Abstract
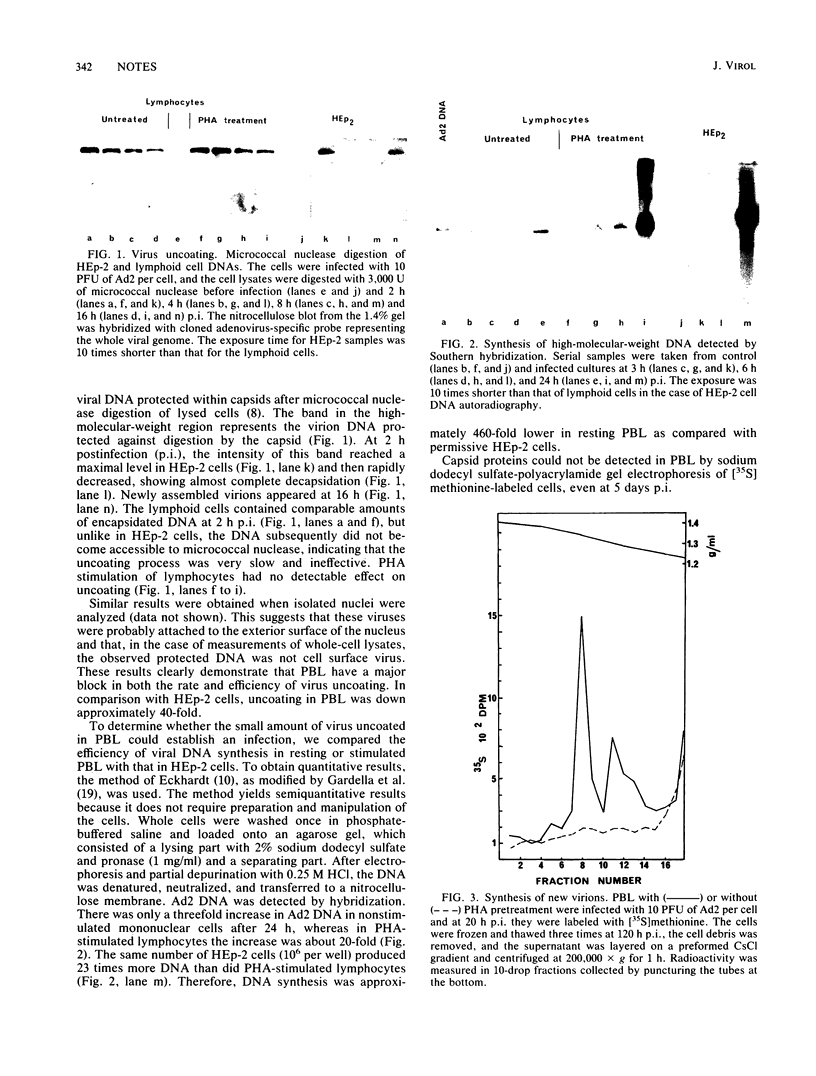
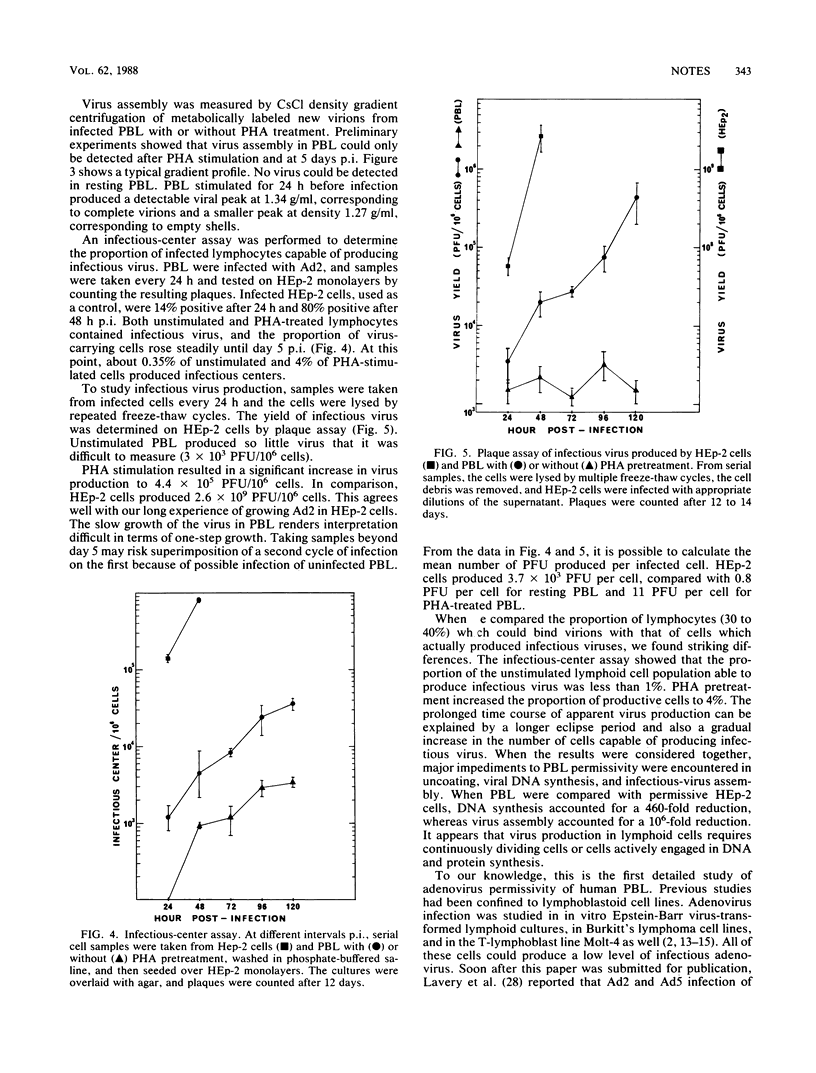
The capacity of freshly explanted human peripheral blood lymphocytes (PBL) to support the replication of human adenovirus type 2 (Ad2) was investigated. Unlike other types of human cells, PBL were found to be highly nonpermissive. Ad2 adsorbed 30 to 40% of both T and non-T cells. Virus uncoating was very slow and inefficient, resulting in a 40-fold reduction compared with HEp-2 cells. On a population basis, viral DNA synthesis was reduced 460-fold and infectious virus production was reduced 10(6)-fold. Only 0.35% of PBL produced infectious centers, yielding 0.8 PFU per infected cell. Phytohemagglutinin stimulation increased DNA synthesis 23-fold, infectious centers 11-fold, and virus yield 14-fold. We conclude that resting human PBL are highly nonpermissive to Ad2 infection and that phytohemagglutinin can only marginally lift this nonpermissiveness.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andiman W. A., Jacobson R. I., Tucker G. Leukocyte-associated viremia with adenovirus type 2 in an infant with lower-respiratory-tract disease. N Engl J Med. 1977 Jul 14;297(2):100–101. doi: 10.1056/NEJM197707142970208. [DOI] [PubMed] [Google Scholar]
- Andiman W. A., Miller G. Persistent infection with adenovirus types 5 and 6 in lymphoid cells from humans and woolly monkeys. J Infect Dis. 1982 Jan;145(1):83–88. doi: 10.1093/infdis/145.1.83. [DOI] [PubMed] [Google Scholar]
- Aterman K., Embil J., Easterbrook K. B., Haldane E. V., Crosby J. Liver necrosis, adenovirus type 2 and thymic dysplasia. Virchows Arch A Pathol Pathol Anat. 1973 Aug 9;360(2):155–171. doi: 10.1007/BF00543226. [DOI] [PubMed] [Google Scholar]
- Boyum A. Separation of blood leucocytes, granulocytes and lymphocytes. Tissue Antigens. 1974;4(4):269–274. [PubMed] [Google Scholar]
- Burgert H. G., Maryanski J. L., Kvist S. "E3/19K" protein of adenovirus type 2 inhibits lysis of cytolytic T lymphocytes by blocking cell-surface expression of histocompatibility class I antigens. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1356–1360. doi: 10.1073/pnas.84.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael G. P., Jr, Zahradnik J. M., Moyer G. H., Porter D. D. Adenovirus hepatitis in an immunosuppressed adult patient. Am J Clin Pathol. 1979 Mar;71(3):352–355. doi: 10.1093/ajcp/71.3.352. [DOI] [PubMed] [Google Scholar]
- Déry C. V., Toth M., Brown M., Horvath J., Allaire S., Weber J. M. The structure of adenovirus chromatin in infected cells. J Gen Virol. 1985 Dec;66(Pt 12):2671–2684. doi: 10.1099/0022-1317-66-12-2671. [DOI] [PubMed] [Google Scholar]
- EVANS A. S. Latent adenovirus infections of the human respiratory tract. Am J Hyg. 1958 May;67(3):256–266. doi: 10.1093/oxfordjournals.aje.a119932. [DOI] [PubMed] [Google Scholar]
- Eager K. B., Williams J., Breiding D., Pan S., Knowles B., Appella E., Ricciardi R. P. Expression of histocompatibility antigens H-2K, -D, and -L is reduced in adenovirus-12-transformed mouse cells and is restored by interferon gamma. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5525–5529. doi: 10.1073/pnas.82.16.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Eron L. Post-transcriptional restriction of human adenovirus expression in monkey cells. J Virol. 1975 May;15(5):1256–1261. doi: 10.1128/jvi.15.5.1256-1261.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucon N., Chardonnet Y., Perrinet M. C., Sohier R. Superinfection with adenovirus of Burkitt's lymphoma cell lines. J Natl Cancer Inst. 1974 Aug;53(2):305–308. doi: 10.1093/jnci/53.2.305. [DOI] [PubMed] [Google Scholar]
- Faucon N., Desgranges C. Persistence of human adenovirus 5 in human cord blood lymphoblastoid cell lines transformed by Epstein-Barr virus. Infect Immun. 1980 Sep;29(3):1180–1184. doi: 10.1128/iai.29.3.1180-1184.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucon N., Ogier G., Chardonnet Y. Changes in human adenovirus 5 propagated in Burkitt's lymphoma cells. J Natl Cancer Inst. 1982 Dec;69(6):1215–1220. [PubMed] [Google Scholar]
- Gallimore P. H. Interactions of adenovirus type 2 with rat embryo cells. Permissiveness, transformation and in vitro characteristics of adenovirus transformed rat embryo cells. J Gen Virol. 1974 Nov;25(2):263–273. doi: 10.1099/0022-1317-25-2-263. [DOI] [PubMed] [Google Scholar]
- Gardella T., Medveczky P., Sairenji T., Mulder C. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J Virol. 1984 Apr;50(1):248–254. doi: 10.1128/jvi.50.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath J., Palkonyay L., Weber J. Group C adenovirus DNA sequences in human lymphoid cells. J Virol. 1986 Jul;59(1):189–192. doi: 10.1128/jvi.59.1.189-192.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horváth J., Kulcsár G., Ugryumov J. P., Dás P., Nász I., Barinsky I. F., Simon G., Ongrádi J. Effect of adenovirus infection on human peripheral lymphocytes. Acta Microbiol Hung. 1983;30(3-4):203–209. [PubMed] [Google Scholar]
- Klessig D. F., Chow L. T. Incomplete splicing and deficient accumulation of the fiber messenger RNA in monkey cells infected by human adenovirus type 2. J Mol Biol. 1980 May 15;139(2):221–242. doi: 10.1016/0022-2836(80)90306-x. [DOI] [PubMed] [Google Scholar]
- Kulcsár G., Sallay K., Dán P., Nász I., Horváth J. Adenovírus-izolálás recidiváló aphthás beteg keringö lymphocytáiból. Fogorv Sz. 1977 Nov;70(11):345–347. [PubMed] [Google Scholar]
- Lambriex M., Van der Veen J. Comparison of replication of adenovirus type 2 and type 4 in human lymphocyte cultures. Infect Immun. 1976 Sep;14(3):618–622. doi: 10.1128/iai.14.3.618-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky R. D., Troy F. A. Possible DNA-RNA tumor virus interaction in human lymphomas: expression of retroviral proteins in Ramos lymphoma lines is enhanced after conversion with Epstein-Barr virus. Proc Natl Acad Sci U S A. 1984 Jan;81(1):33–37. doi: 10.1073/pnas.81.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery D., Fu S. M., Lufkin T., Chen-Kiang S. Productive infection of cultured human lymphoid cells by adenovirus. J Virol. 1987 May;61(5):1466–1472. doi: 10.1128/jvi.61.5.1466-1472.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja G., Winbladh B., Vedin I., Petrini B. Cord blood T lymphocyte subpopulations in premature and full-term infants. Int Arch Allergy Appl Immunol. 1984;75(3):273–275. doi: 10.1159/000233628. [DOI] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Pitha P. M. Herpes simplex virus type-1 can reactivate transcription of latent human immunodeficiency virus. Nature. 1987 Jan 1;325(6099):67–70. doi: 10.1038/325067a0. [DOI] [PubMed] [Google Scholar]
- Myerowitz R. L., Stalder H., Oxman M. N., Levin M. J., Moore M., Leith J. D., Gantz N. M., Hierholzer J. C., Hierholzer J. C. Fatal disseminated adenovirus infection in a renal transplant recipient. Am J Med. 1975 Oct;59(4):591–598. doi: 10.1016/0002-9343(75)90267-3. [DOI] [PubMed] [Google Scholar]
- Nász I., Kulcsár G., Dán P., Sallay K., Vértes L., Geck P., Keskeny S., Horváth J. Aetiological significance of viruses and role of lymphocytes in certain gastrointestinal diseases. Acta Microbiol Acad Sci Hung. 1973;20(3):191–203. [PubMed] [Google Scholar]
- Patterson S., Russell W. C. Ultrastructural and immunofluorescence studies of early events in adenovirus-HeLa cell interactions. J Gen Virol. 1983 May;64(Pt 5):1091–1099. doi: 10.1099/0022-1317-64-5-1091. [DOI] [PubMed] [Google Scholar]
- Persson R., Wohlfart C., Svensson U., Everitt E. Virus-receptor interaction in the adenovirus system: characterization of the positive cooperative binding of virions on HeLa cells. J Virol. 1985 Apr;54(1):92–97. doi: 10.1128/jvi.54.1.92-97.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päbo S., Nilsson T., Peterson P. A. Adenoviruses of subgenera B, C, D, and E modulate cell-surface expression of major histocompatibility complex class I antigens. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9665–9669. doi: 10.1073/pnas.83.24.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schranz V., Kulcsár G., Dán P., Horváth J., Nász I., Barinsky I. F., Ugryumov E. P. Interaction of human lymphocytes and viruses in vitro. Acta Microbiol Acad Sci Hung. 1979;26(1):1–9. [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Shimojo H., Yamashita T. Induction of DNA synthesis by adenoviruses in contact-inhibited hamster cells. Virology. 1968 Nov;36(3):422–433. doi: 10.1016/0042-6822(68)90167-0. [DOI] [PubMed] [Google Scholar]
- Thomasset N., Chardonnet Y. Properties of human adenoviruses 5 and 7 grown in guinea-pig cells. Arch Virol. 1980;66(4):353–358. doi: 10.1007/BF01320631. [DOI] [PubMed] [Google Scholar]
- de Jong P. J., Valderrama G., Spigland I., Horwitz M. S. Adenovirus isolates from urine of patients with acquired immunodeficiency syndrome. Lancet. 1983 Jun 11;1(8337):1293–1296. doi: 10.1016/s0140-6736(83)92411-x. [DOI] [PubMed] [Google Scholar]
- van der Veen J., Lambriex M. Relationship of adenovirus to lymphocytes in naturally infected human tonsils and adenoids. Infect Immun. 1973 Apr;7(4):604–609. doi: 10.1128/iai.7.4.604-609.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]