Abstract
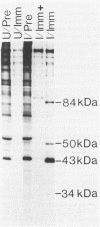
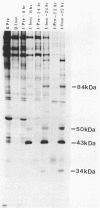
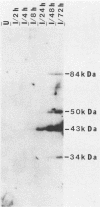
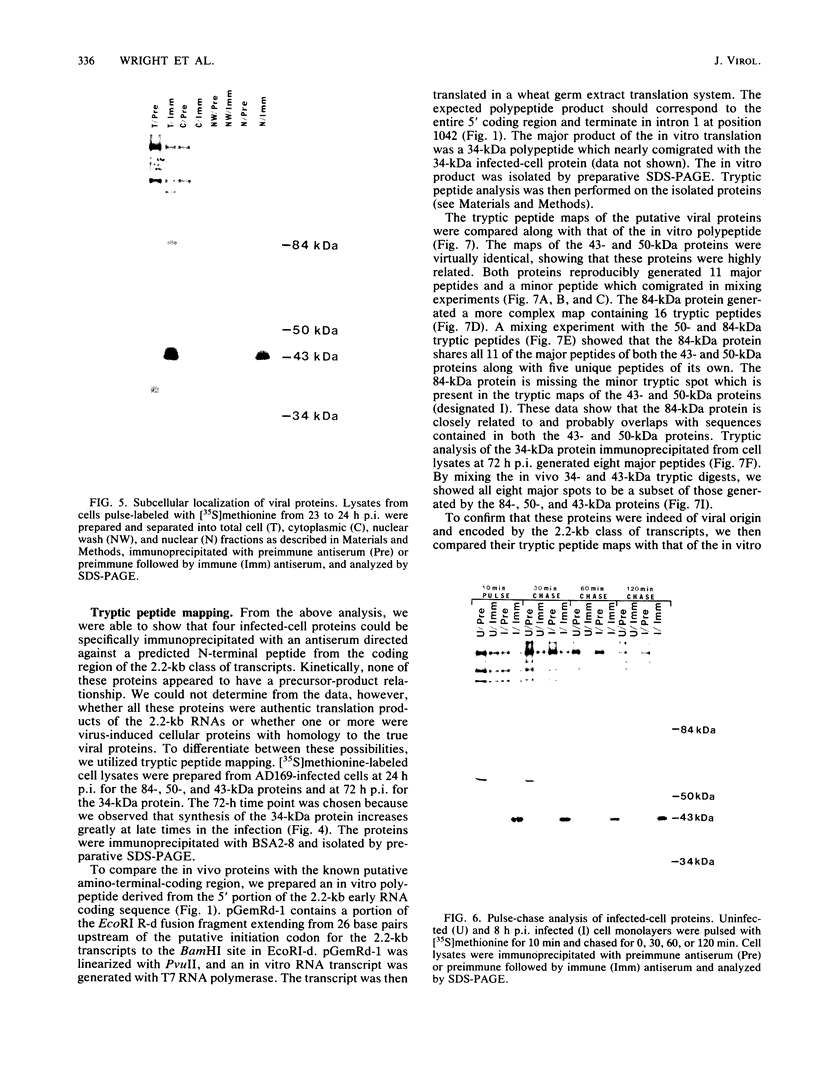
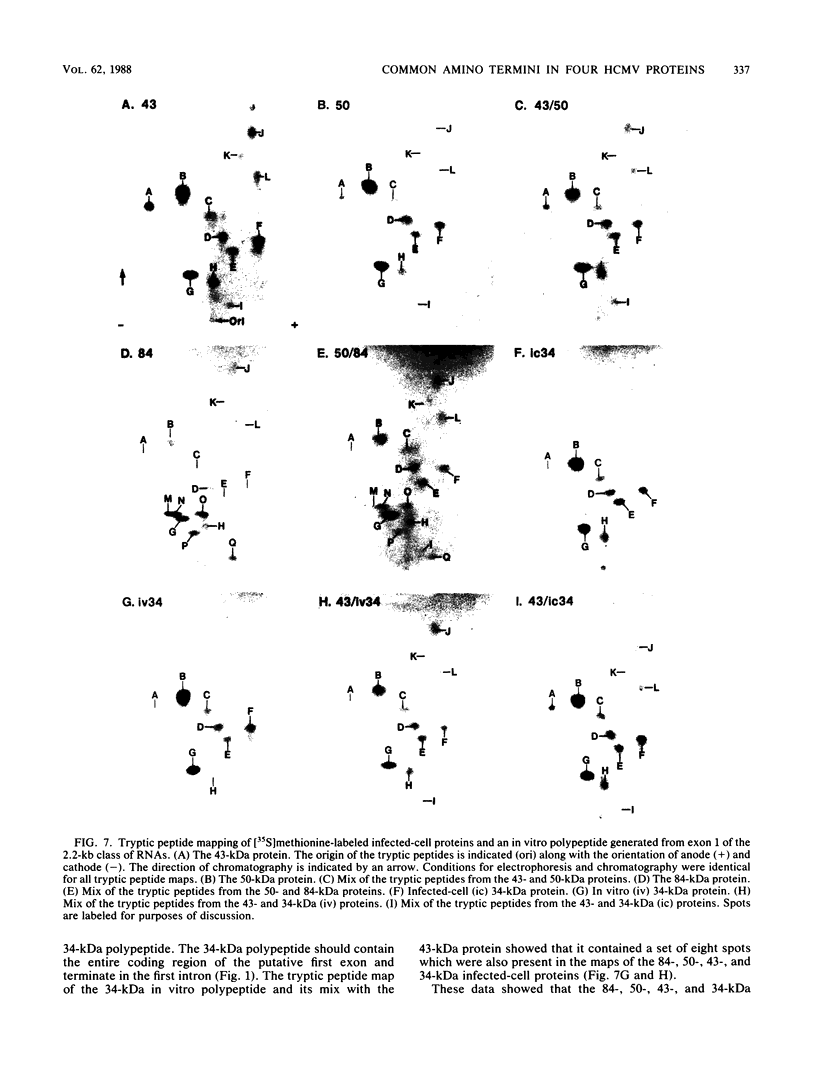
In this report, we identify the proteins encoded by the 2.2-kilobase class of early transcripts arising from a region of the strain AD169 human cytomegalovirus genome (map units 0.682 to 0.713) which contains cell-related sequences. These transcripts, encoded by adjacent EcoRI fragments R and d, have a complex spliced structure with 5' and 3' coterminal ends. Antiserum directed against a synthetic 11-amino-acid peptide corresponding to the predicted amino terminus of the proteins was generated and found to immunoprecipitate four infected-cell proteins of 84, 50, 43, and 34 kilodaltons. These proteins were phosphorylated and were associated predominantly with the nuclei of infected cells. The 43-kilodalton protein was the most abundant of the four proteins, and its level of expression remained relatively constant throughout the infection. Expression of the other proteins increased as the infection progressed. Pulse-chase analysis failed to show a precursor-product relationship between any of the proteins. A comparison of the [35S]methionine-labeled tryptic peptide maps of the four proteins from infected cells and an in vitro-generated polypeptide derived from the putative first exon showed that all four infected-cell proteins were of viral origin and contained a common amino-terminal region.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders D. G., Irmiere A., Gibson W. Identification and characterization of a major early cytomegalovirus DNA-binding protein. J Virol. 1986 May;58(2):253–262. doi: 10.1128/jvi.58.2.253-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisel C., Tanner J., Matsuo T., Thorley-Lawson D., Kezdy F., Kieff E. Two major outer envelope glycoproteins of Epstein-Barr virus are encoded by the same gene. J Virol. 1985 Jun;54(3):665–674. doi: 10.1128/jvi.54.3.665-674.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R., Lewis J. B. Complex splicing patterns of RNAs from the early regions of adenovirus-2. J Mol Biol. 1979 Oct 25;134(2):265–303. doi: 10.1016/0022-2836(79)90036-6. [DOI] [PubMed] [Google Scholar]
- Clanton D. J., Jariwalla R. J., Kress C., Rosenthal L. J. Neoplastic transformation by a cloned human cytomegalovirus DNA fragment uniquely homologous to one of the transforming regions of herpes simplex virus type 2. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3826–3830. doi: 10.1073/pnas.80.12.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarchi J. M. Human cytomegalovirus DNA: restriction enzyme cleavage maps and map locations for immediate-early, early, and late RNAs. Virology. 1981 Oct 15;114(1):23–38. doi: 10.1016/0042-6822(81)90249-x. [DOI] [PubMed] [Google Scholar]
- Geballe A. P., Leach F. S., Mocarski E. S. Regulation of cytomegalovirus late gene expression: gamma genes are controlled by posttranscriptional events. J Virol. 1986 Mar;57(3):864–874. doi: 10.1128/jvi.57.3.864-874.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goins W. F., Stinski M. F. Expression of a human cytomegalovirus late gene is posttranscriptionally regulated by a 3'-end-processing event occurring exclusively late after infection. Mol Cell Biol. 1986 Dec;6(12):4202–4213. doi: 10.1128/mcb.6.12.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Hutchinson M. A., Eckhart W. Translation of polyoma virus T antigens in vitro. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5917–5921. doi: 10.1073/pnas.75.12.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmiere A., Gibson W. Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology. 1983 Oct 15;130(1):118–133. doi: 10.1016/0042-6822(83)90122-8. [DOI] [PubMed] [Google Scholar]
- Jahn G., Knust E., Schmolla H., Sarre T., Nelson J. A., McDougall J. K., Fleckenstein B. Predominant immediate-early transcripts of human cytomegalovirus AD 169. J Virol. 1984 Feb;49(2):363–370. doi: 10.1128/jvi.49.2.363-370.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Barrell B. G. Nucleotide sequence of the transforming region of human cytomegalovirus. Mol Biol Med. 1983 Jul;1(1):47–58. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McDonough S. H., Spector D. H. Transcription in human fibroblasts permissively infected by human cytomegalovirus strain AD169. Virology. 1983 Feb;125(1):31–46. doi: 10.1016/0042-6822(83)90061-2. [DOI] [PubMed] [Google Scholar]
- Nankervis G. A., Kumar M. L. Diseases produced by cytomegaloviruses. Med Clin North Am. 1978 Sep;62(5):1021–1035. doi: 10.1016/s0025-7125(16)31752-7. [DOI] [PubMed] [Google Scholar]
- Nelson J. A., Fleckenstein B., Galloway D. A., McDougall J. K. Transformation of NIH 3T3 cells with cloned fragments of human cytomegalovirus strain AD169. J Virol. 1982 Jul;43(1):83–91. doi: 10.1128/jvi.43.1.83-91.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. A., Fleckenstein B., Jahn G., Galloway D. A., McDougall J. K. Structure of the transforming region of human cytomegalovirus AD169. J Virol. 1984 Jan;49(1):109–115. doi: 10.1128/jvi.49.1.109-115.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricaudet M., Akusjärvi G., Virtanen A., Pettersson U. Structure of two spliced mRNAs from the transforming region of human subgroup C adenoviruses. Nature. 1979 Oct 25;281(5733):694–696. doi: 10.1038/281694a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen R. D., Staprans S. I., Shaw S. B., Spector D. H. Sequences in human cytomegalovirus which hybridize with the avian retrovirus oncogene v-myc are G + C rich and do not hybridize with the human c-myc gene. Mol Cell Biol. 1985 Jun;5(6):1525–1530. doi: 10.1128/mcb.5.6.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S. B., Rasmussen R. D., McDonough S. H., Staprans S. I., Vacquier J. P., Spector D. H. Cell-related sequences in the DNA genome of human cytomegalovirus strain AD169. J Virol. 1985 Sep;55(3):843–848. doi: 10.1128/jvi.55.3.843-848.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Hock L., Tamashiro J. C. Cleavage maps for human cytomegalovirus DNA strain AD169 for restriction endonucleases EcoRI, BglII, and HindIII. J Virol. 1982 May;42(2):558–582. doi: 10.1128/jvi.42.2.558-582.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Spector S. A. The oncogenic potential of human cytomegalovirus. Prog Med Virol. 1984;29:45–89. [PubMed] [Google Scholar]
- Staprans S. I., Spector D. H. 2.2-kilobase class of early transcripts encoded by cell-related sequences in human cytomegalovirus strain AD169. J Virol. 1986 Feb;57(2):591–602. doi: 10.1128/jvi.57.2.591-602.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Thomsen D. R., Stinski M. F. Structural analysis of the major immediate early gene of human cytomegalovirus. J Virol. 1984 Jan;49(1):190–199. doi: 10.1128/jvi.49.1.190-199.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Thomsen D. R., Stenberg R. M., Goldstein L. C. Organization and expression of the immediate early genes of human cytomegalovirus. J Virol. 1983 Apr;46(1):1–14. doi: 10.1128/jvi.46.1.1-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro J. C., Hock L. J., Spector D. H. Construction of a cloned library of the EcoRI fragments from the human cytomegalovirus genome (strain AD169). J Virol. 1982 May;42(2):547–557. doi: 10.1128/jvi.42.2.547-557.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Thomsen D. R., Stinski M. F. Temporal regulation of human cytomegalovirus transcription at immediate early and early times after infection. J Virol. 1981 May;38(2):446–459. doi: 10.1128/jvi.38.2.446-459.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G. W., Akrigg A., Greenaway P. J. Transcription of the immediate early genes of human cytomegalovirus strain AD169. Virus Res. 1984;1(2):101–106. doi: 10.1016/0168-1702(84)90067-4. [DOI] [PubMed] [Google Scholar]
- el-Beik T., Razzaque A., Jariwalla R., Cihlar R. L., Rosenthal L. J. Multiple transforming regions of human cytomegalovirus DNA. J Virol. 1986 Nov;60(2):645–652. doi: 10.1128/jvi.60.2.645-652.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]