Abstract
Behavioral characteristics closely associated with specific physiological profiles present an important area of research in understanding health disparities. In particular, glucocorticoid overproduction may be an important factor moderating disease progression; natural variance in production of this steroid has been proposed as one mechanism underlying individual differences in health and disease. In the current paper, we examined immune parameters in female rats of two different behavioral types previously shown to have differential glucocorticoid production and life spans. We categorized young female rats according to their behavioral response to novelty (high- or low-locomotion), and compared their glucocorticoid production, adrenal size, thymus size, tumor necrosis factor-α (TNF-α) production, tumor development and life span. As expected, high-locomotion females produced more glucocorticoids and had larger adrenal glands during young adulthood than did low-locomotion females. High-locomotion females had significantly smaller thymuses and reduced TNF-α levels compared to low-locomotion, suggesting altered immune function in young adulthood. Finally, high-locomotion females had shorter life spans than did low-locomotion females, and this was particularly true in females that developed pituitary tumors, but not in those that developed mammary tumors. These results, along with other published findings, suggest that high-locomotion rodent females experience life-long elevations in glucocorticoid responses to novelty, and that these elevated levels may be comparable to chronic stress. This naturally-occurring endocrine profile may influence immune responses which in turn could affect disease susceptibility. Variance in immune function across personality types may be partially moderated by natural variance in glucocorticoid production.
Keywords: temperament, personality, glucocorticoids, stress, pro-inflammatory cytokines, tumor development, life span, tumor necrosis factor alpha
It has been proposed that female personality or temperament may play a role in cancer resilience in women (Lutgendorf, 2003; Segerstrom, 2003). However, data to support this proposition are inconclusive (Garssen, 2004; Jadoulle et al., 2004; Nakaya et al., 2005); this discrepancy may reflect the fact that most studies do not measure temperament or personality until after tumor formation and/or because specific underlying biological mechanisms associated with cancer-prone personalities have not been clearly identified. One potential mechanism, physiological stress (i.e., neuroendocrine processes), clearly influences cancer cell functioning (Lutgendorf et al., 2003; Sood et al., 2006). In the current article, we used an animal model of spontaneous tumor development in which female temperament (locomotion in a novel situation) has been associated with natural variance in glucocorticoid production, tumor development and life span (Cavigelli et al., 2006). With this model, we were able to test whether a female behavioral trait associated with glucocorticoid overproduction is related to indicators of immune function. We measured thymus size and circulating tumor necrosis factor-α (TNF-α) in young adults, and monitored onset of palpable mammary tumors, identified tumor location at death, and monitored life span. The overall goal was to determine if naturally occurring variance in female temperament was related to glucocorticoid production and immune function during young adulthood and whether these variables were associated with tumor development and changes in lifespan. Together, these data might provide a potential mechanism for altered tumor development and life span among females with different temperaments.
In the rat model, we have found that female locomotion in a novel setting varies considerably among individuals and that this response is relatively stable over time. A single 5-min test of locomotor response to novelty during infancy or young adulthood predicts individual locomotor responses both 4- and 10-months later (Cavigelli et al., 2006). Low-locomotion females had attenuated glucocorticoid responses following novelty in late adulthood (15 mos) - during ovarian aging - which may have been a byproduct of more rapid degradation of ovarian functioning at the end of life. Finally, for females that developed spontaneous mammary and/or pituitary tumors, low-locomotion females died considerably earlier (about 6 months) than their high-locomotion sisters (Cavigelli et al., 2006). Importantly, this life span effect was reversed when comparing high- vs. low-locomotion females across families as opposed to within families – i.e. females from high-locomotion, high-glucocorticoid families succumbed much earlier than females from low-locomotion, low-glucocorticoid families (Cavigelli, unpublished data). These latter results, in conjunction with prior findings with male mice and rats (Cavigelli and McClintock, 2003; Péréz-Álvarez et al., 2005), suggest that there are important individual differences in the relationship between glucocorticoid overproduction and lifespan. In the present experiment, we hypothesized that female temperaments associated with natural variance in glucocorticoid production would be associated with natural variance in immune function, thereby providing an additional mechanism underlying differential life span among individuals with different behavioral tendencies.
An extended stress response is known to cause thymic atrophy in rodents (Domínguez-Gerpe and Rey-Méndez, 1997; Selye, 1956). Blockade of glucocorticoid receptors (using RU486) during acute infection can partially reverse this atrophy (Roggero et al., 2006). Thymic atrophy has also been reported in response to graft-versus-host disease, a condition associated with elevated glucocorticoid levels and accelerated apoptosis of immature thymocytes. However, glucocorticoid receptor blockade did not slow the atrophic process, suggesting glucocorticoid-independent mechanisms underlying stress-related thymocyte apoptosis (Krenger et al., 2000). We hypothesized that high-locomotion females with naturally-elevated glucocorticoid production may experience greater thymic atrophy, leading to smaller thymus weights in young adulthood. However, it is highly likely that the natural variance in glucocorticoid production between these two kinds of females may not be great enough to lead to significant differences in thymus size.
Tumor necrosis factor was originally identified as a cytokine that promotes tumor necrosis (Carswell et al., 1975; Old, 1985). However, recent data indicate TNF-α promotes tumor development and invasion of surrounding tissue through a variety of cellular mechanisms (Montesano et al., 2005; Moore et al., 1999; Orosz et al., 1993; Suganuma et al., 1999). For example, TNF-deficient mice exposed to okadaic acid, a tumerogenic compound, developed tumors more slowly and tumor size was diminished compared to normal control mice (Suganuma et al., 1999). Treating normal mice with TNF-α blocker inhibited both skin papilloma and breast carcinoma (Scott et al., 2003). Thus, natural variances in TNF-α production may also influence individual tumor susceptibility.
Glucocorticoids (e.g. cortisone) are classically used in the clinical setting to decrease inflammatory responses (e.g. asthma, Van der Velden, 1998; rheumatoid arthritis, Gaffo et al., 2006), though recent research indicates they may have acute pro-inflammatory qualities as well, especially in response to injury (Sorrells and Sapolsky, 2007). Exogenous glucocorticoid administration decreases pro-inflammatory cytokine release, including TNF-α (Barnes, 1998), and glucocorticoid receptor blockade increases TNF-α levels (Roggero et al., 2006). Thus, we hypothesized that high-locomotion, high-glucocorticoid females would have attenuated TNF-α levels at baseline and/or in responses to tail nicking, compared to low-locomotion, low-glucocorticoid females. Decreased TNF-α levels in high-locomotion females may serve as a protective factor for tumor development and progression, allowing them to show later tumor development and longer life spans. Alternatively, it is possible that high-locomotion/high-glucocorticoid females develop glucocorticoid resistance in the immune system (Miller et al., 2005) leading to either no differences in TNF-α production, and/or perhaps greater responses as a result of diminished glucocorticoid regulation.
In the current study, we tested the following hypotheses: 1) During young adulthood, females that move a lot in a novel environment will release more glucocorticoids into circulation following novelty than low-locomotion females; 2) If high-locomotion females chronically produce more corticosterone, we expect them to have enlarged adrenal glands and potentially smaller thymus glands following pubertal involution; 3) Circulating TNF-α levels following tail nicking will differ between high- and low-locomotion females, with lower levels in high-locomotion females unless they have developed glucocorticoid resistance; 4) High-locomotion females will have a shorter life span compared to low-locomotion females; 5) Mammary tumors will appear earlier in high-locomotion females compared to low-locomotion females.
METHODS
Overall Design & Sample
We studied two cohorts of Sprague-Dawley female rats (Charles River Laboratories, Wilmington, MA). The first cohort was used to compare high- and low-locomotion females’ adrenal and thymus weights in young adulthood, and consisted of 49 females from 7 litters. The second cohort was used to compare glucocorticoid responses, circulating TNF-α levels, and life span between the two locomotion types, and consisted of 60 females received in the laboratory at 60 days of age. The following conditions were true for both cohorts. Rats were housed in solid bottom plastic cages (43.5 × 23.5 × 20.5 cm) on a 14L:10D lighting schedule (lights on at 19:00 h EST). Food and water were available ad libitum and cages were cleaned once a week by animal facility personnel trained in animal care and handling. The colony room was maintained at 72°F with ~50% humidity. Rats were handled daily and vaginal cytology data collected during the 2 weeks prior to testing and testing was conducted during the metestrous phase of the estrous cycle. All methods were approved by and conducted in accordance with the Pennsylvania State University Institute for Animal Care and Use Committee.
In the first cohort, females were weaned from mothers at 21 days of age, housed in groups of 3 same-sex siblings, and at 25 days introduced to a novel arena containing rat-sized objects to identify high- vs. low-locomotion females. Females were sacrificed at 4 months of age to compare relative adrenal and thymus weights. For the second cohort, after a 2-week acclimation period, females were tested on one of two novel environments: one that contained novel rat-sized objects (‘non-social’) and one that contained a caged novel female rat (‘social’). Order of testing across the two arenas and within test days were balanced across females. The first test was completed at 2.5 months, the second test at 4 months. Repeat blood samples were collected after the second behavioral test (‘non-social’ for half the females, ‘social’ for the other half) to measure plasma glucocorticoid and TNF-α responses to novelty and tail nicking. Females were allowed to live the natural life span or were sacrificed at 620 days or when they displayed a series of morbidity-related behavioral symptoms. Necropsies were performed to identify location of neoplastic growth.
Behavioral Response to Novelty during Adolescence and Young Adulthood
For both cohorts, behavioral testing was done in the middle of the rats’ active period (1300–1500h, 4–6h after lights off). Rats were tested in a non-colony room illuminated with two 25-watt red bulbs reflected off the walls of the room, which provided 6 lux of light at the center of the test arena, and were videotaped for 5min with a camera above the arena. Rats were introduced into the arenas in a ceramic bowl that was cleaned between successive rats.
Novel Non-Social Arena
The arena, previously described in (Cavigelli and McClintock, 2003), was designed to be minimally anxiety-provoking. The square test arena (120cm × 120cm × 46cm) contained a novel rat-sized object in three corners (a plastic tube, an inverted bowl, or a wire tunnel). To include a familiar odor, the bedding-covered floor included some bedding from all cages in the colony. On the rare occasion that feces were left in the arena during testing, these were removed. To maintain conspecific odors, the arena floor was not cleaned any further.
Novel Social Arena
The novel social arena was the same size and height as the physical arena, but instead of novel physical objects, the arena contained two cages – one empty cage and one cage with an unfamiliar female rat of similar size, reproductive state, and age as the test rat.
For both arenas, locomotion was scored from video recordings by a trained coder who did not know the identity of each rat. To code locomotion, the arena image was superimposed with an 8×8 grid dividing the arena into 64 equally-sized squares. Locomotion was quantified as the cumulative number of squares crossed on the grid.
Thymus and Adrenal Weights in Young Adulthood
Females in cohort 1 were sacrificed, weighed and the left adrenal and thymus glands removed, loosened from connective tissue and fat, and weighed. Each organ was processed by the same investigator who was unaware of females’ behavioral classifications. Adrenal and thymus weights were normalized to individual rat size by dividing by 100g of body weight.
Corticosterone Production in Young Adulthood - Baseline Levels & Response to Novelty
To measure glucocorticoid responses to complex novel experiences, we collected repeated blood samples via tail nick after introduction to the novel arena in cohort 2 females. The glucocorticoid circadian rhythm (e.g. Atkinson and Waddell, 1997; Krieger, 1973) was controlled by initiating all sampling between 1300h and 1500h. After novel-arena testing, a female was brought to a separate room and placed into a new cage comparable to their home cage with access to water. Ten minutes from the beginning of behavioral testing, the first blood sample was taken by removing the tip of the tail (approx. 3mm) with a scalpel. Blood (200μl) was collected into EDTA microtainers (Becton Dickinson and Company) within 3 minutes using gentle tail palpation. After the first sample, the female was returned to the holding cage and repeat samples were collected 40, 80 and 120 min from the beginning of behavioral testing. Thirty minutes following the 120-min sample, rats were returned to their home cage in the colony room. Twenty-four hours after the initial (10-min) blood sample, rats were transported back to the collection room in their home cage and a final (24-h) blood sample collected by gently palpating the tail tip to re-stimulate blood flow.
To measure basal (unstimulated) corticosterone levels, we collected blood samples 8 weeks following the repeated sampling protocol described above. These samples were collected well after to allow ample time for animals to return to basal levels. The protocol for this blood collection was similar to the one described above with only one sample collection between 1300h and 1400h.
For all measures, blood samples were kept on ice for 40 min until centrifuged and plasma collected. Plasma was aliquoted – one portion for corticosterone analysis, a second portion for the TNF-α analysis described below - and all frozen at −80°C until assayed. Corticosterone was measured using a commercial radioimmunoassay kit (Rat & Mice Corticosterone kit, MP Biomedicals, Solon, OH). All samples were run in duplicate across 6 assays, with females balanced across assays according to their locomotion on the novel physical arena. Intra-assay variability for a low and high control was 17.7 and 13.9%; inter-assay variability for these controls was 14.7 and 15.1%.
Tumor Necrosis Factor-α in Young Adulthood
Blood samples collected for corticosterone measures for cohort 2 (as described above), were also measured for circulating TNF-α levels. Specifically, we measured TNF-α at the time of tail clipping and 24-hrs later, during wound healing. Samples were analyzed for 19 high- and 19 low-locomotion females with a commercially-available enzyme immunoassay (Quantikine, R&D, Minneapolis, MN). All samples were tested in duplicate across two plates. The average of the duplicate tests is reported. The assay sensitivity was 5 pg/ml, based on minimum TNF-α concentration required to arrive at two standard deviations from assay A0. Intra-assay coefficient of variation (%CV) computed for the mean of 20 replicate tests was less than or equal to 5.1%. Inter-assay variation computed for the mean of average duplicates for 20 separate runs was less than or equal to 9.7%.
Life Span
Females in cohort 2 were allowed to live their natural life span or until they displayed pre-defined symptoms indicative of non-recoverable health problems or prolonged pain (n=26 and 34, respectively) (i.e. ‘endpoints’: rapid excessive weight loss, dehydration, inability to ambulate and obtain food and water, labored respiration, infected or necrotic tumors, tumors that impair ability to walk with normal gait). Decisions to sacrifice were made by a veterinarian that was unaware of the study hypotheses. The study was terminated when the rats were 620 days of age (21 mos), at which point all surviving females were sacrificed whether they exhibited health problems or not (n=29). For these females, age of death was identified as 620 days and their data were classified as ‘censored’ in survival analyses.
Tumor Development & Identification
To determine age of palpable mammary tumor development in cohort 2, we used a comprehensive technique for repeatedly palpating mammary tissue; three checkers could reliably detect mammary neoplasia as small as 3mm in diameter. Regular checks began just prior to 10 mos of age and were repeated every 2–3 wks until death. We defined onset of tumor detection as the first date at which a growth was reliably palpable. Checkers were not informed as to which females’ had been palpated with tumors in the previous weeks or the behavioral category of each female. Necropsies were performed to determine presence of a pituitary or mammary tumor. Mammary tumors were defined as solid masses larger than 5 mm in all three dimensions. Pituitary tumors were defined by extensive vasculature and enlargement of the gland.
Analyses
Cohort 1
To determine if female locomotion in a novel arena was related to young adult adrenal and/or thymus weight, females were categorized, using a median split, as either HIGH or LOW locomotion based on their response to a novel non-social arena at 25 days of age. T-tests were used to compare organ weights between these two groups. Rat size was controlled for by dividing organ weights by 100g of body weight. Relative thymus weights were log-transformed to arrive at a normal distribution and 7 outliers removed (greater than 2 standard deviations from mean value).
Cohort 2
Correlation analyses were used to determine if female locomotion was stable across different novel environments. To test whether young females with stably higher exploratory behavior had elevated glucocorticoid responses we grouped females into three categories based on their locomotion scores in the two novel arenas. The 3 groups were: 1) those with higher than median locomotion score in both arenas (‘HIGH’), 2) those with lower than median scores in one arena and higher than median in the other arena (‘MIXED’), and 3) those below median in both arenas (‘LOW’). Corticosterone levels were compared among these three groups using repeated-measures analyses of variance (ANOVAs). Corticosterone values were log-transformed to achieve a normal distribution. Because test order and time of day may significantly influence glucocorticoid levels, we tested whether these factors had a significant effect on glucocorticoid levels, and if so, included these variables in the analyses. Five females were removed from the repeated measures corticosterone analyses because they were missing a blood sample at one of the 6 repeated measure time points (2 HIGH, 2 MIXED, and 1 LOW female). For clarity, graphs include data from the HIGH and LOW females only. The text includes information on the MIXED females.
Plasma TNF-α levels at the time of tail nicking and 24-hrs later were compared between HIGH and LOW locomotion females using a repeated-measures ANOVA. Change in levels from tail nicking to 24-hrs later were compared between female groups using a t-test. For all analyses, TNF-α values were log-transformed to achieve a normal distribution. Four samples had to be excluded from analyses because they were grossly hemolyzed, leaving 16 HIGH and 18 LOW locomotion females in the analyses.
RESULTS
Adrenal and Thymus Weight in Young Adulthood
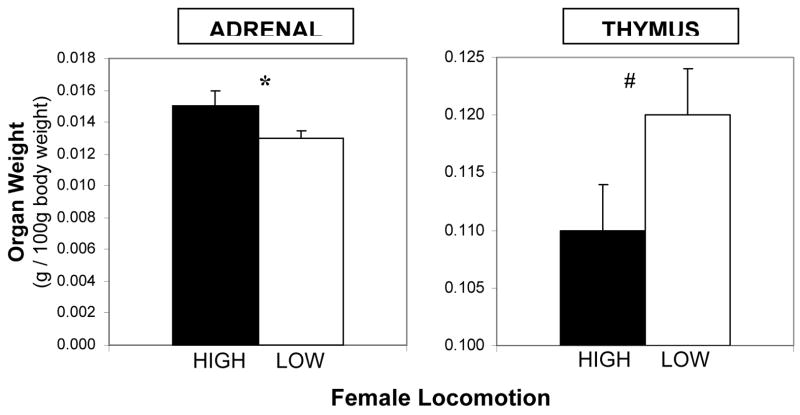
In cohort 1, overall, females with large adrenal glands had smaller thymuses (r (43) = −0.318, p < .05). As hypothesized, adrenal and thymus weights differed between high- and low-locomotion females. At 4 months of age, HIGH locomotion females had significantly larger left adrenal glands than LOW locomotion females (t (47) = 2.07, p < .05; Figure 1). The opposite was the case for the thymus gland (t (41) = 1.86, p = .07; Figure 1). This difference was not quite statistically significant, however, when we compared females in the top and bottom tertiles for locomotion, the high-locomotion tertile had significantly smaller thymuses than the low-locomotion (t (27) = 2.10, p < .05).
Figure 1.
Mean adrenal and thymus weights (relative to 100 g body weight) for high- and low-locomotion young adult females. Error bars indicate S.E.M. * p < .05, # p < .10
Behavioral Response to Novelty during Adolescence and Young Adulthood
In cohort 2, females’ behavioral responses to the two different novel arenas (non- social and social) were not highly related. High locomotion females in one arena were not necessarily high locomotion females in the second arena (r (58) = 0.11, ns). Of the 60 females tested, 22 had above median locomotion on both arenas (classified as ‘HIGH locomotion’), 22 had below median locomotion on both arenas (‘LOW locomotion’), and the remaining 16 were classified as MIXED (above median on one arena, below median on the other).
Corticosterone Production in Young Adulthood - Baseline Levels & Response to Novelty
Corticosterone production differed between high- and low-locomotion females in the direction expected. In cohort 2, very active females - in both novel arenas (HIGH locomotion) - had the greatest glucocorticoid rise following novelty, while LOW locomotion females had the lowest rise and MIXED females had an intermediate response (repeated measures ANOVA for 10–120min: F (2,52) = 4.04, p < .05; post-hoc t-tests reveal the greatest difference between HIGH and LOW females at 40- and 80-min; Figure 2). HIGH locomotion females did not maintain these elevated corticosterone levels 24-hrs after novelty or 8 weeks later (repeated measures ANOVA F (2,53) = 0.95, ns; Figure 2).
Figure 2.
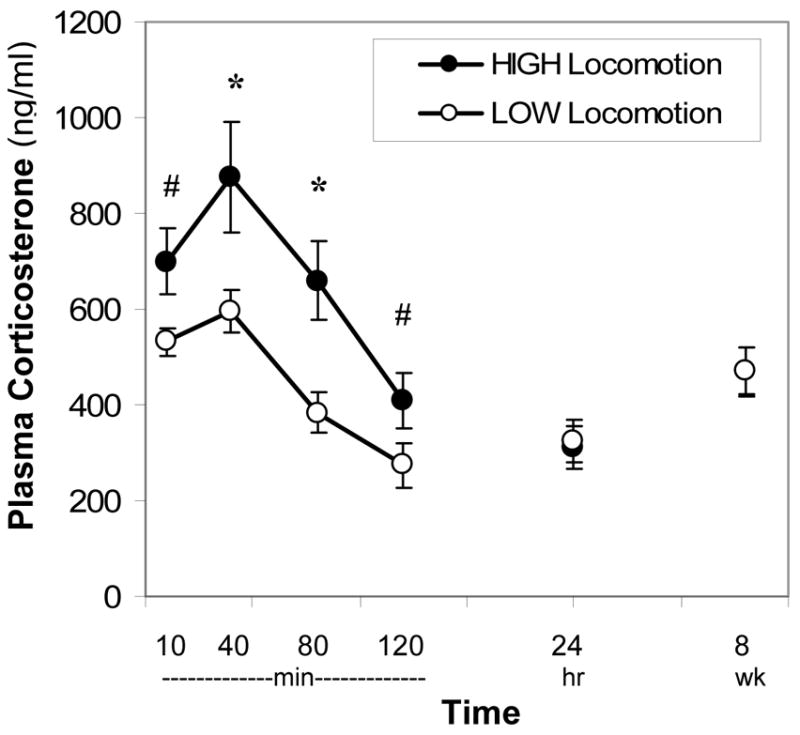
High- and low-locomotion females circulating corticosterone response to a complex novel stimulus (behavioral testing, housing in novel room and tail clip procedure) at 4 months of age and levels 24-hrs and 8-wks later. Error bars indicate S.E.M. * p < .05, # p < .10
Tumor Necrosis Factor-α in Young Adulthood
Circulating TNF-α levels were relatively low, both at the time of tail nicking and 24-hrs after tail nick (range: 5–39 pg/ml). Across both female types, TNF-α levels increased significantly, albeit slightly, from the time of tail nick to 24-hrs later (approximately 8 pg/ml; repeated measures ANOVA: F (1,32) = 10.83, p < .01). As hypothesized, HIGH locomotion females had slightly lower levels than LOW locomotion females, although this difference was not quite statistically significant (repeated measures ANOVA: F (1,32) = 3.52, p = .07). If we limited analyses to females that were in the top and bottom locomotion tertiles across both arenas, HIGH locomotion females had significantly lower TNF-α levels than LOW locomotion females (repeated measures ANOVA: F (1,16) = 6.43, p < .05; Figure 3). HIGH and LOW locomotion females showed no difference in the amount of change in circulating TNF-α levels from the time of tail nicking to 24-hrs later (t (32) = 0.54, ns; calculated with tertiles: t (16) = 1.69, ns).
Figure 3.
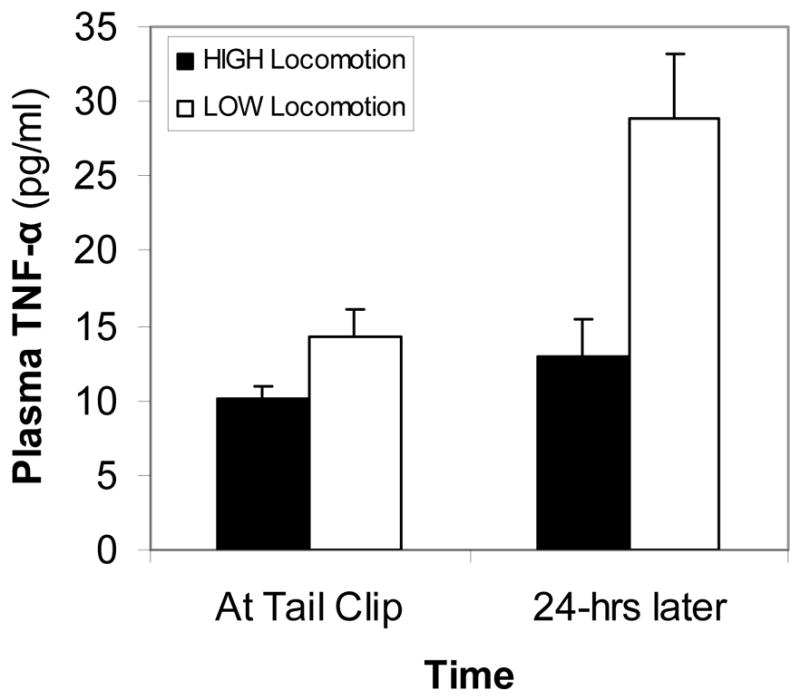
High- and low-locomotion female plasma TNF-α levels at tail clip and 24-hrs later during young adulthood. Error bars indicate S.E.M. See text for statistically significant differences.
Tumor Development & Life Span
Seventy percent (42/60) of females developed a mammary and/or pituitary tumor prior to death (15 with only a mammary tumor, 16 with only a pituitary tumor, and 11 with both a mammary and pituitary tumor). Females with a tumor died at the same age as females without (median life span: 605 vs. 616 days; χ2 = 2.26, ns). As predicted, across the entire population, HIGH locomotion females died significantly earlier than LOW locomotion females (median life spans: 558 vs. 620 days; χ2 = 4.42, p < .05; Figure 4a). This was particularly true when comparing females that developed pituitary tumors (χ2 = 6.66, p < .01; Figure 4c), but not significant for female that developed mammary tumors (χ2 = 0.89, ns; Figure 4b). HIGH locomotion females’ mammary tumors were palpable slightly earlier than LOW locomotion females, but this difference was not statistically significant (χ2 = 1.10, ns). Mammary and pituitary tumor weights at death did not differ between HIGH and LOW locomotion females (mammary: t (17) = 0.90, ns; pituitary: t (13) = 0.78, ns).
Figure 4.
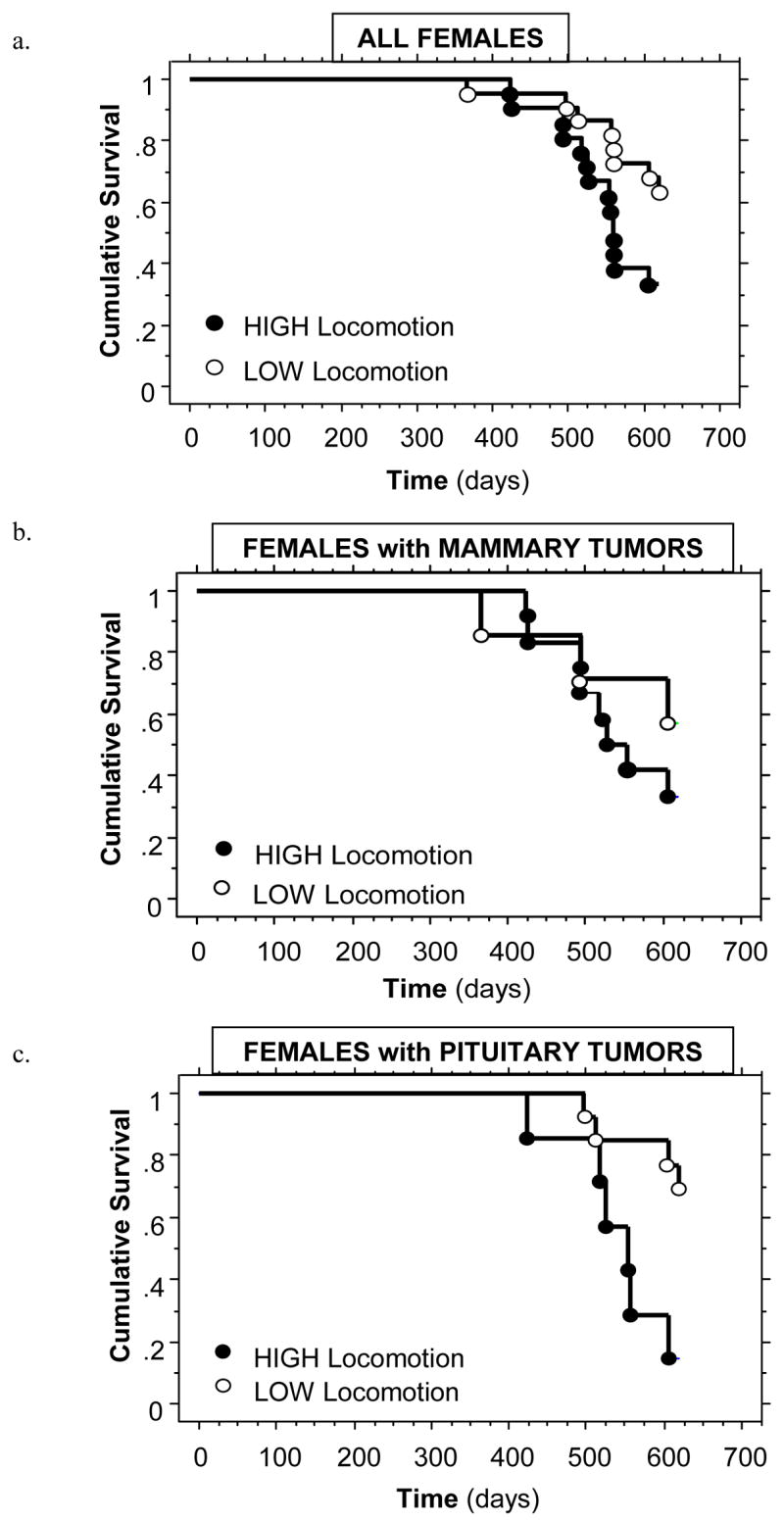
High- and low-locomotion female life spans for (a) all females in the study population, (b) females that developed mammary neoplasia, and (c) female that developed pituitary neoplasia. See text for statistical comparisons.
DISCUSSION
In the present study, young females that were highly active in novel settings (‘high-locomotion females’) were morphologically and physiologically distinct from less active females (‘low-locomotion females’). These distinctions may begin to account for differential life span between these females. Specifically, during young adulthood, high-locomotion females had enlarged adrenal glands and produced more corticosterone in response to a complex novel experience (being removed from the home cage, exposed to a large novel arena, placed in a novel colony room in a new cage, and having the tip of the tail cut), suggesting they may stimulate the hypothalamic pituitary adrenal (HPA) axis more readily throughout the life span than low-locomotion females. High-locomotion females also showed signs of dampened immune capacity at this young age (i.e., decreased thymus size and TNF-α in circulation), when looking at the extreme HIGH and LOW tertile animals. Finally, high-locomotion females died more rapidly than did low-locomotion females; and this differential life span was most pronounced in females that developed pituitary tumors. These results provide compelling support for the fact that early expressed behavioral differences, associated with natural variance in HPA axis activity, are also associated with differential immune function, potentially making high-glucocorticoid-producing animals more susceptible to specific disease processes at the end of life.
Glucocorticoid responses to novelty were significantly elevated in high-locomotion females during young adulthood, compared to low-locomotion females. These results support previous findings in which high-locomotion females were shown to have elevated glucocorticoid levels in late middle age (Cavigelli et al., 2006). The present findings extend these results, showing that elevated glucocorticoid production is already present during young adulthood for high-locomotion females, indicating this elevated HPA activity is not a result of previously documented differences in rates of ovarian aging during middle adulthood. Furthermore, this early difference in HPA activity between high- and low-locomotion females suggests these glucocorticoid differences may exist throughout the life span and may mimic effects of chronic stressor exposure. Although single-housed laboratory animals may not experience the frequency and/or magnitude of novel situations experienced by free-ranging animals, they regularly experience husbandry procedures (cage changes, door openings, etc) that have been shown to cause significant stress responses, particularly in single-housed female rats (Sharp et al., 2003). This interpretation is supported by the enlarged adrenal glands in young adult high-locomotion females. Further studies are required to address other physiological repercussions that may exist later in life as a result of this differential HPA axis activity, and to determine the extent to which HPA axis activity is a stable trait within individuals over time (Adam et al., 2006; Burleson et al., 2003; Capitanio et al., 1998; Cohen and Hamrick, 2003).
Several hormones other than glucocorticoids have been proposed to affect the genesis and progression of cancers; among these are epinephrine (Epi) and norepinephrine (NE) (Lutgendorf et al., 2003; Sood et al., 2006). For example, Epi and NE have been shown to increase invasiveness in three cancer cell lines, while cortisol only increased invasiveness in one (Sood et al., 2006). Furthermore, Dobbs and colleagues (1993) have shown both a glucocorticoid and catecholamine mechanism for immune suppression under stress. Most likely, glucocorticoid-independent mechanisms of thymic atrophy (Krenger et al., 2000) are related to catecholamine action (see Madden, 2003 for a comprehensive review of catecholamine effects on immune function). The effects of catecholamines on thymus weight and lifespan may be mediated by glucocorticoids in that NE production can stimulate interleukin-1 in turn stimulating the HPA axis (Shintani et al., 1995). Further work will have to include measures of personality-linked variance in catecholamines to better understand mechanisms underlying a relationship between personality, immune function and cancer susceptibility/progression (Elenkov & Chrousos, 1999). Catecholamines, as compared to glucocorticoids, may be a more likely functional link between female locomotor responses to novelty and immune function.
High-locomotion females, compared to low-locomotion females, had reduced thymus sizes and reduced circulating TNF-α levels in young adulthood. These results are in the direction predicted given increased glucococorticoid production in high-locomotion females. If these differences hold true in other studies, this may provide another potential mechanism for differential survival between high- and low-glucocorticoid individuals (Aspinall, 2000). Overall, TNF-α levels were relatively low in the current study, indicating tail-nicking did not provide a potent immune challenge or that cytokine levels should be measured later into the healing process. In addition, Sprague-Dawley rats tend to be less reactive to mild stressors than other rat strains (e.g. Fischer 344; Dhabar et al., 1995), thus differences in immune activation may be more apparent in more reactive rat strains. Based on these data, it is hard to conclude whether slight differences in TNF-α levels could differentially influence tumor development in high- vs. low-locomotion females. Perhaps elevated TNF-α levels in low-locomotion, low-glucocorticoid individuals may serve as a protective advantage for certain disease processes like parasite infections (Derouich-Guergour et al., 2001), and/or a risk factor for others diseases, like rheumatoid arthritis, multiple sclerosis, and irritable bowel disease (Inglis et al., 2005; Issekutz et al., 1994; Kollias, 1999). A stronger immune challenge with longer monitoring of pro-inflammatory cytokines would provide more clear results, and immune measures later in the life span after repeated elevated glucocorticoid exposure may provide the most accurate picture of potential immune function alteration in females with different behavioral temperaments (Cacioppo et al., 1998). The suggestion that thymus size and plasma TNF-α levels may differ among young females of different behavioral tendencies provides support for further work in this area.
In a prior study comparing life span among female siblings, high-locomotion sisters survived longer than low-locomotion females (Cavigelli et al., 2006). Within this same study, results across families were the opposite – with females from high-locomotion families surviving shorter than those from low-locomotion families (Cavigelli, Yee, McClintock, unpublished data). The current results support the latter family-level survival effects and present a curious anomaly in terms of within-litter life span effects. In the present study, females were housed individually, and therefore never came in contact with females of the ‘opposite’ behavioral temperament. In the prior study, females were housed socially with sisters that had opposite behavioral traits (i.e. high-locomotion and low-locomotion in the same cage). Although we do not know the familial relationship for females in the current life span study, they do confirm prior findings across families and suggest that daily interactions between females with different behavioral predispositions may introduce an important moderating variable that may override the biological risk-factors identified in the current paper. We have evidence that high- and low-locomotion females take on different social roles within a group and that these roles may have a strong influence on life span (Yee, Cavigelli, McClintock, unpublished data).
An animal model of spontaneous tumor development, with documented variance in life span associated with temperament and HPA activity, provides an ideal system for prospective studies on the relationship between early behavior/temperament/personality, physiology, and later tumor susceptibility/resilience. These models provide a means to study early interventions. In human research, environmental conditions (e.g. socioeconomic status, environmental pollutants, etc.) are often confounded with behavioral and physiological differences among individuals (Brody et al., 2007). A key benefit of this model is the removal of these confounding variables. In addition, differential health behaviors (exercise, smoking, diet) between individuals with differential personalities and/or HPA activity are removed. However, of course, limitations of applicability must be considered. The biological basis of tumor development across species is quite variable (Rudel et al., 2007), and behavioral profiles among species do not necessarily represent homologous traits.
In summary, we have shown that an early-emerging behavioral trait in female rats (activity in a novel environment) is associated with glucocorticoid response to novelty and certain immune parameters. This behavioral trait may be analogous to certain human personality traits associated with naturally-elevated glucococorticoid production in certain individuals (Arranz et al., 2007; Zobel et al., 2004). The results of the current study suggest that females with such personalities may physiologically mimic the effects of chronic environmental stress. In turn, this chronic stress could alter immune system functioning (Peters et al., 2003), leading to specific disease susceptibility (Segerstrom and Miller, 2004; Yang and Glaser, 2003). Alternatively, immune functioning might influence glucocorticoid functioning and/or behavioral traits. Further research on the relationship between personality and physiological biases (neural, endocrine, immune, etc.) will provide greater insight into potential links between personality and disease susceptibility/resilience.
Acknowledgments
We thank A.C. Ross and J.F. Sheridan for advice on possible immune measures, and R. Anolik, C. Barrett, A. Bruscke, M. Diep, K. Haskins, A. Jefferson, C. Kovacsics, C. Mateo, J. McGovern, and K. Mehta, J. Quinn, and R. Smull for expert assistance with data collection. This research was supported by NIMH R03 MH071406 (SAC) and internal Pennsylvania State University Funding (SAC and LCK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci USA. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arranz L, Guayerbas N, De la Fuente M. Impairment of several immune functions in anxious women. J Psychosom Res. 2007;62:1–8. doi: 10.1016/j.jpsychores.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Aspinall R. Longevity and the immune response. Biogerontology. 2000;1:273–278. doi: 10.1023/a:1010046532657. [DOI] [PubMed] [Google Scholar]
- Atkinson HC, Waddell BJ. Circadian variation in basal plasma corticosterone and adrenocorticotrophin in the rat: Sexual dimorphism and changes across the estrous cycle. Endocrinology. 1997;138:3842–3848. doi: 10.1210/endo.138.9.5395. [DOI] [PubMed] [Google Scholar]
- Barnes P. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer: epidemiologic studies. Cancer. 2007;109:2667–2711. doi: 10.1002/cncr.22655. [DOI] [PubMed] [Google Scholar]
- Burleson MH, Poehlmann KM, Hawkley LC, Ernst JM, Bernston GG, Malarkey WB, Kiecolt-Glaser JK, Glaser R, Cacioppo JT. Neuroendocrine and cardiovascular reactivity to stress in mid-aged and older women: Long-term temporal consistency of individual differences. Psychophysiology. 2003;40:358–369. doi: 10.1111/1469-8986.00039. [DOI] [PubMed] [Google Scholar]
- Cacioppo J, Berntson GG, Malarkey WB, Kiecolt-Glaser JK, Sheridan JF, Poehlmann KM, Burleson MH, Ernst JM, Hawkley LC, Glaser R. Autonomic, neuroendocrine, and immune responses to psychological stress: the reactivity hypothesis. Ann NY Acad Sci. 1998;840:664–673. doi: 10.1111/j.1749-6632.1998.tb09605.x. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Lerche NW. Individual differences in peripheral blood immunological and hormonal measures in adult male rhesus macaques (Macaca mulatta): evidence for temporal and situational consistency. Am J Primatol. 1998;44:29–41. doi: 10.1002/(SICI)1098-2345(1998)44:1<29::AID-AJP3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavigelli SA, McClintock MK. Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. Proc Natl Acad Sci USA. 2003;100:16131–16136. doi: 10.1073/pnas.2535721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavigelli SA, Yee JR, McClintock MK. Infant temperament predicts life span in female rats that develop spontaneous tumors. Horm Behav. 2006;50:454–462. doi: 10.1016/j.yhbeh.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hamrick N. Stable individual differences in physiological response to stressors: implications for stress-elicited changes in immune related health. Brain Behav Immun. 2003;17:407–414. doi: 10.1016/s0889-1591(03)00110-7. [DOI] [PubMed] [Google Scholar]
- Derouich-Guergour D, Brenier-Pinchart M, Ambroise-Thomas P, Pelloux H. Tumour necrosis factor alpha receptors: role in the physiopathology of protozoan parasite infections. Int J Parasitol. 2001;31:763–769. doi: 10.1016/s0020-7519(01)00194-1. [DOI] [PubMed] [Google Scholar]
- Dhabar F, Miller A, McEwen B, Spencer R. Differential activation of adrenal steroid receptors in neural and immune tissues of Sprage-Dawley, Fischer 344, and Lewis rats. J Neuroimmunol. 1995;56:77–90. doi: 10.1016/0165-5728(94)00135-b. [DOI] [PubMed] [Google Scholar]
- Dobbs C, Vasquez M, Glaser R, Sheridan J. Mechanisms of stress-induced modulation of viral pathogenesis and immunity. J Neuroimmunol. 1993;48:151–160. doi: 10.1016/0165-5728(93)90187-4. [DOI] [PubMed] [Google Scholar]
- Domínguez-Gerpe L, Rey-Méndez M. Time-course of the murine lymphoid tissue involution during and following stressor exposure. Life Sci. 1997;61:1019–1027. doi: 10.1016/s0024-3205(97)00606-1. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab. 1999;10:359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- Gaffo A, Saag KG, Curtis JR. Treatment of rheumatoid arthritis. Am J Health Syst Pharm. 2006;63:2451–2465. doi: 10.2146/ajhp050514. [DOI] [PubMed] [Google Scholar]
- Garssen B. Psychological factors and cancer development: evidence after 30 years of research. Clin Psychol Rev. 2004;24:315–338. doi: 10.1016/j.cpr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Hinson JP, Vinson GP, Whitehouse BJ, Price G. Control of zona glomerulosa function in the isolated perfused rat adrenal gland in situ. J Endocrinol. 1985;104:387–395. doi: 10.1677/joe.0.1040387. [DOI] [PubMed] [Google Scholar]
- Inglis J, Nissim A, Lees D, Hunt S, Chernajovsky Y, Kidd B. The differential contribution of tumour necrosis factor to thermal and mechanical hyperalgesia during chronic inflammation. Arthritis Res Ther. 2005;7:807–816. doi: 10.1186/ar1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz A, Meager A, Otterness I, Issekutz T. The role of tumer necrosis factor-alpha and IL-1 in polymorphonuclear leucocyte and T lymphocyte recruitment to joint inflammating in adjuvant arthritis. Clin Exp Immunol. 1994;97:26–32. doi: 10.1111/j.1365-2249.1994.tb06574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadoulle V, Ogez D, Rokbani L. Cancer, a defect of the psyche? Bull Cancer. 2004;91:249–256. [PubMed] [Google Scholar]
- Kollias G, Douni E, Kassiotis G, Kontoyiannis D. The role of tumor necrosis factor alpha and receptors in modelf of multi-organ inflammation, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Ann Rheum Dis. 1999;58:32–39. doi: 10.1136/ard.58.2008.i32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenger W, Rossi S, Hollander GA. Apoptosis of thymocytes during acute graft-versus-host disease is independent of glucocorticoids. Transplantation. 2000;69:2190–2193. doi: 10.1097/00007890-200005270-00040. [DOI] [PubMed] [Google Scholar]
- Krieger DT. Effect of ocular enucleation and altered lighting regimens at various ages on the circadian periodicity of plasma corticosteroid levels in the rat. Endocrinology. 1973;93:1077–1091. doi: 10.1210/endo-93-5-1077. [DOI] [PubMed] [Google Scholar]
- Lutgendorf S. Individual differences and immune function: Implications for cancer. Brain Behav Immun. 2003;17:106–108. doi: 10.1016/s0889-1591(02)00075-2. [DOI] [PubMed] [Google Scholar]
- Lutgendorf S, Cole S, Costanzo E, Bradley S, Coffin J, Jabbari S, Rainwater K, Ritchie J, Yang M, Sood A. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003;9:4514–4521. [PubMed] [Google Scholar]
- Madden K. Catecholamines, sympathetic innervation, and immunity. Brain Behav Immun. 2003;17:5–10. doi: 10.1016/s0889-1591(02)00059-4. [DOI] [PubMed] [Google Scholar]
- Miller G, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom Med. 2005;67:679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- Montesano R, Soulié P, Eble JA, Carrozzino F. Tumor necrosis factor α confers an invasive, transformed phenotype on mammary epithelial cells. J Cell Sci. 2005;118:3487–3500. doi: 10.1242/jcs.02467. [DOI] [PubMed] [Google Scholar]
- Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M, Kollias G, Balkwill F. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5:828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- Nakaya N, Tsubono Y, Nishino Y, Hosokawa T, Fukudo S, Shibuya D, Akizuki N, Yoshikawa E, Kobayakawa M, Fujimori M, Saito-Nakaya K, Uchitomi Y, Tsuji I. Personality and cancer survival: the Miyagi cohort study. Br J Cancer. 2005;92:2089–2094. doi: 10.1038/sj.bjc.6602610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old LJ. Tumor necrosis factor. Science. 1985;230:630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Orosz P, Echtenacher B, Falk W, Ruschoff J, Weber D, Mannel DN. Enhancement of experimental metastasis by tumor necrosis factor. J Exp Med. 1993;177:1391–1398. doi: 10.1084/jem.177.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péréz-Álvarez L, Baeza I, Arranz L, Marco EM, Borcel E, Guaza C, Viveros MP, de la Fuente M. Behavioral, endocrine, and immunological characteristics of a murine model of premature aging. Dev Comp Immunol. 2005;11:965–976. doi: 10.1016/j.dci.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Peters ML, Godaert GL, Ballieux RE, Heijnen CJ. Moderation of physiological stress responses by personality traits and daily hassles: less flexibility of immune system responses. Biol Psychol. 2003;65:21–48. doi: 10.1016/s0301-0511(03)00096-6. [DOI] [PubMed] [Google Scholar]
- Roggero E, Pérez AR, Tamae-Kakazu M, Piazzon I, Nepomnaschy I, Besedovsky HO, Bottasso OA, del Rey A. Endogenous glucocorticoids cause thymus atrophy but are protective during acute Trypanosoma cruzi infection. J Endocrinol. 2006;190:495–503. doi: 10.1677/joe.1.06642. [DOI] [PubMed] [Google Scholar]
- Rudel R, Attfield K, Schifano J, Brody JG. Chemicals causing mammary gland tumors in animals signal new directions for epidemiology, chemicals testing, and risk assessment for breast cancer prevention. Cancer. 2007;109:2635–2666. doi: 10.1002/cncr.22653. [DOI] [PubMed] [Google Scholar]
- Scott KA, Moore RJ, Arnott CH, East N, Thompson RG, Scallon BJ, Shealy DJ, Balkwill FR. An anti-tumor necrosis factor-alpha antibody inhibits the development of experimental skin tumors. Mol Cancer Ther. 2003;2:445–451. [PubMed] [Google Scholar]
- Segerstrom S. Individual differences, immunity, and cancer: Lessons from personality psychology. Brain Behav Immun. 2003;17:92–97. doi: 10.1016/s0889-1591(02)00072-7. [DOI] [PubMed] [Google Scholar]
- Segerstrom S, Miller G. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H. The stress of life. McGraw-Hill; New York: 1956. [Google Scholar]
- Sharp J, Zammit T, Azar T, Lawson D. Stress-like responses to common procedures in individually and group-housed female rats. Contemp Top Lab Anim Sci. 2003;42:9–18. [PubMed] [Google Scholar]
- Shintani F, Nakaki T, Kanba S, Sato K, Yagi G, Shiozawa M, Aiso S, Kato R, Asai M. Involvement of interleukin-1 in immobilization stress-induced increase in plasma adrenocorticotropic hormone and in release of hypothalamic monoamines in the rat. J Neurosci. 1995;15:1961–1970. doi: 10.1523/JNEUROSCI.15-03-01961.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood A, Bhatty R, Kamat A, Landed C, Han L, Thaker P, Li Y, Gershenson D, Lutgendorf S, Coles S. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells S, Sapolsky R. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav Immun. 2007;21:259–272. doi: 10.1016/j.bbi.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma M, Okabe S, Marino MW, Sakai A, Sueoka E, Fujiki H. Essential role of tumor necrosis factor α (TNF-α) in tumor promotion as revealed by TNF-α deficient mice. Cancer Res. 1999;59:4516–4518. [PubMed] [Google Scholar]
- Van der Velden V. Glucocorticoids: mechanisms of action and anti-inflammatory potential in asthma. Mediators Inflamm. 1998;7:229–237. doi: 10.1080/09629359890910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E, Glaser R. Stress-induced immunomodulation: Implications for tumorigenesis. Brain Behav Immun. 2003;17:37–40. doi: 10.1016/s0889-1591(02)00064-8. [DOI] [PubMed] [Google Scholar]
- Zobel A, Barkow K, Schulze-Rauschenbach S, von Widdern O, Metten M, Pfeiffer U, Schnell S, Wagner M, Maier W. High neuroticism and depressive temperament are associated with dysfunctional regulation of the hypothalamic-pituitary-adrenocortical system in healthy volunteers. Acta Psychiatrica Scandinavica. 2004;109:392–399. doi: 10.1111/j.1600-0447.2004.00313.x. [DOI] [PubMed] [Google Scholar]