Abstract
Projections from the cochlear nuclear complex to the inferior colliculus in the gerbil (Meriones unguiculatus) were studied using anterograde tracing methods based on axonal transport. Methods were developed to map the results onto comparable sets of sections through the inferior colliculus so that the patterns of termination in different animals could be compared directly. Projections to the contralateral inferior colliculus are widespread and most, if not all of them, are topographically organized. Axons terminate throughout the central nucleus and also in at least three distinct regions outside the central nucleus: a caudomedial region in the dorsal cortex, the ventrolateral nucleus and the rostral pole nucleus. Projections from the dorsal and ventral cochlear nuclei appear to overlap almost completely, although those from the dorsal cochlear nucleus may be slightly more widespread at the boundaries of the central nucleus. Projections from the ipsilateral cochlear nuclei arise in both the dorsal and ventral divisions and are largely restricted to the dorsal (low-frequency) part of the inferior colliculus. In this region, the pattern of ipsilateral and contralateral projections is similar, although the terminal fields from the two sides do not appear to overlap completely. The methods developed to display the results form a framework for comparisons with the distribution of inputs from the other major sources of input to the inferior colliculus.
Keywords: auditory pathways, superior olivary complex, neuroanatomy, biotinylated dextran amine
The inferior colliculus (IC) is an important site of integration and transformation of auditory information (e.g., Ehret, 1997; Casseday et al., 2002; Oliver, 2005). Multiple sources of ascending, descending and commissural input converge in the IC (reviewed by Cant, 2005; Schofield, 2005; Saldaña and Merchán, 2005; Winer, 2005), although the terminal fields formed by the major sources of input do not overlap completely. Ascending projections from the cochlear nuclei and the lateral and medial superior olivary nuclei terminate mainly in the central nucleus (CNIC), but the projections of axons from the cochlear nuclei are more widespread than are those from the superior olive (Henkel and Spangler, 1983; Oliver et al., 1997; Loftus et al., 2004; Cant and Benson, 2006). Commissural and descending projections partially overlap the ascending projections, but they also terminate extensively outside the CNIC (e.g., Saldaña and Merchán, 1992; Saldaña et al., 1996; Winer et al., 1998). Interpretation of physiological results in terms of circuitry depends on knowing the extent to which the different sources of input to the IC converge to form synapses on specific populations of cells (cf. Oliver, 2005). One essential step in acquiring the necessary information is to determine the spatial extent of the terminal fields formed by each source of input within the IC, as this will help to delimit the potential sphere of influence of any given projection.
We have undertaken a series of studies of the gerbil IC with the long-term goal of understanding the termination patterns of its major sources of input. In the present study, we addressed three issues related to the organization of inputs from the cochlear nucleus. First, we compared the distribution of the inputs from the three main divisions of the cochlear nucleus and determined, to the extent possible with our methods, their degree of overlap in the IC. Second, we compared the distribution of the terminal fields formed by ipsilateral and contralateral projections. Finally, we evaluated the degree to which the boundaries of the cochlear nucleus projection zone match the boundaries of the central nucleus of the IC in the gerbil, as defined in a previous study (Cant and Benson, 2006).
EXPERIMENTAL PROCEDURES
Animals
Female gerbils (Meriones unguiculatus) were obtained from Charles River Laboratories when they were approximately eight weeks old and were housed in Duke University animal facilities until use. Procedures involving animals were in compliance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Duke University Institutional Animal Care and Use Committee. All efforts were made to minimize the suffering of the animals under our care and to use as few animals as possible compatible with the goals of the study.
Surgery and histology
Twelve gerbils, aged 9 to 13 weeks at the time of surgery, were anesthetized with sodium pentobarbital (Nembutal; 50–70 mg/kg, i.p.). In 11 of the gerbils, an incision was made in the scalp and a small hole was opened in the skull overlying the right cerebellum. Glass micropipettes (20 – 30 µm inside tip diameter) containing 10% biotinylated dextran amine (BDA; MW 10,000; Molecular Probes, Inc. D-1956) in saline were lowered through the cerebellum into the cochlear nucleus. In one gerbil, an incision was made behind the left pinna, a small hole was made in the temporal bone, and the pipette was advanced into the anteroventral cochlear nucleus through the paraflocculus (Frisina et al., 1982). Multiple injections (usually three) of BDA were made iontophoretically (5 µA positive current pulses, 7 seconds on / 7 seconds off for 10 – 12 minutes) at slightly different depths in the cochlear nucleus. Following surgery, the skin was sutured and the gerbils were allowed to recover.
After a 7 – 10 day survival period, animals received an overdose of Nembutal (>100 mg/kg, i.p.). When reflexes were absent and breathing had just ceased, the animals were perfused through the heart with a rinse of 0.1 M phosphate buffer (pH 7.4) followed by a fixative of 4% paraformaldehyde in the same buffer. The brains were removed the next day and stored in 30% sucrose in phosphate buffer at 4°C. One to four days later, frozen sections 40 µm thick were cut in the horizontal plane on a sliding microtome and collected in phosphate buffer in two alternating series. One series was reacted for BDA using avidin-biotin-peroxidase histochemistry (ABC Elite Kit, Vector Laboratories, Burlingame, CA) with metal enhancement (Adams, 1981). The second series of sections was reacted for cytochrome oxidase (Wong-Riley, 1979). Sections were mounted on glass slides, allowed to dry overnight, dehydrated in a series of alcohols, cleared in xylene and sealed under coverslips with Permount (Fisher Scientific).
Data analysis
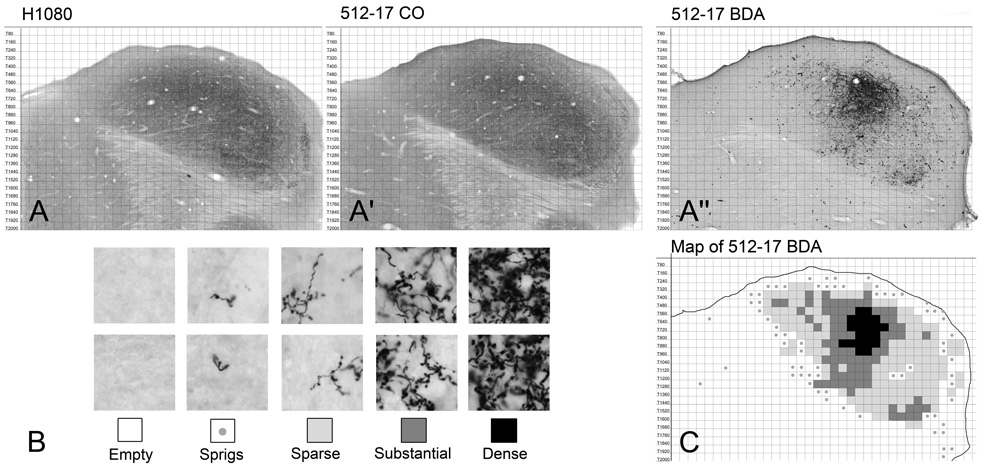
A Zeiss AxioCam HRc digital camera attached to a Zeiss AxioSkop 2 compound microscope and controlled by Zeiss AxioVision software was used to acquire digital images. Every BDA-reacted section containing labeled axons in the inferior colliculus and every adjacent CO-reacted section were photographed. The digital images were imported into Adobe Photoshop CS for subsequent analysis. We used an atlas of the gerbil IC (Cant and Benson, 2005) to develop a method of data collection that allowed direct comparisons of the patterns of terminal labeling in comparable sections from different cases (Fig. 1). First, the CO sections from each case were matched to the most closely corresponding atlas sections (Fig. 1A, A’). Sections were re-sized if necessary, but all sections in any given case were changed uniformly and by the same amount. The BDA sections were then re-sized to match the adjacent CO sections (and, consequently, the atlas CO sections), allowing the atlas grid to be superimposed onto the experimental material (Fig. 1A”). The atlas grid was then used as a framework to reduce the data to a form that could be easily compared across cases (Figure 1B, C). The extent of labeling in each grid square was scored according to the criteria illustrated in Figure 1B. The gerbil IC is approximately 2.2 mm in its dorsal-to-ventral extent, so each case included approximately 27 BDA-reacted sections. For each animal, thirteen of these sections at evenly spaced levels were included in the analysis.
Figure 1.
Methods used to represent the distribution and density of terminal labeling. A-A”. Horizontal sections through the approximate middle of the IC. A. Section H1080 from an IC atlas (Cant and Benson, 2005) reacted for cytochrome oxidase. A’. Comparable CO-reacted section through the IC in case 512. A”. BDA-reacted section adjacent to the CO section in panel A’. B. Criteria used to summarize the extent of terminal label. Each grid square that contained labeled elements was scored as follows: sprigs, no more than one piece of an axon or questionable whether labeling was present; sparse, only a few axons or terminals present. The two examples represent the maximum amount of labeling that would have received this score. This designation was also used for grid squares that contained only axons and no terminal labeling (e.g., in the lateral lemniscus); substantial, labeled terminals cover a large portion of the grid square; dense, labeled terminals fill the grid square making individual boutons difficult or impossible to resolve. C. Map for the section shown in panel A”. Grid squares are filled according to the density of labeling as defined in panel B.
RESULTS
The cochlear nuclei project widely in the brainstem (reviewed, e.g., by Cant and Benson, 2003), and, depending on the location of the injections, many or all of the projections were labeled through anterograde axonal transport. Only the projections from the cochlear nuclei to the IC are described in this report.
Injection sites
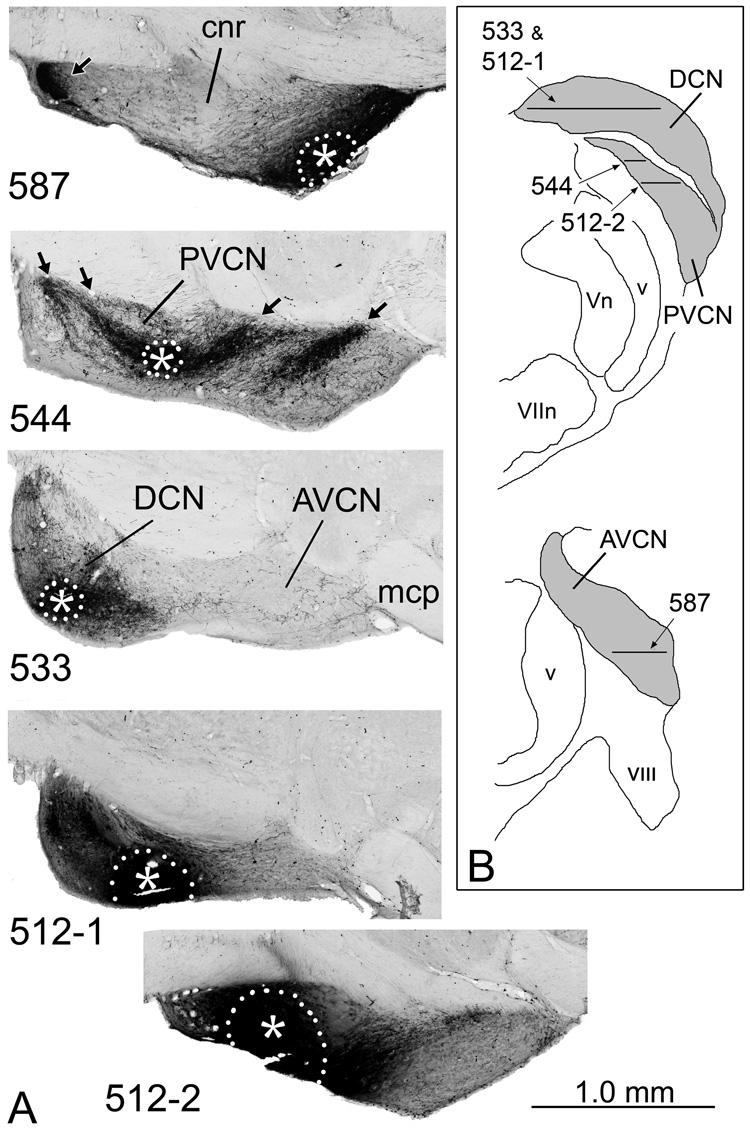
The cases fell roughly into four groups, depending on which subdivisions were included in the injections (Table 1; Fig. 2). In two cases (e.g., Fig. 2, case 587), the injections were located in the AVCN. In three cases (e.g., Fig. 2, case 544) the injections were located in the PVCN. In five cases (e.g., Fig. 2, case 533), the injections were confined entirely or almost entirely to the DCN, although neurons in the VCN that project to the IC could also be labeled by these injections (see discussion). In cases 512 (Fig. 2) and 513, distinct injection sites were present in both the DCN and PVCN.
Table 1.
Summary of injection sites
| Case Number | Subdivision with injection site(s) | Location of injection site (Dorsal, middle or ventral) |
|---|---|---|
| 587 | AVCN | Ventral |
| 564 | AVCN | Dorsal |
| 5341 | PVCN, PVCN | Ventral & Middle, Dorsal |
| 544 | PVCN | Middle |
| 570 | PVCN | Middle |
| 5662 | DCN | Ventral |
| 533 | DCN | Ventral |
| 590 | DCN | Middle |
| 594 | DCN | Dorsal |
| 589 | DCN | Dorsal |
| 5121, 2 | DCN, PVCN | Ventral, Middle |
| 5131 | DCN, PVCN | Ventral, Middle |
In these three cases, the pattern of terminal labeling led to the interpretation that there were injections in two separable locations within the cochlear nucleus (discussed further in text).
In all cases with injections in the DCN, labeled neurons were present in the VCN. These could have contributed to terminal labeling in the IC (see text).
Figure 2.
A. BDA injection sites in the cochlear nucleus in four representative cases. The injection site in 587 was confined to the AVCN; that in 544, to the PVCN; and that in 533, to the DCN. In 512, distinct injection sites were located in both the DCN (512-1) and PVCN (512-2). The approximate dorsal-to-ventral position of each section is indicated on the drawings in panel B. The injection sites, which appear solid black because of accumulation of the BDA reaction product, are outlined by white dots with the approximate center indicated by asterisks. Terminal label in other subdivisions is evident (arrows) because the tracer is taken up by interneurons and auditory nerve fibers and transported to other parts of the complex. In this and subsequent figures, illustrations of sections through case 587, in which the injection site was on the left side, are rotated about the midline for easier comparisons to the other cases, in which the injection sites were on the right. In these horizontal sections, rostral is toward the right and medial is toward the top. The figure was assembled in Adobe Photoshop. Brightness and contrast were adjusted globally and background elements outside the brainstem were erased. B. Drawings of transverse sections through the caudal (top) and rostral (bottom) cochlear nuclear complex (indicated by gray fill) to illustrate the positions of the sections shown in panel A. Lateral is toward the left; dorsal is toward the top.
The apparent injection site was not confined to the cochlear nucleus in three cases (564, 589 and 594), but also included parts of the vestibular complex, the spinal tract and nucleus of the trigeminal nerve and/or the restiform body. Of these regions, only the trigeminal nucleus projects to the IC, a projection restricted to the external cortex (Zhou and Shore, 2006). The possibility that labeling in the external cortex can be accounted for by projections from somatosensory nuclei was taken into account in analyzing the cases. Injections in two cases (Table 1, cases 570 and 590) resulted in only limited labeling in the IC; they are not included in the data analysis.
Terminal labeling in the contralateral inferior colliculus
BDA injections in the cochlear nucleus resulted in Golgi-like labeling of axons and terminals in the contralateral IC. In every case, the labeling was quite dense in some areas and relatively sparse or absent in others (e.g., Fig. 1A”, B). The areas of dense labeling contained numerous axons and terminals of various sizes. A comprehensive description of the terminal morphology is beyond the scope of this report, but in general, it was the same as that described in other species (e.g., Malmierca et al., 2005).
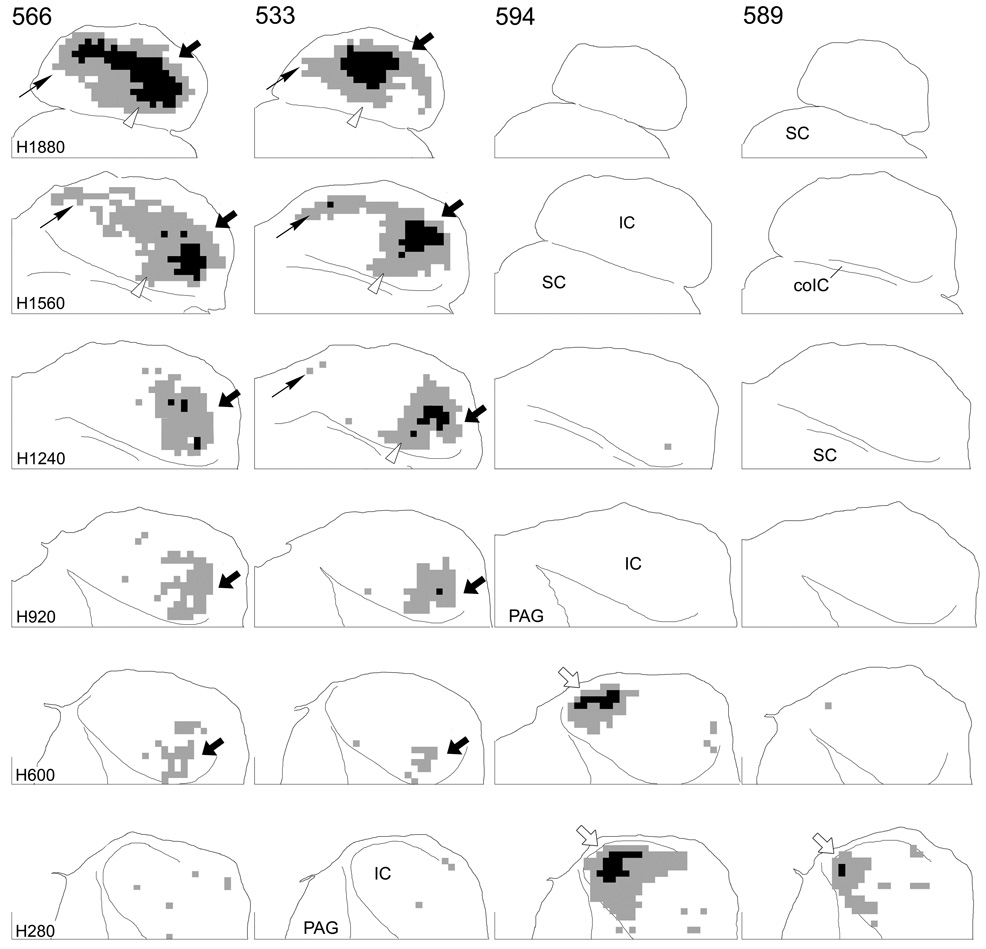
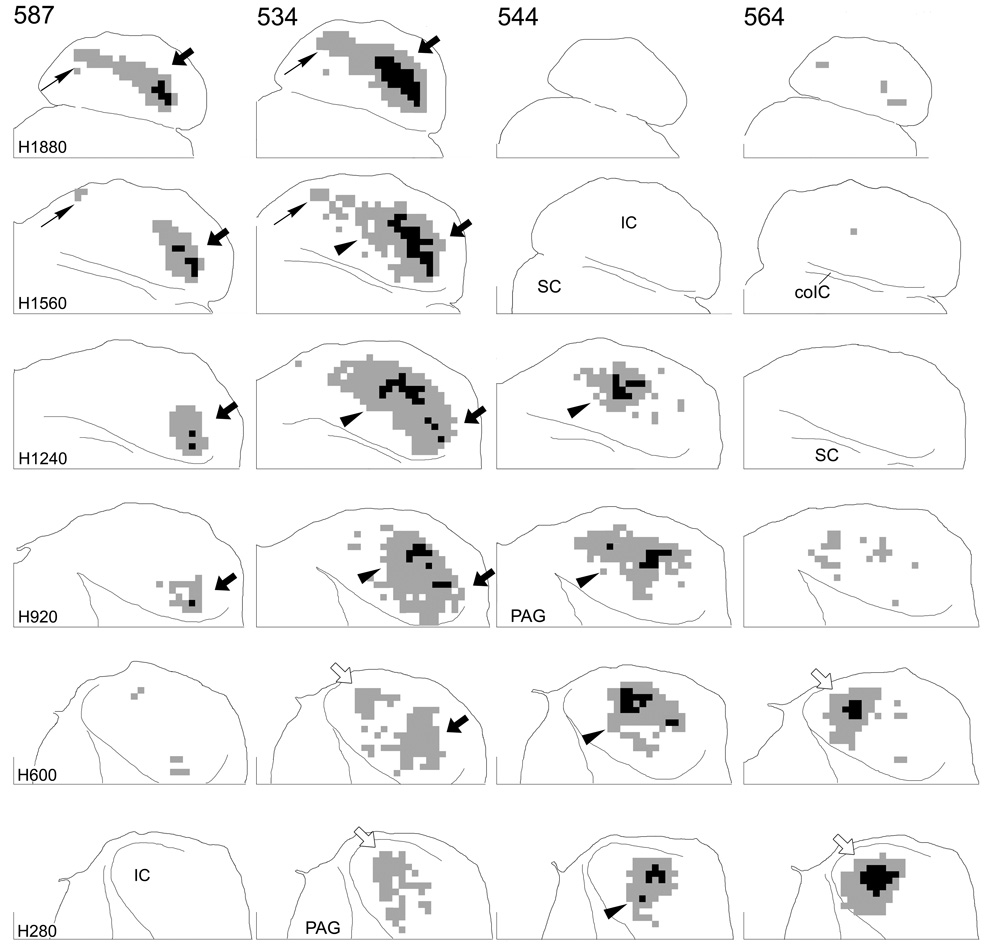
Results from nine cases are illustrated in Figure 3–Figure 5. Terminal labeling in the contralateral IC after injections in the DCN (Fig. 3) or VCN (Fig. 4) is plotted in six horizontal IC sections matched to the indicated atlas sections. As expected, the location of labeled terminal fields depended on the location of the injection sites. Injections in the ventral part of either the DCN (Fig. 3, cases 566 and 533) or VCN (Fig. 4, case 587) gave rise to extensive labeling in the dorsal part of the IC. At the most dorsal levels in these cases (Fig. 3, Fig. 4, level H1880, arrows), labeled terminals spread throughout most of the IC. At more ventral levels, this labeled field began to break up into two ‘patches’ of terminals, one located in the rostrolateral part of the IC (Fig. 3, Fig. 4, H1560, large arrows) and the other located in the caudomedial IC (Fig. 3, 4, H1560, thin arrows). The rostrolateral field was continuous into relatively ventral levels (Fig. 3, Fig. 4, H1560, H920, H600, arrows), but the caudomedial field was confined to dorsal levels. In contrast, when the injections were located in the middle part of the VCN (none of the injections encroached significantly into the middle DCN), the region between the two patches of label seen with the ventral injections was filled in with terminals (Fig. 4, case 544, arrowheads). In case 534, the injection site included both ventral and middle parts of the PVCN and, consequently, the labeled terminal field is continuous at dorsal levels including the rostrolateral (large arrows), caudomedial (thin arrows) and middle (arrowheads) aspects of the IC. Finally, injections in the dorsal part of the DCN or VCN resulted in terminal labeling in the most ventral aspect of the IC (Fig. 3, cases 589 and 594; Fig. 4, case 564, open arrows). Labeled axons in the ventral IC in case 534 (Fig. 4, levels H600 and H280, open arrows) suggests that the injection in this case also included some cells in the most dorsal part of the PVCN.
Figure 3.
Representations of terminal label in four cases in which the BDA injection was confined to the DCN. Each column contains sections through the IC at levels comparable to the levels of the atlas sections indicated at the left of each row. Grid squares that contained “dense” accumulations of terminals are colored black and grid squares that contained “substantial” accumulations of terminals are colored gray (see Fig. 1). Areas with sparse terminal label are not shown. For all sections, the caudal direction is toward the top; the lateral direction is toward the right. The midline is indicated by the line at the medial edge of each section. The line at the bottom of each section indicates transverse level T1680 in the IC atlas. Black arrows (both thick and thin) indicate terminal fields arising from injections in the ventral part of the cochlear nucleus; open arrows indicate terminal fields arising from injections in the dorsal part of the cochlear nucleus; arrows are referenced where appropriate in the text.
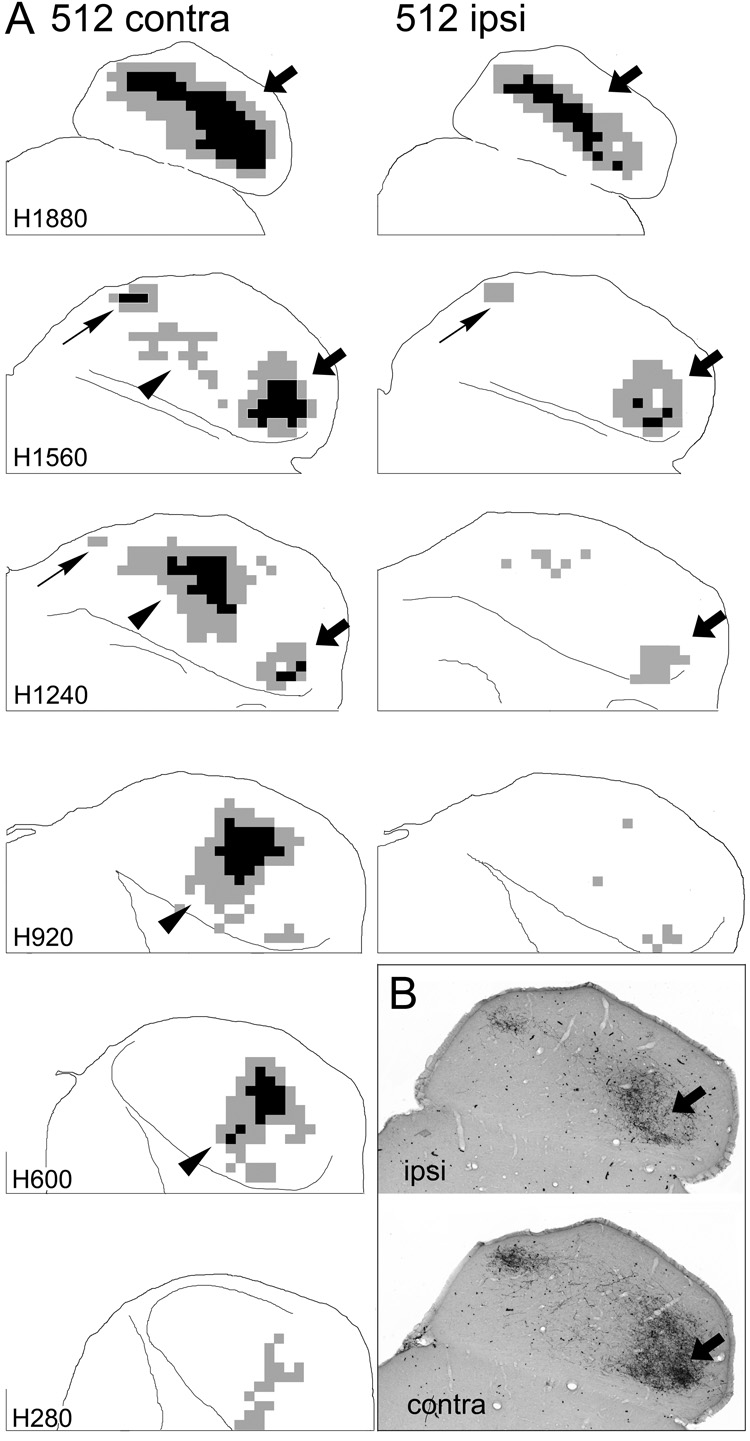
Figure 5.
Representation of terminal label in the ipsilateral (ipsi) and contralateral (contra) inferior colliculi in one case in which distinct injection sites were located in both the DCN and PVCN. A. Sections from the two sides are arranged as in Figure 3 and Figure 4 except that the most ventral levels are not shown for the ipsilateral IC. (Levels more ventral than those shown contained very little ipsilateral labeling.) The sections through the ipsilateral IC were rotated about the midline so that the results from the two sides are more easily compared. Arrows indicate patches of labeled elements as in Figure 3 and Figure 4 and are referenced where appropriate in the text. B. Digital images at one horizontal level (comparable to atlas level H1720) through the IC in the same case shown in panel A. The arrows indicate a comparable area on the two sides that exhibits heavy terminal labeling contralaterally but not ipsilaterally.
Figure 4.
Representations of terminal label in four cases in which the injection site was confined to the VCN. Arrangement of the figure is as for Figure 3; arrowheads indicate terminal fields arising from injections in the middle part of the cochlear nucleus.
In every case, a region of dense terminal labeling was surrounded by regions of substantial terminal labeling (Fig. 3, Fig. 4, black squares vs. gray squares). The area of substantial label was, in turn, generally surrounded by regions of sparse label (not shown), which consisted of both axons and terminals or only axons. (The patterns of sparse labeling do not alter the conclusions reached on the basis of the data presented here.) The region of densest labeling was restricted to relatively lateral or caudal positions in the terminal field in almost all sections.
For the most part, the distribution pattern of projections after injections in the DCN (Fig. 3) was very similar to that when injections were confined to the VCN (Fig. 4). However, especially with injections in the ventral DCN vs. the ventral VCN (compare cases 566 and 533 [DCN] to cases 587 and 534 [VCN]), projections arising in the DCN appeared to spread somewhat more rostrally (Fig. 3, cases 566, 533, open arrowheads) and also perhaps more laterally as well.
Terminal labeling in the ipsilateral inferior colliculus
Significant terminal labeling was observed in the ipsilateral IC only in those cases in which ventral parts of either the DCN or VCN were included in the injection site. Comparison of the two sides in case 512 (Fig. 5), in which there were distinct injections in the ventral DCN and in the middle VCN, illustrates the difference between ipsilateral and contralateral projection fields with injections in these two locations. At dorsal levels, the termination patterns of axons arising from the injection in the ventral part of the DCN were similar on both sides of the IC (Fig. 5, levels H1880, H1560, arrows), although the ipsilateral terminal field did not appear to be as widespread. In more ventral sections, however, a prominent contralateral terminal field in the middle of the IC arising from the injection in the middle of the VCN (e.g., H1240, H920, contra, arrowheads) was not matched by a comparable terminal zone on the ipsilateral side. Ipsilateral labeling was present in all cases with injections in the ventral parts of the cochlear nucleus but quite sparse (although not completely absent) in all cases with injections confined to middle or dorsal parts of the cochlear nucleus.
Although the overall pattern of labeling on the two sides in case 512 was similar in the dorsal and rostrolateral (low-frequency) IC, the terminal fields overlapped only partially (Fig. 5B). Restricted areas containing dense accumulations of terminals on the contralateral side contained only scattered terminals on the ipsilateral side (Fig. 5B, arrows). The converse also appeared to be true although dense patches of labeled terminals on the ipsilateral side were considerably smaller than those on the contralateral side. A similar pattern was seen in all cases with injections in the ventral parts of the cochlear nucleus.
Distribution of terminal fields arising from the cochlear nucleus with respect to the major subdivisions of the IC
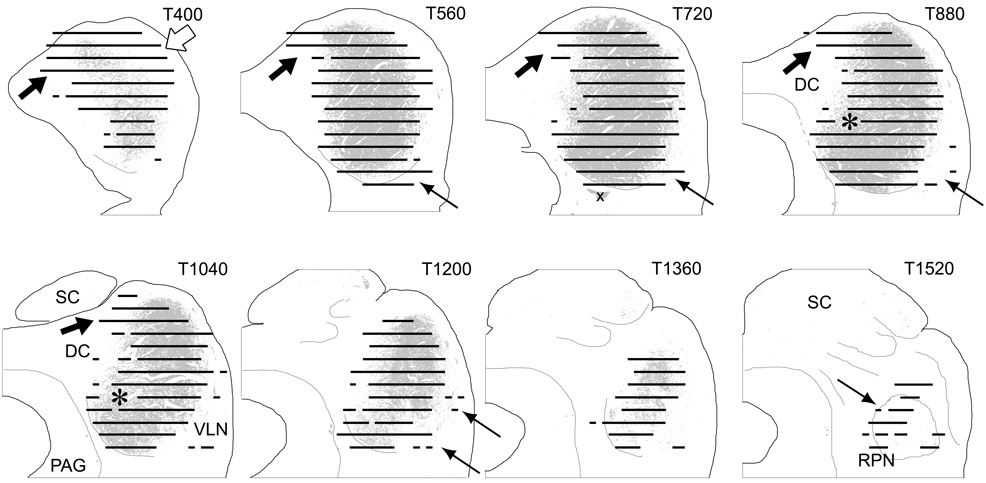
To determine the extent to which the terminal field formed by projections from the cochlear nuclei was congruent with the CNIC as defined in a previous study in the gerbil (Cant and Benson, 2006), we pooled the data from all of the cases and plotted them on sections from the IC atlas. In Figure 6, the summary data are plotted on transverse atlas sections with the CNIC depicted in gray fill. Even with the inaccuracy introduced by pooling the data, the distribution of the terminal fields from the cochlear nucleus matches the area defined as the central nucleus remarkably well. Only a small region in the ventral half of the IC remained unlabeled (Fig. 6, asterisks on levels T880 and T1040). The results also indicate, however, that there are three areas where the cochlear nuclear projections (from both VCN and DCN) extend beyond the boundaries of the CNIC. First, terminations lie well beyond the boundaries of the CNIC in the caudal IC (e.g., Fig. 6, level T400, arrows) and extend rostrally into the dorsomedial part of the dorsal cortex (Fig. 6, black arrows, levels T400–T1040). A second region targeted by the cochlear nucleus appears to be equivalent to the ventrolateral nucleus (VLN) of the lateral IC (Loftus et al., 2008). Terminal labeling in the VLN was seen in most cases (Fig. 6, upward pointing arrows, levels T560–T880, T1200), although it was generally sparse and only rarely rose to the level of “substantial.” Finally, a small region located in the most rostral part of the ventral IC contained labeled terminals (Fig. 6, level T1520, arrow). This region is presumably equivalent to the region identified as the rostral pole nucleus in the cat (Morest and Oliver, 1984). Only sparse terminations were present in the outer layers of the lateral cortex or in the ventral parts of the dorsal cortex in any of the cases.
Figure 6.
Representation of the terminal field formed by projections from the cochlear nuclei with respect to the boundaries of the CNIC. Results from ten cases were pooled and plotted onto atlas sections cut in the transverse plane. Sections from the IC atlas are arranged with the most caudal section (T400) in the upper left panel and the most rostral section (T1520) in the lower right panel. Areas occupied by “dense” or “substantial” label in at least one of the cases are depicted by the black lines drawn at each of the 13 horizontal levels included in the analysis. The CNIC is indicated by the gray fill on each section. Arrows indicate areas outside the CNIC that contain labeled terminals; asterisks indicate a small area within the CNIC that did not contain substantial labeling in any of the cases. The x on level T720 indicates a staining artifact.
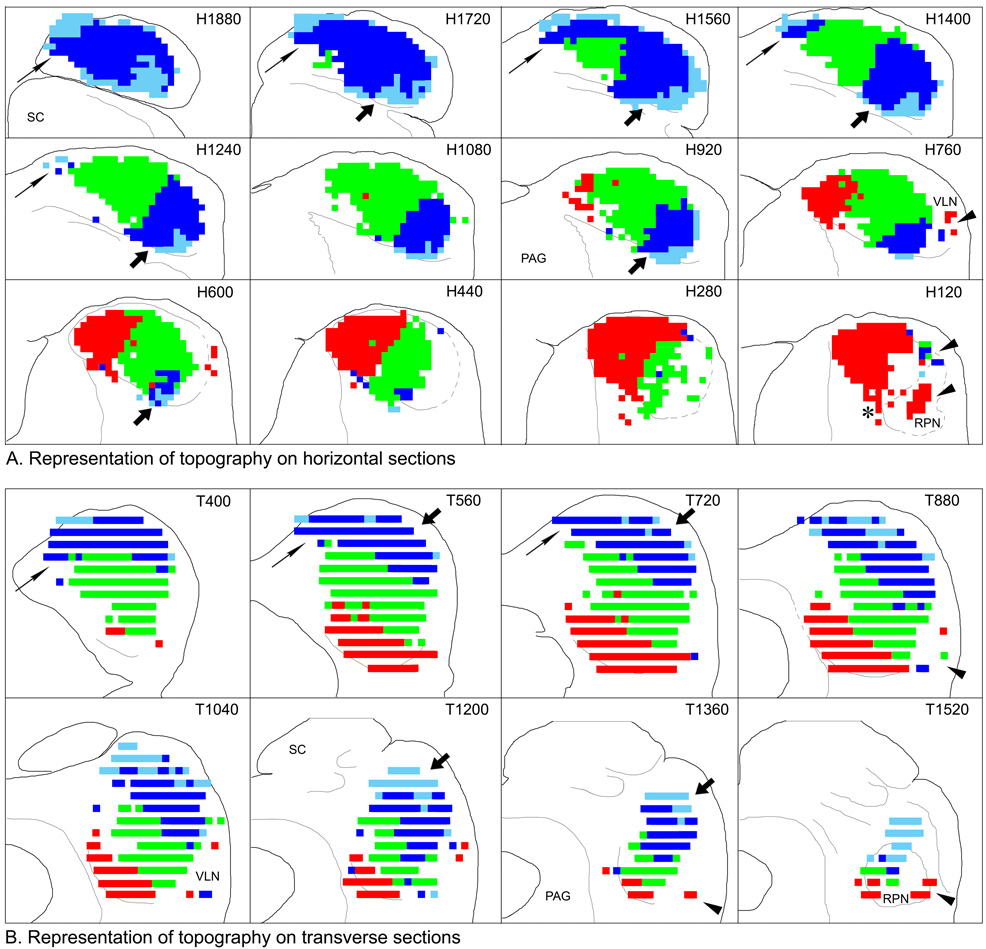
Topography of the projections
As illustrated in Figure 3–Figure 5, each injection gave rise to labeled terminals within a restricted part of the IC. In Figure 7, results from ten cases have been pooled and replotted on both horizontal (Fig. 7A) and transverse (Fig. 7B) atlas sections to illustrate the topographic progression with different injection locations in the cochlear nuclei. Terminal fields from individual cases and combinations of cases have been color-coded to distinguish areas innervated in cases with injections in different locations. In this summary, dark blue represents the labeled terminal field in case 533. The injection in this case was centered in the ventral DCN, but it did not extend into its most ventral part. Consequently, the labeled axons and terminals were distributed throughout most of the dorsal IC but did not extend as far dorsally (not shown), caudally or rostrally as those from cases 566, 587, 534 and those arising from the DCN injections in cases 512 and 513. Areas of terminal labeling that did not overlap that in 533 in these cases is colored light blue. The location of terminal fields from cases with injections in the most dorsal part of the DCN (589, 594) or VCN (564) is colored in red. The green color represents the location of labeling in cases 544, 534, and the component of labeling in 512 and 513 attributed to injections in the PVCN where they did not overlap the other areas. (There was considerable overlap among the cases, and they could be combined in a number of ways, but the essential result remained the same.) The topographic organization of the terminations from the cochlear nuclei is related to the tonotopic organization of the IC as discussed below.
Figure 7.
Representation of the topographic (and presumed tonotopic) organization of the projections from the cochlear nucleus to the inferior colliculus. A. Topographic organization in the horizontal plane plotted on the IC atlas. The most dorsal section (H1880) is in the upper left panel; the most ventral section (H120) is in the lower right panel. Caudal is toward the top; lateral is toward the right. B. The data plotted in panel A re-plotted onto transverse sections from the IC atlas. The most caudal section (T400) is in the upper left panel; the most rostral section (T1520) is in the lower right panel. Dorsal is toward the top; lateral is toward the right. The maps were color-coded based on the distribution of terminal label as described in the text. Only dense and substantial labeling were considered in constructing the maps. Symbols are referenced where appropriate in the discussion.
DISCUSSION
Thirty-five years ago, Osen (1972) published a comprehensive neuroanatomical study of the projections from the cochlear nuclei to the inferior colliculus. Using degeneration methods in the cat, she demonstrated that both the dorsal and ventral cochlear nuclei project to the contralateral IC topographically and that the terminations from these two subdivisions appear to overlap throughout the central nucleus. Subsequent studies of the projections using both light and electron microscopic techniques were summarized in a recent review (Cant, 2005) and will not be repeated here. The intrinsic neuronal organization of the IC has been reviewed by Oliver (2005); many of the issues alluded to in the following discussion are examined in more detail there. The main points considered here are related to specific issues mentioned in the introduction.
Projections from the different divisions of the cochlear nuclear complex give rise to overlapping terminal fields
In a recent study with restricted tracer injections located throughout the IC (Cant and Benson, 2006), all three main divisions of the cochlear nuclear complex (DCN, PVCN and AVCN) contained retrogradely labeled cells in 73 of 74 cases in which the injection was centered in some part of the central nucleus. The anterograde results reported here support the conclusion that the inputs from the dorsal and ventral divisions of the cochlear nucleus overlap almost completely throughout the central nucleus. In several of the cases with injections confined to the DCN, however, the terminal fields do seem to spread slightly more widely with respect to the boundaries of the central nucleus than do those from the VCN. Possibly related to this finding, the proportion of neurons labeled in the DCN relative to neurons labeled in the VCN increased when retrograde tracer injections were located near the margins of the central nucleus (Cant and Benson, 2006). The cases in the present study did not include injections in the middle of the DCN. If the suggestion that the terminal field formed by DCN axons is more widespread than that of the VCN, it is possible that projections from the middle DCN would fill in the gap in terminal labeling near the medial boundary of the CNIC apparent in the drawings in Figure 6 (i.e., locations indicated by asterisks).
With the BDA method, we could not address the question whether there are areas within the central nucleus that receive inputs from the VCN but not from the DCN. Neurons whose axons enter an injection site may transport BDA retrogradely and then anterogradely through collaterals that arise from those neurons (Chen and Aston-Jones, 1998; Doucet and Ryugo, 2003). Because neurons in the VCN that project to the IC also project to the DCN (e.g., Adams, 1983), it is possible that terminal labeling after injections confined to the DCN actually arise in the VCN. Although our retrograde results cited above suggest considerable overlap, projections from the VCN and DCN might interdigitate or otherwise form non-overlapping terminal fields at a local level of organization (cf. Shneiderman and Henkel, 1987; Gabriele et al., 2000; Loftus et al., 2004). At any rate, it does seem that most neurons in the CNIC of the gerbil are potentially subject to influences from both the DCN and the VCN. Whether inputs from both divisions terminate on individual cells will require experiments at a higher level of resolution than those reported here (cf. Oliver, 2005).
Anterograde labeling studies of projections to the IC from the PVCN have not been previously reported. Two cases in the present series (534 and 544) had injection sites confined to the PVCN and two others (512 and 513) had one injection site in the PVCN and another in the DCN. (The two injection sites in the latter cases were in different locations with respect to the topographical organization of the nucleus so that the distribution of terminations from each injection site was easily interpreted.) Although terminal labeling throughout the cochlear nucleus was extensive in these cases (e.g., Fig. 2), only a few labeled cells were present in the AVCN so that collateral labeling with the tracer does not appear to be a major consideration in interpreting the results of these injections. Consistent with the results of our retrograde study (Cant and Benson, 2006), the distribution of inputs from the PVCN appears to overlap the distribution of inputs from the AVCN. Whether the neuronal population in the PVCN that projects to the IC represents a different population from that in the AVCN remains an open question.
Organization of projections from the cochlear nucleus to the ipsilateral inferior colliculus
Ipsilateral projections arise from neurons in the ventral (low frequency) regions of both the VCN and DCN in the gerbil (Nordeen et al., 1983; Cant and Benson, 2006). The distribution of terminations within the ipsilateral IC was first described in the gerbil by Moore and Kitzes (1985), who used degeneration techniques to visualize the projections. Our results are in complete agreement with theirs. Ipsilateral labeling was less extensive than the corresponding contralateral labeling. Labeling on both sides occupied comparable locations in the rostrolateral CNIC and in the caudomedial field in the dorsal part of the dorsal cortex. Very little labeling was present in the middle and ventral parts of the CNIC, although it was not completely absent. In agreement with Oliver (1987), we also found that the ipsilateral terminations could be as dense as those from the contralateral side within limited parts of the termination zone. However, the terminal fields from the two sides do not appear to overlap completely in the lateral part of the CNIC. Regions with dense terminal labeling from the contralateral side appear to correspond to areas of sparse labeling ipsilaterally and vice versa.
Ipsilateral projections from the cochlear nucleus to the inferior colliculus were not seen in the degeneration studies reported by Osen (1972), but even her largest lesions did not appear to include the most ventral parts of the VCN or DCN. Oliver (1984) did not find projections to the ipsilateral IC from the DCN when his tracer deposits were confined to that division. Because the part of the DCN devoted to low frequencies is disproportionately small in the cat (Spirou et al., 1993), it seems possible that the finding of weak or absent ipsilateral projections from the DCN to the dorsal CNIC in the cat might be explained by non-inclusion of the most ventral part of the DCN in the injection sites.
Combined with the finding that the projections from the medial and lateral superior olivary nuclei do not terminate uniformly within the low frequency laminae of the CNIC (Loftus et al., 2004; unpublished results in the gerbil), the present results support the suggestion that the synaptic organization within this frequency region may be quite complex and that different neurons that lie quite close together may receive synaptic inputs from different combinations of sources (see Oliver, 2005). Detailed maps of the organization of the projections from each source combined with maps of the distribution of different neuronal types and their dendritic orientation (Oliver and Morest, 1984; Oliver et al., 1994; Malmierca et al., 1993) are important for guiding higher resolution studies of different “synaptic domains” (Oliver, 2005) within a frequency layer.
Organization of projections inside and outside the central nucleus
Although we defined the central nucleus of the inferior colliculus in the gerbil using criteria independent of those used in the classical definitions in cat and rat (gerbil: Cant and Benson, 2006; cat: Oliver and Morest, 1984; rat: Faye-Lund and Osen, 1985; Malmierca, 2003), the organization of inputs with respect to the boundaries of the central nucleus in the gerbil are remarkably similar to that documented in the other species. The cochlear nucleus projects throughout the CNIC, although, as also noted by Moore and Kitzes (1985), the projections do not appear to be uniformly distributed. The highest density of terminals was found in rostrolateral and caudal parts of the CNIC, whereas a somewhat lower density of terminals was characteristic of its relatively rostromedial parts. Differences in the density of inputs from the cochlear nucleus to different parts of the CNIC may be related to differences in distribution of inputs from other sources (e.g., Oliver et al., 1997).
Three areas outside the boundaries of the central nucleus receive inputs from the cochlear nuclei: a restricted part of the dorsal cortex, the ventrolateral nucleus, and the rostral pole nucleus. Projections from the cochlear nucleus into the dorsal cortex were first described in the cat (Oliver, 1987; Oliver et al., 1997). At dorsal levels through the IC of the gerbil, a part of the dorsal cortex in the caudomedial part of the nucleus receives heavy terminations from the cochlear nucleus. The projections seem biased toward the low frequencies. Indeed, we could not be sure whether there were projections into the dorsal cortex from intermediate and higher frequency regions in the cochlear nucleus. Although they do not comment on a frequency bias, it also appears, based on the figures in the studies from Oliver’s group, that there is more spread of terminations into the dorsal cortex when the tracer injections are in ventral (low-frequency) parts of the cochlear nuclei than when they are in more dorsal locations. The part of the dorsal cortex that receives this input in the gerbil also receives a heavy projection from the auditory cortex (Bajo and Moore, 2005; unpublished results from our laboratory).
The outer layers of the external (lateral) cortex received very little input from the cochlear nuclei, although sparse labeling was sometimes present over areas that contained relatively high CO activity (i.e., the “neurochemical modules” described by Chernock et al., 2004). Projections to the external cortex from the cochlear nucleus have been described in the rat (Coleman and Clerici, 1987; Oliver et al., 1999), mouse (Ryugo et al., 1981; Ryugo and Willard, 1985), and opossum (Willard and Martin, 1983), all species whose audiograms are biased toward the higher frequencies. Loftus et al. (2008) in this volume suggest that the part of the external nucleus that receives input from the cochlear nucleus is a distinct region defined as the ventrolateral nucleus, which is relatively large in the rat compared to species that hear lower frequencies. Consistent with their analysis, the comparable region in the gerbil is relatively small and terminations from the cochlear nucleus are most obvious in the cases with injections in dorsal (high-frequency) regions. Like the dorsal cortex, the ventrolateral nucleus may also receive inputs from the auditory cortex (based on Figure 8 in Bajo and Moore, 2005). In addition, it receives inputs from somatosensory nuclei in the brainstem, which appear to overlap the projections from the cochlear nucleus to a certain extent (Zhou and Shore, 2006).
A third region outside the CNIC that receives projections from the cochlear nuclei, the rostral pole nucleus, forms a small rostral extension of the IC. It is also a target of both the dorsal and ventral cochlear nuclei in the cat (Oliver, 1984, 1987). The rostroventral process, described by Osen (1972) in the cat and also present in the gerbil (e.g., Fig. 7A, level H120, asterisk) might be considered to be the part of the rostral pole nucleus that contains neurons responsive to the higher frequencies.
Predicted tonotopic organization of the gerbil IC
Translation of the color-coded regions illustrated in Figure 7 to relative frequency regions in the IC is straightforward because they can be related to the results of previously published functional studies in the gerbil IC (Ryan et al., 1982, 1992; Brückner and Rübsamen, 1995). The areas colored light blue, located rostrolaterally and dorsomedially in the most dorsal levels of the IC should contain neurons responsive to the lowest frequencies in the gerbil audiogram. Based on functional studies, the light and dark blue areas combined should contain neurons that respond to frequencies below about 2–3 kHz. About a quarter of the way down from the dorsal surface, frequencies above about 2–3 kHz should start to appear (green color). Representation of the middle frequencies extends ventrally in the lateral IC, but more medially, the highest frequencies would be represented (red color) Based on the known physiology, we estimate that the area shown in red would respond to tones above approximately 15–20 kHz. The results further suggest that the entire frequency range would be represented in both the VLN and the RPN (Fig. 7A, B, arrowheads), and there is some indication that these regions would also be organized tonotopically.
In the more dorsal sections, it appears that there may be more than one representation of the lower frequencies, one in the caudomedial IC (Fig. 7, thin arrows) and one in the rostrolateral IC (Fig. 7, thick arrows). The pattern of labeling suggests that there would be a shallow frequency reversal in the tuning of neurons along an electrode track that moved from rostrolateral to caudomedial in the horizontal plane at dorsal levels of the IC.
The orientation of the lines between areas of different colors provide an estimate of the orientation of the fibrodendritic laminae characteristic of the IC in all species. In the horizontal plane, the line between the dark and light blue colors in the rostral IC forms a curved line such that the lowest frequencies are collected in the most rostral part of the representation. This curvature, which is hinted at in the summary drawing but which is obvious in the individual cases, is reminiscent of the arrangement in this region described in Golgi studies of the cat (Rockel and Jones, 1973). It is apparent that the lower frequencies should extend quite ventrally in the rostral IC. The borders between the blue and green label in the lateral IC and between the green and red areas in the ventral IC form approximately 45 degree angles with respect to the long axis of the brain stem in both the horizontal and transverse sections (Fig. 7A, B, respectively).
Conclusion
A major objective of auditory neuroscience is to understand the principles of organization and function of the neural circuits that make up the auditory system. Studies by Kirsten Osen and other pioneers set a high standard for this research, pointing out the supreme importance of describing the circuits in terms of the individual neuronal types that form them. The goal of our studies of the gerbil IC is to produce comprehensive maps of the terminal fields arising from its major sources of input and of the cell populations that project to its different targets. Such maps can provide a guide for higher resolution neuroanatomical studies and also serve as a template for mapping the results of functional studies so that they can be interpreted in light of the circuitry in any particular region.
ACKNOWLEDGEMENTS
We are grateful to Dr. Manuel Malmierca and an anonymous reviewer for their constructive critiques of an earlier version of the manuscript. The research was supported by a grant from the National Institutes on Deafness and Other Communication Disorders, DC00135.
LIST OF ABBREVIATIONS
- AVCN
anteroventral cochlear nucleus
- BDA
biotinylated dextran amine
- CNIC
central nucleus of the inferior colliculus
- cnr
cochlear nerve root
- coIC
commissure of the inferior colliculus
- CO
cytochrome oxidase
- DC
dorsal cortex (of the inferior colliculus)
- DCN
dorsal cochlear nucleus
- gcl
granule cell layer
- IC
inferior colliculus
- icp
inferior cerebellar peduncle
- LN
lateral nucleus (of the inferior colliculus)
- mcp
middle cerebellar peduncle
- PAG
periaqueductal gray matter
- PVCN
posteroventral cochlear nucleus
- RPN
rostral pole nucleus (of the inferior colliculus)
- SC
superior colliculus
- VCN
ventral cochlear nucleus
- v
spinal tract of the fifth nerve
- vg
vestibular ganglion
- VLN
ventrolateral nucleus (of the inferior colliculus)
- Vn
spinal nucleus of the fifth nerve
- VIIn
motor nucleus of the seventh nerve
- VIII
eighth nerve root
- vnr
vestibular nerve root
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams JC. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981;29:775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Adams JC. Multipolar cells in the ventral cochlear nucleus project to the dorsal cochlear nucleus and the inferior colliculus. Neurosci Lett. 1983;37:205–208. doi: 10.1016/0304-3940(83)90431-7. [DOI] [PubMed] [Google Scholar]
- Bajo VM, Moore DR. Descending projections from the auditory cortex to the inferior colliculus in the gerbil, Meriones unguiculatus. J Comp Neurol. 2005;486:101–116. doi: 10.1002/cne.20542. [DOI] [PubMed] [Google Scholar]
- Brückner S, Rübsamen R. Binaural response characteristics in isofrequency sheets of the gerbil inferior colliculus. Hearing Res. 1995;86:1–14. doi: 10.1016/0378-5955(95)00048-9. [DOI] [PubMed] [Google Scholar]
- Cant NB. Projections from the cochlear nuclear complex to the inferior colliculus. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. New York: Springer; 2005. pp. 115–131. [Google Scholar]
- Cant NB, Benson CG. Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull. 2003;60:457–474. doi: 10.1016/s0361-9230(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. An atlas of the inferior colliculus of the gerbil in three dimensions. Hearing Res. 2005;206:12–27. doi: 10.1016/j.heares.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Organization of the inferior colliculus of the gerbil (Meriones unguiculatus): differences in distribution of projections from the cochlear nucleus and the superior olivary complex. J Comp Neurol. 2006;495:511–528. doi: 10.1002/cne.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casseday JH, Fremouw T, Covey E. The inferior colliculus: a hub for the central auditory system. In: Oertel D, Fay RR, Popper AN, editors. Integrative Functions in the Mammalian Auditory Pathway. Springer Handbook of Auditory Research. vol 15. New York: Springer; 2002. pp. 238–318. [Google Scholar]
- Chen S, Aston-Jones G. Axonal collateral-collateral transport of tract tracers in brain neurons: false anterograde labeling and useful tool. Neuroscience. 1998;82:1151–1163. doi: 10.1016/s0306-4522(97)00336-9. [DOI] [PubMed] [Google Scholar]
- Chernock ML, Larue DT, Winer JA. A periodic network of neurochemical modules in the inferior colliculus. Hearing Res. 2004;188:12–20. doi: 10.1016/S0378-5955(03)00340-X. [DOI] [PubMed] [Google Scholar]
- Coleman JR, Clerici WJ. Sources of projections to subdivisions of the inferior colliculus in the rat. J Comp Neurol. 1987;262:215–226. doi: 10.1002/cne.902620204. [DOI] [PubMed] [Google Scholar]
- Doucet JR, Ryugo DK. Axonal pathways to the lateral superior olive labeled with biotinylated dextran amine injections in the dorsal cochlear nucleus of rats. J Comp Neurol. 2003;461:452–465. doi: 10.1002/cne.10722. [DOI] [PubMed] [Google Scholar]
- Ehret G. The auditory midbrain, a “shunting yard” of acoustical information processing. In: Ehret G, Romand R, editors. The Central Auditory System. New York: Oxford University Press; 1997. pp. 259–316. [Google Scholar]
- Faye-Lund H, Osen KK. Anatomy of the inferior colliculus in rat. Anat Embryol. 1985;171:1–20. doi: 10.1007/BF00319050. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Chamberlain SC, Brachman ML, Smith RL. Anatomy and physiology of the gerbil cochlear nucleus: An improved surgical approach for microelectrode studies. Hearing Res. 1982;6:259–275. doi: 10.1016/0378-5955(82)90059-4. [DOI] [PubMed] [Google Scholar]
- Gabriele ML, Brunso-Bechtold JK, Henkel CK. Plasticity in the development of afferent patterns in the inferior colliculus of the rat after unilateral cochlear ablation. J Neurosci. 2000;20:6939–6949. doi: 10.1523/JNEUROSCI.20-18-06939.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel CK, Spangler KM. Organization of the efferent projections of the medial superior olivary nucleus in the cat as revealed by HRP and autoradiographic tracing methods. J Comp Neurol. 1983;221:416–428. doi: 10.1002/cne.902210405. [DOI] [PubMed] [Google Scholar]
- Loftus WC, Bishop DC, Saint Marie RL, Oliver DL. Organization of binaural excitatory and inhibitory inputs to the inferior colliculus from the superior olive. J Comp Neurol. 2004;472:330–344. doi: 10.1002/cne.20070. [DOI] [PubMed] [Google Scholar]
- Loftus WC, Malmierca MS, Bishop DC, Oliver DL. The cytoarchitecture of the inferior colliculus revisited: a common organization of the lateral cortex in rat and cat. Neuroscience. 2008;current volume doi: 10.1016/j.neuroscience.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca MS. The structure and physiology of the rat auditory system: an overview. Int Rev Neurobiol. 2003;56:147–211. doi: 10.1016/s0074-7742(03)56005-6. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Blackstad TW, Osen KK, Karagüelle T, Molowny RL. The central nucleus of the inferior colliculus in rat: A golgi and computer reconstruction of neuronal and laminar structure. J Comp Neurol. 1993;333:1–27. doi: 10.1002/cne.903330102. [DOI] [PubMed] [Google Scholar]
- Malmierca MS, Saint Marie RL, Merchán MA, Oliver DL. Laminar inputs from dorsal cochlear nucleus and ventral cochlear nucleus to the central nucleus of the inferior colliculus: two patterns of convergence. Neuroscience. 2005;136:883–894. doi: 10.1016/j.neuroscience.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Moore DR, Kitzes LM. Projections from the cochlear nucleus to the inferior colliculus in normal and neonatally cochlea-ablated gerbils. J Comp Neurol. 1985;240:180–195. doi: 10.1002/cne.902400208. [DOI] [PubMed] [Google Scholar]
- Morest DK, Oliver DL. The neuronal architecture of the inferior colliculus in the cat: defining the functional anatomy of the auditory midbrain. J Comp Neurol. 1984;222:209–236. doi: 10.1002/cne.902220206. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Killackey HP, Kitzes LM. ascending auditory projections to the inferior colliculus in the adult gerbil, Meriones unguiculatus. J Comp Neurol. 1983;214:131–143. doi: 10.1002/cne.902140203. [DOI] [PubMed] [Google Scholar]
- Oliver DL. Dorsal cochlear nucleus projections to the inferior colliculus in the cat: A light and electron microscopic study. J Comp Neurol. 1984;224:155–172. doi: 10.1002/cne.902240202. [DOI] [PubMed] [Google Scholar]
- Oliver DL. Projections to the inferior colliculus from the anteroventral cochlear nucleus in the cat: possible substrates for binaural interaction. J Comp Neurol. 1987;264:24–46. doi: 10.1002/cne.902640104. [DOI] [PubMed] [Google Scholar]
- Oliver DL. Neuronal organization in the inferior colliculus. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. New York: Springer; 2005. pp. 69–114. [Google Scholar]
- Oliver DL, Morest DK. The central nucleus of the inferior colliculus in the cat. J Comp Neurol. 1984;222:237–264. doi: 10.1002/cne.902220207. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Beckius GE, Bishop DC, Kuwada S. Simultaneous anterograde labeling of axonal layers from lateral superior olive and dorsal cochlear nucleus in the inferior colliculus of cat. J Comp Neurol. 1997;382:215–229. doi: 10.1002/(sici)1096-9861(19970602)382:2<215::aid-cne6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Ostapoff E-M, Beckius GE. Direct innervation of identified tectothalamic neurons in the inferior colliculus by axons from the cochlear nucleus. Neuroscience. 1999;93:643–658. doi: 10.1016/s0306-4522(99)00143-8. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Winer JA, Beckius GE, Saint Marie RL. Morphology of GABAergic neurons in the inferior colliculus of the cat. J Comp Neurol. 1994;340:27–42. doi: 10.1002/cne.903400104. [DOI] [PubMed] [Google Scholar]
- Osen KK. Projection of the cochlear nuclei on the inferior colliculus in the cat. J Comp Neurol. 1972;144:355–372. doi: 10.1002/cne.901440307. [DOI] [PubMed] [Google Scholar]
- Rockel AJ, Jones EG. The neuronal organization of the inferior colliculus of the adult cat. I. The central nucleus. J Comp Neurol. 1973;147:22–60. doi: 10.1002/cne.901470103. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Woolf NK, Sharp FR. Tonotopic organization in the central auditory pathway of the Mongolian gerbil: a 2-deoxyglucose study. J Comp Neurol. 1982;207:369–380. doi: 10.1002/cne.902070408. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Axelsson GA, Woolf NK. Central auditory metabolic activity induced by intense noise exposure. Hearing Res. 1992;61:24–30. doi: 10.1016/0378-5955(92)90032-i. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Willard FH. The dorsal cochlear nucleus of the mouse: a light microscopic analysis of neurons that project to the inferior colliculus. J Comp Neurol. 1985;242:381–396. doi: 10.1002/cne.902420307. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Willard FH, Fekete DM. Differential afferent projections to the inferior colliculus from the cochlear nucleus in the albino mouse. Brain Res. 1981;210:342–349. doi: 10.1016/0006-8993(81)90907-0. [DOI] [PubMed] [Google Scholar]
- Saldaña E, Merchán MA. Intrinsic and commissural connections of the inferior colliculus. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. New York: Springer; 2005. pp. 155–181. [Google Scholar]
- Saldaña E, Merchán MA. Intrinsic and commissural connections of the rat inferior colliculus. J Comp Neurol. 1992;319:417–437. doi: 10.1002/cne.903190308. [DOI] [PubMed] [Google Scholar]
- Saldaña E, Feliciano M, Mugnaini E. Distribution of descending projections from primary auditory neocortex to inferior colliculus mimics the topography of intracollicular projections. J Comp Neurol. 1996;371:15–40. doi: 10.1002/(SICI)1096-9861(19960715)371:1<15::AID-CNE2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Schofield BR. Superior olivary complex and lateral lemniscal connections of the auditory midbrain. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. New York: Springer; 2005. pp. 132–154. [Google Scholar]
- Shneiderman A, Henkel CK. Banding of lateral superior olivary nucleus afferents in the inferior colliculus: a possible substrate for sensory integration. J Comp Neurol. 1987;266:519–534. doi: 10.1002/cne.902660406. [DOI] [PubMed] [Google Scholar]
- Spirou GA, May BJ, Wright DD, Ryugo DK. Frequency organization of the dorsal cochlear nucleus in cats. J Comp Neurol. 1993;329:36–52. doi: 10.1002/cne.903290104. [DOI] [PubMed] [Google Scholar]
- Willard FH, Martin GF. The auditory brainstem nuclei and some of their projections to the inferior colliculus in the North American opossum. Neuroscience. 1983;10:1203–1232. doi: 10.1016/0306-4522(83)90109-4. [DOI] [PubMed] [Google Scholar]
- Winer JA. Three systems of descending projections to the inferior colliculus. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. New York: Springer; 2005. pp. 231–247. [Google Scholar]
- Winer JA, Larue DT, Diehl JJ, Hefti BJ. Auditory cortical projections to the cat inferior colliculus. J Comp Neurol. 1998;400:147–174. [PubMed] [Google Scholar]
- Wong-Riley MTT. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Zhou J, Shore S. Convergence of spinal trigeminal and cochlear nucleus projections in the inferior colliculus of the guinea pig. J Comp Neurol. 2006;495:100–112. doi: 10.1002/cne.20863. [DOI] [PubMed] [Google Scholar]