Abstract
Background
The study was conducted to determine whether the phosphodiesterase (PDE) 3 inhibitor ORG 9935 prevents the resumption of meiosis in primate oocytes during natural menstrual cycles.
Study design
Regularly-cycling adult female macaques (n=8) were followed during the follicular phase and then started on a 2-day treatment regimen of human recombinant gonadotropins to control the timing of ovulation. Monkeys received no further treatment (controls), or ORG 9935. Oocytes were recovered by laparoscopic follicle aspiration 27 hours after an ovulatory stimulus, cultured in vitro in the absence of inhibitor, and inseminated. The primary outcome was the meiotic stage of the oocyte.
Results
In 6 ORG 9935 cycles; 5 of the recovered oocytes were germinal vesicle (GV)-intact, and 1 exhibited GV-breakdown (GVBD). In contrast, all 3 oocytes recovered during control cycles were GVBD (p < 0.05). None of the ORG 9935-treated oocytes underwent fertilization compared with 2/3 (67%) from controls.
Conclusions
These results demonstrate that ORG 9935 blocks resumption of meiosis in the naturally-selected dominant follicle in primates, and suggest that PDE 3 inhibitors have potential clinical use as contraceptives in women.
Keywords: oocyte, meiosis, contraception, phosphodiesterase inhibitor, macaque
1. Introduction
Despite the widespread availability of contraceptives, an unacceptably high rate of unintended pregnancy occurs. According to recent data from the 2002 United States National Survey of Family Growth, 7.4% of sexually active couples in thte United States use no method of contraception, an increase of 2.2% from the last survey of 1995 [1]. Moreover, the number of unintended pregnancy is distributed almost equally between this group of nonusers, and the larger group of contraceptive users who become pregnant due to inconsistent or incorrect use or failure of their method [2]. Since most of the highly effective methods of contraception use synthetic steroid hormones, and real or perceived side-effects of hormones limit their acceptability [3]. the development of highly effective non-coitally related non-hormonal methods could increase the acceptability of contraception and reduce the number of unintended pregnancies, unwanted births, and abortions.
In mammals, meiosis is arrested at prophase I in oocytes of resting (primordial) and growing follicles. A preovulatory surge in gonadotropins triggers reinitiation of oocyte meiotic maturation, such that a fertilizable metaphase II-stage oocyte is available at the time of ovulation. Experiments in rodents demonstrated that a decrease in intracellular cAMP occurs in the oocyte prior to germinal vesicle-breakdown (GVBD) and the resumption of meiosis, and that the enzyme responsible for the drop in cAMP is phosphodiesterase (PDE) 3A [4, 5]. Divergence of PDE isoform expression exists in the ovary; PDE3 in the oocyte and PDE4 in somatic cells represent the primary isoforms expressed within the follicle [4,6,7]. This observed compartmentalization suggests the basis for a novel contraceptive strategy: selective treatment with a PDE3 inhibitor should result in ovulation of a non-fertilizable, immature oocyte without affecting the development or rupture of the follicle, subsequent development of a functional corpus luteum, or normal menstrual cyclicity. Experiments in rodents demonstrating that PDE3 inhibitors prevent oocyte maturation in vitro and in vivo, and prevention of pregnancy in chronically treated females supports this hypothesis [8]. Subsequent studies confirmed that the PDE3 inhibitor ORG 9935 selectively blocks the spontaneous resumption of meiosis that occurs in vitro in rhesus macaque [9] and in human [10] oocytes. More recently, we reported inhibition of gonadotropin-induced oocyte maturation in vivo during controlled ovarian stimulation cycles in macaques [11]. However, conditions of the controlled ovarian stimulation model (e.g., heterogeneity of follicles (and presumably oocytes) produced using supra-physiologic levels of gonadotropins and GnRH antagonists designed to support development of a pool of follicles that would normally undergo apoptosis as the naturally selected dominant follicle develops) limit the interpretation of these results [12].
Ideally, investigations of the cellular and molecular events surrounding oocyte maturation, would utilize the naturally-selected dominant follicle of the spontaneous menstrual cycle. Unfortunately, normal variation in the interval for follicle maturation (e.g., the length of the follicular phase) and the timing of the pre-ovulatory luteinizing hormone (LH) surge among nonhuman primates and women makes follicle sampling during the spontaneous cycle logistically difficult. The technique of controlled ovulation (COv) overcomes the difficulties inherent in studying development of the naturally-selected dominant follicle [13]. Under this protocol, menstrual cycles of rhesus monkeys are monitored, and a 2-day treatment consisting of a GnRH antagonist plus gonadotropins is initiated after dominant follicle selection, but before ovulation. Administration of an ovulatory stimulus allows for precise timing of surgery for retrieval of tissues to study events in the peri-ovulatory follicle. This protocol has been used to precisely time tissue recovery during experiments investigating the role of gonadotropins and local factors in the ovulatory process and luteal development [13]. We recently reported on a novel technique of simultaneous follicle aspiration/irrigation to assist in the retrieval of the single oocyte from the dominant follicle during COv cycles [14].
To determine whether a PDE3 inhibitor has potential use as a contraceptive agent, we designed an experiment to test the hypothesis that oral administration of the PDE3 inhibitor ORG 9935 to rhesus macaques during COv protocols in the natural menstrual cycle will block oocyte maturation but not ovulation and luteal function.
2. Materials and methods
2.1. Controlled ovulation (COv) protocol
The general care and housing of rhesus monkeys at the Oregon National Primate Research Center (ONPRC) was described previously [15]. The ONPRC Institutional Animal Care and Use Committee approved all study protocols and experiments prior to initiation. After a period of observation to confirm occurrence of normal menstrual cycles, adult female rhesus macaques (n=8) weighing 5-6 kg underwent daily venipuncture during the follicular phase [13,16] until serum estradiol levels reached levels ≥80 but ≤120 pg/mL. At this point, animals were started on a 2-day treatment regimen of human recombinant gonadotropins to control the timing of ovulation: On day 1 of treatment, a GnRH antagonist (Antide; Ares Serono Group, Ltd; Geneva, Switzerland, 0.5 mg/kg) and r-hFSH and r-hLH (30 IU each; Repronex, Ferring Pharmaceuticals, Saint-Prex, Switzerland) were administered at 12:00, and gonadotropins alone at 20:00. On day 2, GnRH antagonist and an ovulatory stimulus (1000 IU hCG, Ovidrel, Ares Serono Group, Ltd) were administered at 0600 with laparoscopy performed 27 h later.
Serum concentrations of estradiol and progesterone were determined by specific electrochemoluminescent assay using a Roche Elecsys 2010 analyzer (F. Hoffmann-La Roche Ltd, Basel Switzerland) by the Endocrine Services Core Laboratory, ONPRC [17].
2.2. Treatment with the PDE3 inhibitor ORG 9935
Monkeys either received no further treatment (controls, n=4), or the oral administration of the PDE3 inhibitor ORG 9935 (n=6). In order to avoid side effects (e.g. inappetance) associated with acute dosing, treatment was initiated at a dose of 50 mg/kg/d in the early follicular phase and gradually increased to 200 mg/kg/d by the first day of gonadotropin treatment. This dose regimen had been established as effective in prior studies during controlled ovarian stimulation cycles [11]. Once initiated, treatment with ORG 9935 was continued until laparoscopic egg retrieval, 7-10 days later depending on the time needed for estrogen to reach the threshold level to begin the COv protocol. Since ORG 9935 is completely insoluble, animals received the compound in food treats (e.g. cookie dough). Monkeys that refused the food treat were administered the compound as a suspension in a flavored sweetened water-based drink following light sedation. We based this dose on pharmacokinetic studies (data not shown) done in macaques in our laboratory that demonstrated trough levels of 1000 nmol/L (a dose shown to be effective in preventing GVBD in vitro) [9] associated with a once daily dose of 200 mg/kg orally.
2.3. Single follicle aspiration
Follicle aspiration was performed via laparoscopy on anesthetized animals [18] using a double-needle, continuous irrigation technique to recover the oocyte from the single dominant follicle [14]. The follicular contents were collected in a physiologic buffered solution (TALP-Hepes with 0.3% bovine serum albumin (BSA) and 0.5% heparin), which was also used as the irrigant.
2.4. Oocyte isolation, culture, and in vitro fertilization
Follicular fluid and flush media were pooled and inspected under a dissecting microscope (Olympus SZ-11, Melville, NY) for the oocyte. The oocyte was isolated using a 140 μm pipette tip (Flexipet, Cook Women's Health, Spencer, IN). If the oocyte retained adherent cumulus cells, it was briefly (30 s) treated in a 100 microliter drop of 1% hyaluronidase (Sigma Chemical Co., St. Louis, MO) in TALP-Hepes with 0.3% BSA, to facilitate assessment of nuclear maturation [15]. Denuded oocytes were examined under an inverted microscope (Olympus CK-40, Melville, NY) at 200x, and classified as germinal vesicle (GV)-intact, metaphase I (MI, no GV and no polar body), metaphase II (MII, extruded polar body in perivitelline space), or degenerate (presence of fragmentation or vacuoles in ooplasm and/or aspherical shape) [19].
Recovered oocytes were transferred to 100 μL microdrops of media (TALP with 0.3% BSA) in sterile culture dishes [15]. Prior to, and following the addition of oocytes, the microdrops were equilibrated under mineral oil (Cooper Surgical, Trumbull, CT) at 37°C in a humidified CO2 atmosphere. Oocytes were transferred to fresh media after 24 h. An inverted microscope at 200x was used to assess the meiotic status of oocytes following the initial recovery (T=0), and at 6, 24, and 48 h of incubation.
All oocytes that showed evidence of resumption of meiosis (e.g., MI or MII) on initial recovery underwent in vitro fertilization 6-8 h later using activated fresh sperm from male macaques of proven fertility [20]. Oocytes were re-inspected 18 h later (T=24 h), and the following day (T=48 h) for evidence of further meiotic development, or in the case of the inseminated oocytes, fertilization or embryo development. Those oocytes that underwent GVBD between 0 and 24 h were inseminated at 24 h, with fertilization evaluated at 48 h. The objective criteria for normal fertilization were the observation of the male and female pro-nuclei, and the presence of two polar bodies.
2.5. Outcomes and statistical analysis
The primary outcome measurement was the number of oocytes that had resumed meiosis prior to initial recovery (T=0). The objective criterion for resumption of meiosis was GVBD, indicating progression to the MI stage. Extrusion of the first polar body was considered an objective sign indicating progression to the MII stage. Frequencies of GVBD and fertilization among oocytes exposed to ORG 9935 and controls at the initial recovery (T=0), and at 6, 24, and 48 h were analyzed with Fisher's exact test. All the tests applied two-sided statistics with alpha set at 0.05 and analyses were performed using SPSS for Windows (version 15.0, Chicago, Ill.)
3. Results
Regularly cycling female macaques (n=8) underwent monitoring of hormone levels by cycle Day 2 or 3 following the onset of a total of 23 menstrual cycles. During 8 of these cycles, estrogen failed to rise above 80 pg/mLby cycle Day 10, so the cycle was considered abnormal and abandoned. Gonadotropin therapy was not initiated during 3 cycles when the estradiol level increased from below 80 pg/mLto greater than 120 pg/mL within one day of observation, and in one animal where the estradiol exceeded 120 pg/mL on cycle Day 4. In the remaining 12 cycles where animals received gonadotropins and the COv protocol, a single unruptured dominant follicle was noted at the time of laparoscopy in 10/12 (91%). In one monkey, the duration of gonadotropin treatment was extended by 2 days (to facilitate timing of surgery) and multiple follicles were noted consistent with a typical controlled ovarian stimulation cycle. Estradiol did not rise appropriately during the other failed COv protocol, and a dominant follicle was not seen at laparoscopy.
In all 10 of the successful cycles, monkeys had initiation of the standard COV protocol beginning on or after cycle Day 5. A single dominant follicle was noted at laparoscopy, with an oocyte recovered by follicle aspiration in all but one (90%) of these trials. During four of the successful experiments, animals received no additional treatments (controls). In one control experiment the oocyte was not recovered, due to a malfunction of the suction equipment during the follicle aspiration. As expected, all (3/3) of the oocytes recovered from control COv cycles were noted to have resumed meiosis. Monkeys received treatment with ORG 9935 during six COv cycles. Significantly, 5/6 (83%) of the ORG 9935-treated oocytes were arrested at the GV-intact stage, consistent with results seen during controlled ovarian stimulation cycles (p < 0.05, Fisher's exact test) [11]. (see Table 1).
Table 1.
Maturation Stage, before and after 48 hours of culture, and results of in vitro fertilization of oocytes collected from control and PDE3 inhibitor-treated monkeys during COv protocols.
| Oocyte Stage | ||||
|---|---|---|---|---|
| Initial | 24 h | 48 h | Normal Fertilization | |
| ORG 9935 | GV | M1 | 0PB/1PN | No |
| GV | GV | GV | No | |
| GV | M1 | M1 | No | |
| GV | GV | GV | No | |
| GV | M1 | M1 | No | |
| M1 | 1PB/0PN | Fragmented | No | |
|
| ||||
| Control | M1 | M1 | ATR | No |
| M2 | 2PB/2PN | 4 cell | Yes | |
| M1 | 2PB/2PN | Fragmented | Yes | |
GV = germinal vesicle intact; M1 = metaphase 1; M2 = metaphase 2; PB = polar body; PN= pronucleus; ATR= atretic
Following isolation and initial scoring, recovered oocytes were incubated in the absence of the inhibitor, and observed for subsequent in vitro maturation. Oocytes that progressed to MI or MII underwent insemination. By 24 hours, three additional ORG 9935 treated oocytes underwent GVBD. However, none of the ORG 9935 treated oocytes progressed to MII or underwent normal fertilization after insemination. In contrast, two of the three oocytes recovered from control COv cycles showed evidence of normal fertilization (2 polar body, 2 pronuclei) (Table 1) after sperm exposure.
As noted above, the COv protocol was not initiated in 4 animals because estrogen levels rose above the desired window (≥120 pg/mL). One of these monkeys received ORG 9935 throughout the follicular phase and underwent measurement of ovarian hormones throughout a natural menstrual cycle. Laparoscopy was performed three days after the peak estrogen level. At surgery, an ovulatory stigmata was identified (Fig. 1). Steroid hormone assays demonstrated a biphasic pattern of estrogen followed by progesterone, suggesting development of a corpus luteum which functioned for the expected 14-day interval during the menstrual cycle (Fig. 2).
Fig. 1.
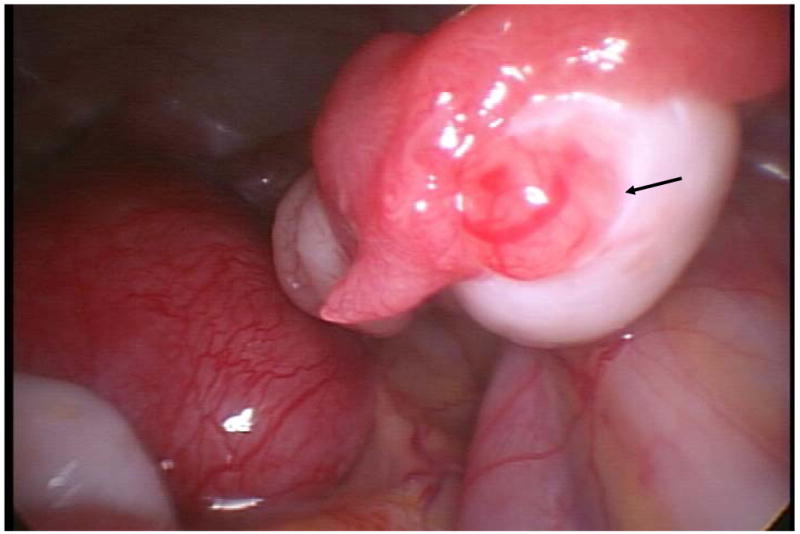
Ovulation site (arrow) on ovary from an ORG 9935 treated rhesus macaque examined by laparoscopy 3 days following the peak estradiol level.
Fig. 2.
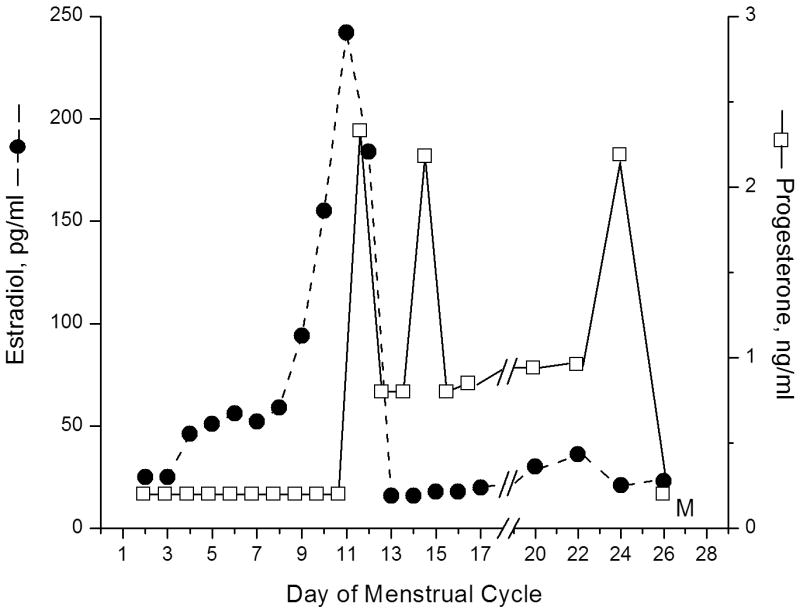
Serum estrogen (dotted line) and progesterone (solid line) values obtained from an animal treated with ORG 9935 200 mg/kg/day throughout the follicular phase (cycle days 2-13). A biphasic pattern of estrogen followed by progesterone secretion is seen, suggesting ovulation. Samples were not collected days 18 and 19.
4. Discussion
The COv protocol represents an important advance in our ability to study events occurring within the naturally-selected dominant follicle during the peri-ovulatory interval. This protocol allows investigators to take control of the timing of ovulation so as to obtain tissue, including the cumulus-oocyte complex (current study) from the naturally-selected dominant follicle at precise intervals following the ovulatory (gonadotropin) stimulus.
Our results using the COv protocol demonstrate that the PDE3 inhibitor ORG 9935 prevents oocyte maturation in the naturally-selected dominant follicle of spontaneous menstrual cycles in rhesus macaques. As expected, oocytes recovered at the end of the peri-ovulatory interval from control animals resumed meiosis, while most oocytes from animals treated with the meiotic inhibitor ORG 9935 did not exhibit GVBD. While 2 of the 3 oocytes from control animals underwent normal fertilization following insemination, the ORG 9935-treated oocytes did not undergo fertilization or progress to MII. These results are consistent with findings observed with primate oocytes failing to undergo spontaneous maturation in vitro during exposure to ORG 9935 [9] and from oocytes not initiating gonadotropin-induced maturation in vivo when obtained from animals treated with ORG 9935 during controlled ovarian stimulation cycles [11].
For conception to occur, cytoplasmic and nuclear maturation of the oocyte must be timed with follicle rupture and ovulation to present a competent MII oocyte to the reproductive tract. Conception is unlikely if the oocyte is not fertilized within 24 hours of ovulation. Therefore, it is possible that delaying oocyte maturation during the gonadotropin-regulated peri-ovulatory interval can interfere with fertilization even if GVBD occurs, and meiosis resumes shortly before, or following ovulation. However, short-term culture of immature human oocytes with ORG 9935 to delay spontaneous nuclear maturation in vitro results in an improvement in fertilization rates compared with oocytes allowed to undergo GVBD in the absence of an inhibitor [21]. These experiments suggest that nuclear and cytoplasmic maturation are highly coordinated events in reproduction and that disruption of either process may affect successful fertilization and embryo development.
Our experiments are the first to extend the model of COV to study of the oocyte. The COv protocol requires careful monitoring of hormone levels in the early follicular phase of the cycle. Young et al. [13] found that when gonadotropin treatment was initiated at estradiol levels above 80 but ≤120 pg/mL, the LH surge was prevented, but with estradiol levels >120 pg/mL, neither the endogenous LH surge, ovulation, or luteal function were controlled. An estradiol level that rose rapidly beyond this 120 pg/ml limit represented the most common reason for cycle cancellation during our experiments. However, cancellation did not prevent continued evaluation of hormonal patterns: these results (Fig. 2) indicate that continuous exposure to ORG 9935 did not alter the characteristic pattern of ovarian steroid hormone in the natural cycle.
A weakness of our results is the small number of animals studied. Nonhuman primates represent a scarce and expensive animal resource. Obtaining the experimental unit in these studies (the single oocyte of the naturally-selected dominant follicle) requires careful coordination of animal husbandry, laboratory follow-up of steroid assays, and surgical services. Still, the limited data supports a significant inhibition of nuclear maturation in oocytes obtained from ORG 9935-treated animals. Additional experiments are needed to support an effect on subsequent fertilization.
These results demonstrate that treatment of macaques with the PDE3 inhibitor ORG 9935 prevents nuclear maturation of the oocyte within the naturally-selected dominant follicle without affecting ovulation or function of the corpus luteum. COV represents a feasible research strategy for study of agents that affect meiosis in the naturally-selected dominant follicle. Further experiments involving long-term treatment of animals in breeding groups will be needed to establish the feasibility of PDE3 inhibitors as novel contraceptive agents.
Acknowledgments
ORG 9935 was provided as a gift of N.V. Organon through the kind assistance of Pieter M. Verbost, PhD. The authors wish to thank the staff of the Division of Animal Resources at ONPRC for excellent surgical and animal care, and David Hess, PhD, and the Endocrine Services Core Laboratory for steroid assays. John Fanton, DVM, is recognized posthumously for his contribution to developing the technique of single follicle aspiration. This research was supported by NICHD/NIH through R01-042710, and by U54 HD18185; U54 HD 031398, and NCRR RR00163 the primate center core grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chandra A, Martinez GM, Mosher WD, et al. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital and health statistics. 2005:1–160. [PubMed] [Google Scholar]
- 2.Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspec Sex Reproduc Health. 2006;38:90–6. doi: 10.1363/psrh.38.090.06. [DOI] [PubMed] [Google Scholar]
- 3.Peipert JF, Gutmann J. Oral contraceptive risk assessment: a survey of 247 educated women. Obstet Gynecol. 1993;82:112–7. [PubMed] [Google Scholar]
- 4.Tsafriri A, Chun SY, Zhang R, et al. Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: studies using selective phosphodiesterase inhibitors. Develop Biol (Orlando) 1996;178:393–402. doi: 10.1006/dbio.1996.0226. [DOI] [PubMed] [Google Scholar]
- 5.Conti M, Andersen CB, Richard FJ, et al. Role of cyclic nucleotide phosphodiesterases in resumption of meiosis. Molec Cell Endocrinol. 1998;145:9–14. doi: 10.1016/s0303-7207(98)00187-7. [DOI] [PubMed] [Google Scholar]
- 6.Mayes MA, Sirard MA. Effect of type 3 and type 4 phosphodiesterase inhibitors on the maintenance of bovine oocytes in meiotic arrest. Biol Reprod. 2002;66:180–4. doi: 10.1095/biolreprod66.1.180. [DOI] [PubMed] [Google Scholar]
- 7.Conti M, Andersen CB, Richard F, et al. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol. 2002;187:153–9. doi: 10.1016/s0303-7207(01)00686-4. [DOI] [PubMed] [Google Scholar]
- 8.Wiersma A, Hirsch B, Tsafriri A, et al. Phosphodiesterase 3 inhibitors suppress oocyte maturation and consequent pregnancy without affecting ovulation and cyclicity in rodents. J Clin Invest. 1998;102:532–7. doi: 10.1172/JCI2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen JT, Schwinof KM, Zelinski-Wooten MB, et al. Phosphodiesterase 3 inhibitors selectively block the spontaneous resumption of meiosis by macaque oocytes in vitro. Hum Reprod. 2002;17:2079–84. doi: 10.1093/humrep/17.8.2079. [DOI] [PubMed] [Google Scholar]
- 10.Nogueira D, Albano C, Adriaenssens T, et al. Human oocytes reversibly arrested in prophase I by phosphodiesterase type 3 inhibitor in vitro. Biol Reprod. 2003;69:1042–52. doi: 10.1095/biolreprod.103.015982. [DOI] [PubMed] [Google Scholar]
- 11.Jensen JT, Zelinski-Wooten MB, Schwinof KM, et al. The phosphodiesterase 3 inhibitor ORG 9935 inhibits oocyte maturation during gonadotropin-stimulated ovarian cycles in rhesus macaques. Contraception. 2005;71:68–73. doi: 10.1016/j.contraception.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Stouffer RL, Zelinski-Wooten MB. Overriding follicle selection in controlled ovarian stimulation protocols: quality vs quantity. Reprod Biol Endocrinol. 2004;2:32. doi: 10.1186/1477-7827-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young KA, Chaffin CL, Molskness TA, et al. Controlled ovulation of the dominant follicle: a critical role for LH in the late follicular phase of the menstrual cycle. Hum Reprod. 2003;18:2257–63. doi: 10.1093/humrep/deg467. [DOI] [PubMed] [Google Scholar]
- 14.Jensen JT, Stanley JE, Zelinski MB, et al. Use of controlled ovulation of the dominant follicle to assess oocyte maturation during natural menstrual cycles in rhesus macaques. Fertil Steril. 2007;87:1477–9. doi: 10.1016/j.fertnstert.2006.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf DP, Thomson JA, Zelinski-Wooten MB, et al. In vitro fertilization-embryo transfer in nonhuman primates: The technique and its applications. Mol Reprod Dev. 1990;27:261–80. doi: 10.1002/mrd.1080270313. [DOI] [PubMed] [Google Scholar]
- 16.Zelinski-Wooten MB, Hutchison JS, Trinchard-Lugan I, et al. Initiation of periovulatory events in gonadotrophin-stimulated macaques with varying doses of recombinant human chorionic gonadotrophin. Hum Reprod. 1997;12:1877–85. doi: 10.1093/humrep/12.9.1877. [DOI] [PubMed] [Google Scholar]
- 17.Young KA, Hennebold JD, Stouffer RL. Dynamic expression of mRNAs and proteins for matrix metalloproteinases and their tissue inhibitors in the primate corpus luteum during the menstrual cycle. Mol Hum Reprod. 2002;8:833–40. doi: 10.1093/molehr/8.9.833. [DOI] [PubMed] [Google Scholar]
- 18.Hibbert ML, Stouffer RL, Wolf DP, et al. Midcycle administration of a progesterone synthesis inhibitor prevents ovulation in primates. Proc Natl Acad Sci USA. 1996;93:1897–1901. doi: 10.1073/pnas.93.5.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zelinski-Wooten MB, Lanzendorf SE, Wolf DP, et al. Titrating luteinizing hormone surge requirements for ovulatory changes in primate follicles. I. Oocyte maturation and corpus luteum function. J Clin Endocrinol Metab. 1991;73:577–83. doi: 10.1210/jcem-73-3-577. [DOI] [PubMed] [Google Scholar]
- 20.Wolf DP, Vandevoort CA, Meyer-Haas GR, et al. In vitro fertilization and embryo transfer in the rhesus monkey. Biol Reprod. 1989;41:335–46. doi: 10.1095/biolreprod41.2.335. [DOI] [PubMed] [Google Scholar]
- 21.Vanhoutte L, De Sutter P, Nogueira D, et al. Nuclear and cytoplasmic maturation of in vitro matured human oocytes after temporary nuclear arrest by phosphodiesterase 3-inhibitor. Hum Reprod. 2007;22:1239–46. doi: 10.1093/humrep/dem007. [DOI] [PubMed] [Google Scholar]


