancer: prognosis and response to tamoxifen treatment. J Natl Cancer Inst 92:120–127 [DOI] [PubMed] [Google Scholar]
- Chu I, Arnaout A, Loiseau S, Sun J, Seth A, McMahon C, Chun K, Hennessy B, Mills GB, Nawaz Z, Slingerland JM 2007 Src promotes estrogen-dependent estrogen receptor α proteolysis in human breast cancer. J Clin Invest 117:2205–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreihofer DA, Resnick EM, Lin VY, Shupnik MA 2001 Ligand-independent activation of pituitary ER: dependence on PKA-stimulated pathways. Endocrinology 142:3361–3368 [DOI] [PubMed] [Google Scholar]
- Wood TJ, Sliva D, Lobie PE, Goullieux F, Mui AL, Groner B, Norstedt G, Haldosen LA 1997 Specificity of transcription enhancement via the STAT responsive element in the serine protease inhibitor 2.1 promoter. Mol Cell Endocrinol 130:69–81 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.