Abstract
Our purpose was to determine predictors of endothelial function and potential association with cardiovascular risk in women with sedentary occupations, in whom obesity-associated risk factors may contribute to excess morbidity and mortality. Ninety consecutive women (age range 22-63 years; 22 overweight (body-mass index [BMI] ≥25 - 29.9 kg/m2), 42 obese [BMI ≥ 30 kg/m2) underwent measurements of vital signs, lipids, insulin, glucose, C-reactive protein (hsCRP) and sex hormones. Endothelial function was determined by brachial artery flowmediated dilation following 5 minutes of forearm ischemia. Treadmill stress testing was performed with gas exchange analysis at peak exercise (peak VO2) to assess cardiorespiratory fitness. Brachial artery reactivity was negatively associated with Framingham risk score (r=-0.3542, P=0.0007). Univariate predictors of endothelial function included peak VO2 (r=0.4483, P<0.0001), age (r=-0.3420, P=0.0010), body-mass index (r=-0.3065, P=0.0035) and hsCRP (r=-0.2220, P=0.0400). By multiple linear regression analysis with stepwise modeling, peak VO2 (P=0.0003) was the best independent predictor of brachial artery reactivity, with age as the only other variable reaching statistical significance (P=0.0436) in this model. In conclusion, endothelial function is significantly associated with cardiovascular risk in women with sedentary occupations, who were commonly overweight or obese. Even in the absence of routine exercise, cardiorespiratory fitness--rather than conventional risk factors or body mass--is the dominant predictor of endothelial function and suggests a modifiable approach to risk.
Keywords: endothelium, exercise, nitric oxide, obesity, women's health
Endothelial dysfunction is increasingly recognized to be a biomarker of cardiovascular risk in apparently healthy subjects as well as in patients with coronary artery disease.1-4 The endothelium is an active organ system which regulates vascular tone and circulatory homeostasis, largely mediated by autocrine, paracrine and endocrine effects of nitric oxide.2 Mechanism of endothelial dysfunction may include injury from hypertension, dyslipidemia, diabetes and cigarette smoking, with possible contribution of genetic determinants. In this regard, an inverse association between endothelial function, measured by brachial artery flowmediated dilation following 5 minutes of forearm ischemia (flow-mediated dilation), and Framingham risk score was reported by Hill et al 5 in 45 middle-aged men without known cardiovascular disease. The purpose of our study was to determine whether endothelial function as a biomarker of risk may also be relevant to women without known cardiovascular disease, targeting those with sedentary occupations anticipated to have high prevalence of obesityassociated cardiovascular risk factors.6-8
Methods
This study was conducted at the Clinical Center of the National Institutes of Health, following approval by the institutional review board of the National Heart, Lung and Blood Institute, in a consecutive series of women who provided informed written consent to participate in this protocol. Study participants meeting eligibility criteria were office or laboratory workers and were non-pregnant, had no known coronary artery or other vascular disease. All subjects underwent a focused cardiovascular physical examination. All testing was performed in the fasting state, except for water. Venous blood samples were drawn for lipid profile, glucose, insulin, estradiol, follicle-stimulating hormone (FSH) and C-reactive protein (high-sensitivity assay, hsCRP). Cardiovascular risk estimates were calculated using the Framingham Heart Study Prediction Score Sheet for women based on low-density lipoprotein (LDL) cholesterol level (www.nhlbi.nih.gov/about/framingham/riskabs.htm).
Ninety women ranging in age from 22-63 years (average 44±11 years) were enrolled in this study (Table 1). The majority of participants were Caucasian (58%), but with significant participation by African-American (31%) and Asian (12%) racial groups. Two-thirds of the cohort were either overweight (22 women) or obese (42 women). Fifty-four women were of reproductive age with regular menses and of these, 8 were on hormone contraceptives. Thirtysix women were postmenopausal and of these, 5 were on hormone therapy. Compared with postmenopausal women--and consistent with self-reporting of hormonal status--premenopausal women had significantly higher levels of estradiol (107.4±109.3 versus 48.0±37.8 pg/mL, P<0.005) and lower levels of FSH (6.2±5.3 versus 55.4±27.6 units/L, P<0.001).
Table 1.
Clinical characteristics and testing data for study participants (n=90)
| Variable | |
|---|---|
| Age (years) (range) | 44 ± 11 (22-83) |
| Weight (kg) | 81.2 ± 22 |
| Body-mass index | 30.3 ± 8.0 |
| Resting heart rate (bpm) | 71 ± 10 |
| Systolic blood pressure (mmHg) | 118 ± 16 |
| Diastolic blood pressure (mmHg) | 74 ± 10 |
| Total cholesterol (mg/dL) | 190 ± 34 |
| Triglycerides (mg/dL) | 100 ± 67 |
| Low-density lipoprotein cholesterol (mg/dL) | 125 ± 33 |
| High-density lipoprotein cholesterol mg/dL) | 61 ± 14 |
| Glucose (mg/dL) | 93 ± 23 |
| Insulin (micro-international units/mL) | 12.2 ± 9.0 |
| Estradiol (pg/mL) | 84.4 ± 93.1 |
| Follicle stimulating hormone (international units/L) | 25.2 ± 29.8 |
| High sensitivity c-reactive protein (mg/L) (n=86) (median, range) | 4.9 ± 5.2 (2.8, 0-21.8) |
| Exercise testing | |
| Duration (seconds) | 498 ± 131 |
| Respiratory exchange ratio (RQ) | 1.16 ± 0.1 |
| Ventilatory threshold (mL/kg/min) | 18.78 ± 3.8 |
| Peak oxygen consumption (mL/kg/min) | 25.8 ± 6.5 |
Forty-three women (48%) had ≥ 1 risk factor for atherosclerotic cardiovascular disease by history or determined during screening: diabetes- 6, current cigarette smoker- 6, blood pressure >140/90 mmHg- 25, LDL cholesterol >160 mg/dL- 21, high-density lipoprotein (HDL) cholesterol <40 mg/dL- 3. Obese women were more likely to have risk factors (29 of 42; 69%) than lean women (5 of 26; 19%, P=0.002). Of the 19 women on treatment for hypertension, 6 were taking angiotensin-converting enzyme inhibitors, 5 were taking angiotensin receptor blocking agents and the remainder were taking diuretics, beta blockers or calcium channel blockers. Of the 9 women on treatment for dyslipidemia, 8 were on statins and 1 was taking ezetimibe. Twentyseven women were not on pharmacologic treatments for risk factors, including 9 with LDL cholesterol >160 mg/dL, 6 with blood pressure >140/90 mmHg, and one with fasting glucose >126 mg/dL.
Brachial artery flow-mediated dilator responsiveness as an index of endothelial function was performed by an experienced technician, who also performed this testing in the male cohort reported previously from our department.5 Imaging of the left brachial artery proximal to the antecubital fossa was performed using a high-resolution ultrasound (12.5 MHz linear-array transducer) following 10 minutes of rest. Vasodilation was determined as the maximum increase in diameter of the brachial artery during reactive hyperemia created by an inflated cuff (200 mmHg for 5 minutes) on the forearm, distal to the measurement site. Arterial diameter was measured in millimeters from the leading edge of the intima-lumen interface of the near wall (echo zone three) to the leading edge of the lumen-intima interface of the far wall (echo zone five), coincident with the R-wave on the electrocardiogram (i.e., end-diastole). The brachial artery vasodilator response was calculated as: % vasodilation = ([post-ischemia - baseline diameter] X 100)/ baseline diameter. The variability of this analysis (measured twice in blinded fashion by a single operator) was assessed in 16 subjects (including obese women) by this technician; coefficient of variation (standard deviation divded by average of measurements) was 128% for measurement 1 and 134% for measurement 2.
Symptom-limited treadmill exercise testing was performed using the standard Bruce protocol. Respiratory gas analysis was performed using a breath-by-breath analysis of O2 and CO2 on a SensorMedics VMAX 229c instrument (Yorba Linda, CA). Peak oxygen consumption relative to body weight (peak VO2) as a marker of cardiorespiratory fitness and peak respiratory exchange ratio (VCO2/VO2) as a measure of exercise effort were expressed as the highest 20 second averaged samples during the last stage of the exercise test.
Data are reported as mean value ± standard deviation. As prespecified in the protocol, flowmediated dilation of brachial artery as a bioassay for endothelial nitric oxide bioactivity was chosen as the primary measure of vascular health. The covariates of interest as predictors of flow-mediated dilation were divided into three categories: 1) clinical characteristics and laboratory variables, 2) hormonal variables and 3) exercise performance variables. The covariates were first investigated as univariate predictors of brachial artery flow-mediated dilation using simple linear regression and correlation analysis (hsCRP data were logtransformed). Subsequently, the potential confounding effects of age and BMI were investigated separately for each univariate predictor in a 2-factor model with interaction. Multiple regression models for explaining flow-mediated dilation within each predictor category were then constructed using the forward, backward, and stepwise model-building approaches. These model-building approaches were also used to investigate a global multiple regression model for flow-mediated dilation taken over all covariates in the three predictor categories. All analyses were performed using the SAS statistical analysis package, utilizing the STEPWISE, RSQUARE, GLM, and MEANS procedures (SAS User's Guide: Statistics, Version 9 Edition: SAS Institute Inc, Cary, NC). All reported P values are based on two-sided t-tests for continuous data or chi-square analysis for proportions.
Results
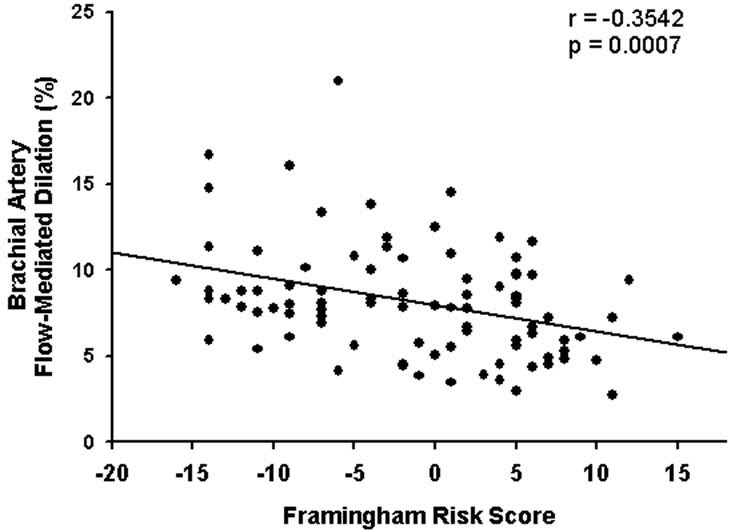
Brachial artery testing revealed baseline arterial diameter of 3.2 ± 0.4 mm and flow-mediated dilation of 8.1±3.3% (range 2.7% to 21.0%). Women with ≥ 1 cardiovascular risk factor had lower values than women without risk factors (7.0±2.1% versus 9.2±3.7%, P=0.0010). Flowmediated dilation was higher in premenopausal women compared with postmenopausal women (8.7± 3.5% versus 7.3±2.7%), but of borderline statistical significance (P=0.0537). Brachial artery flow-mediated dilation correlated inversely with Framingham risk score (Figure 1), with similar correlation coefficients for Caucasian (r=-0.3666), African-American (r=-0.4205) and Asian (r=-0.3414) women.
Figure 1.
Brachial artery flow-mediated dilation is negatively associated with Framingham risk score (www.nhlbi.nih.gov/about/framingham/riskabs.htm), with higher Framingham risk score indicating greater 10-year cardiovascular risk.
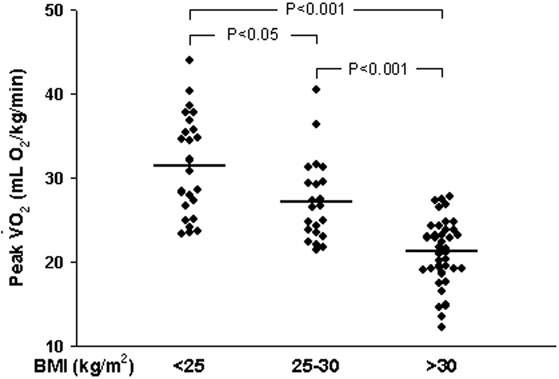
Study participants as a group averaged over 8 minutes during treadmill exercise testing using the standard Bruce protocol (Table 1), but ranged from 3.2 minutes to 16.2 minutes. Peak oxygen consumption ranged from 12.3 mL O2/kg/min to 44.0 mL O2/kg/min. An average respiratory exchange ratio >1.1 is consistent with maximal effort during testing by the group as a whole.9 Exercise duration and peak oxygen consumption declined with increasing BMI, such that exercise performance of obese women (BMI > 30 kg/m2) was approximately one-third less than women with BMI < 25 kg/m2, although many obese women had similar levels of fitness as lean women (Figure 2). For the cohort, peak oxygen consumption was predictive of Framingham Risk Score (r=-0.4455, P<0.0001). Peak oxygen consumption was also predictive of brachial artery flow-mediated dilation (r=0.4483, P<0.0001). Because blood pressure or cholesterollowering medications or estrogen-based drugs may affect endothelial function, correlation between brachial artery flow-mediated dilation and peak VO2 was determined for the 56 women taking neither medications for blood pressure or cholesterol, nor estrogen-based therapies; the association remained strong (r=0.4729, P<0.0001).
Figure 2.
Level of fitness as determined by peak oxygen consumption (Peak VO2) during exercise stress (standard Bruce protocol) is stratified by body-mass index (BMI) as a measure of adiposity.
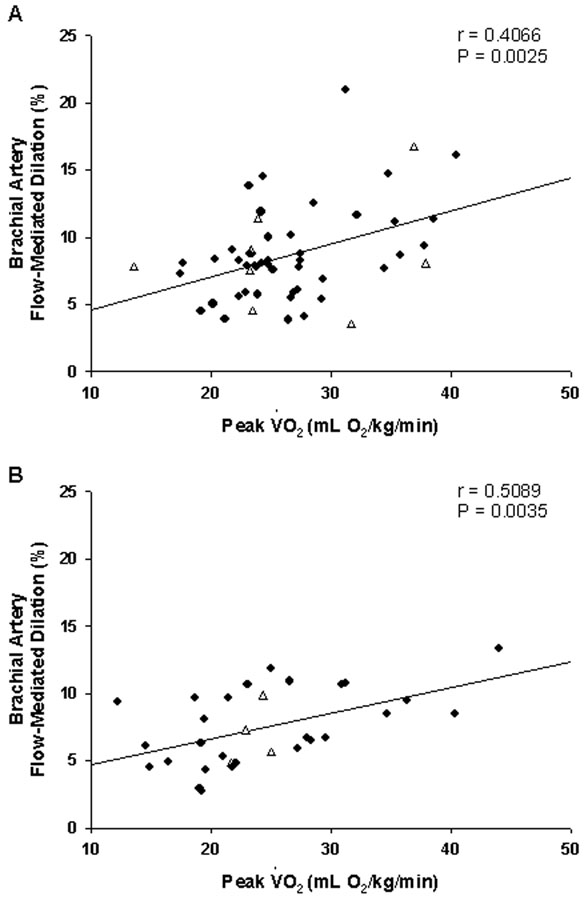
In univariate analyses, age, BMI, hsCRP, peak oxygen consumption, exercise duration and ventilatory threshold were all statistically significant predictors of brachial artery flow-mediated dilation, with FSH, total cholesterol and systolic blood pressure of borderline significance (Table 2). In multivariate analysis of the clinical characteristics, laboratory variables and hormonal variables, the best predictive model for flow-mediated dilation consisted of the two factors age (P= 0.0048) and BMI (P= 0.0093), with no statistically significant interaction between them. In this model, no hormonal status variables were found to be significant predictors of flowmediated dilation. Because of the statistical significance of age and BMI in determining brachial artery flow-mediated dilation with respect to clinical, laboratory and hormonal variables, multivariate analysis of exercise performance variables included age and BMI in the predictive models for flow-mediated dilation. By this process, peak oxygen consumption during exercise (P= 0.0003) and age (P= 0.0436), but not BMI, were together selected for the best predictive model. Since there was evidence of a borderline interaction (P=0.0520) between peak oxygen consumption and age, a stratified analysis of premenopausal (average age 37 years; range 22- 51 years) and postmenopausal women (average age 55 years; range 42-63 years) was performed: The relation between flow-mediated dilation and peak oxygen consumption was significant for both groups (Figure 3).
Table 2.
Univariate Predictors of Brachial Artery Flow-Mediated Dilation
| r value |
p value |
||
|---|---|---|---|
| Demographics and Laboratory Variables | |||
| Age | -0.3420 | 0.0001 | |
| Body-mass index | -0.3065 | 0.0035 | |
| High sensitivity c-reactive protein | -0.2220 | 0.0400 | |
| Total cholesterol | -0.1952 | 0.0684 | |
| Systolic blood pressure | -0.1803 | 0.0909 | |
| Triglycerides | -0.1765 | 0.1000 | |
| Insulin | -0.1687 | 0.1161 | |
| Low-density lipoprotein cholesterol | -0.1650 | 0.1246 | |
| Fasting glucose | -0.1479 | 0.1692 | |
| Diastolic blood pressure | -0.0816 | 0.4446 | |
| High-density lipoprotein cholesterol | 0.0149 | 0.8906 | |
| Hormonal Status Variables | |||
| Follicle stimulating hormone | -0.2090 | 0.0506 | |
| Estradiol/Follicle stimulating hormone | -0.0565 | 0.6014 | |
| Estradiol | 0.0414 | 0.7030 | |
| Exercise Performance Variables | |||
| Peak oxygen consumption | 0.4483 | <0.0001 | |
| Duration of stress test | 0.3819 | 0.0003 | |
| Ventilatory threshold | 0.2500 | 0.0218 | |
Figure 3.
Relationship between brachial artery flow-mediated dilation and peak oxygen consumption (Peak VO2) during treadmill exercise for premenopausal women (Panel A) and postmenopausal women (Panel B). Open triangle symbols indicate women taking hormonal therapies for contraception (Panel A) or for menopausal symptoms (Panel B).
In a global model of all covariates, the forward, backward, and stepwise model-building procedures converged to the same two-factor model including peak oxygen consumption during exercise and age as the best predictive model for brachial artery flow-mediated dilation. The final model was optimal in that 1) it has the largest model R-squared among all two-factor models (R-squared = 0.2404), 2) peak oxygen consumption and age are both strong univariate predictors of brachial artery flow-mediated dilation (P< 0.0001 and P= 0.0010, respectively) that maintain statistical significance when entered together into the same model (P=0.0003 and P= 0.0436, respectively), and 3) there is no additional third predictor that can be entered into the model with statistical significance at the 0.05 level.
Discussion
In our cohort of women with sedentary occupations at the National Institutes of Health, most were overweight or obese and almost half had one or more risk factors for atherosclerotic cardiovascular disease. Consistent with a previous study performed in our department with middle-aged men5, endothelial function, as measured by brachial artery flow-mediated dilation, was inversely related to Framingham risk scores in our cohort and was similar for Caucasian, African-American and Asian women. Consistent with observations in men4, we found significant inverse association between levels of CRP, measured by high-sensitivity assay, and brachial artery flow-mediated dilation. Because of previous reports demonstrating exercise capacity to be a predictor of cardiovascular risk, we measured fitness by peak oxygen consumption during treadmill exercise. Not surprisingly, lean women demonstrated greater exercise fitness than obese women, but with considerable overlap in individual values. By multivariate linear regression analysis with forwards, backwards and stepwise modeling entering demographic, laboratory, hormonal and exercise performance covariates, only exercise fitness and, to a lesser degree, age remained as the best independent predictors of brachial artery endothelial function.
Because our cohort was female, we considered whether hormonal status or treatment might have impacted the results because of potential direct effects on of estrogen on endothelium, as shown in animal models and in treatment trials of postmenopausal women.10-14 In our cohort, premenopausal women had greater brachial artery flow-mediated dilation than postmenopausal women and, by univariate analysis, levels of FSH (but not estradiol) correlated significantly with flow-mediated dilation. By multivariate analysis, however, when adjusted for age and cardiorespiratory fitness, hormonal variables were not significant predictors of endothelial function. A potential limitation of our study was that brachial artery testing was not performed during a uniform phase of the menstrual cycle of premenopausal women, which may have contributed to variability in this measurement. Nonetheless, a significant association between exercise fitness and brachial artery endothelial function was apparent for premenopuasal women as was the case for postmenopausal women, without obvious impact of estrogen-based therapies in either group.
It is possible that women in our cohort with the greatest level of fitness, despite having sedentary occupations, were more active in work and leisure life and thus had better endothelial function by virtue of repetitive stimulation of endothelium by shear stress and up-regulation of endothelial nitric oxide synthase.15,16 In support of this possibility is that lean women, who might be anticipated to be the most active, demonstrated significantly greater exercise performance than obese women, who might be anticipated to be less active. By multivariate analysis, however, body mass did not remain an independent predictor of brachial artery flowmediated dilation as a measure of endothelial function or of peak oxygen consumption during exercise as a measure of cardiopulmonary fitness. In this regard, although obese women as a group had worse exercise performance than lean women, many obese women had similar fitness as lean women. Alternative to the notion that activity-related level of fitness accounts for variability in endothelial function is that endothelial dysfunction, related in part to associated risk factors known to impair endothelial function, might reduce perfusion of skeletal muscles during exercise because of limited nitric oxide bioactivity.17 Against this possibility is the experimental finding that administration of N-monomethyl-L-arginine (an NO-synthase inhibitor) did not alter exercise duration or oxygen consumption in healthy subjects undergoing exercise testing.18
Our determination that cardiorespiratory fitness is a major predictor of cardiovascular risk in women--assessed by Framingham risk score and measurement of endothelial function--is consistent with cohort studies in which exercise performance was measured and associated with mortality risk, including the Aerobics Center Longitudinal Study19 and the St. James Women Take Heart Projec.20 Our study extends these findings by providing mechanistic insight, linking level of fitness with endothelial function, even in individuals who do not engage in regular exercise and are commonly overweight or obese. Our data emphasizing fitness over body mass with respect to cardiovascular risk are consistent with the recent report from the Cooper Clinic, in which cardiorespiratory fitness, measured by maximal treadmill exercise, was a significant mortality predictor in older adults, independent of BMI or abdominal adiposity.21
Analysis of associations among body mass and physical activity with death among participants in the Nurses' Health Study indicated that adiposity--estimated by BMI, as in our study-- predicted a higher risk of death regardless of the level of physical activity as selfreported by their cohort.22 In that study, however, fitness was not assessed by objective measure, in contrast to determination of peak oxygen during graded treadmill exercise stress in our study. Because women in our cohort were selected to be relatively sedentary, it is possible that self-reporting of physical activity in our study and in the Nurses' Health Study missed the contribution of usual daily activities to level of fitness, as suggested by overlap of peak oxygen consumption during exercise among lean, overweight and obese participants in our study. Conversely, there may exist determinants of fitness other than adiposity and the quantity of daily physical activity.
Our study suggests that more fit women, regardless of adiposity, may have better endothelial function than less fit women, with increased nitric oxide bioactivity anticipated to have anti-inflammatory and anti-thrombotic properties that are relevant to the pathophysiology of atherosclerosis.2,3
Footnotes
No author has any conflict of interest related to this study.
References
- 1.Yeboah J, Crouse JR, Hsu F-C, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 2.Lerman A, Zeiher AM. Endothelial function. Cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 3.Anderson TJ. Prognostic significance of brachial flow-mediated vasodilation. Circulation. 2007;115:2372–2375. doi: 10.1161/CIRCULATIONAHA.107.697045. [DOI] [PubMed] [Google Scholar]
- 4.Fichtlscherer S, Rosenberger G, Walter DH, Breuer S, Dimmeler, Zeiher AM. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation. 2000;102:1000–1006. doi: 10.1161/01.cir.102.9.1000. [DOI] [PubMed] [Google Scholar]
- 5.Hill JM, Zalos G, Halcox JPJ, Shenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 6.US Dep. Health Hum. Serv. Physical Activity and Health. A Report of the Surgeon General. US Dep. Health Hum. Serv.: Cent. Dis. Control Prev.; Atlanta, GA: 1996. [Google Scholar]
- 7.McGinnis JM. The public health burden of a sedentary lifestyle. Med Sci Sports Exerc. 1992;24:S196–200. [PubMed] [Google Scholar]
- 8.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 9.Issekutz B, Rodahl K. Respiratory quotient during exercise. J Appl Physiol. 1961;16:606–610. doi: 10.1152/jappl.1961.16.4.606. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi T, Yamada K, Esaki T, Kuzuya M, Satake S, Ishikawa T, Hidaka H, Iguchi A. Estrogen increases endothelial nitric oxide by a receptor-mediated system. Biochem Biophys Res Commun. 1995;214:847–855. doi: 10.1006/bbrc.1995.2364. [DOI] [PubMed] [Google Scholar]
- 11.Arnal JF, Clamens S, Pechet C, Negre-Salvayre A, Allera C, Girolami JP, Salvayre R, Bayard F. Ethinylestradiol does not enhance the expression of nitric oxide synthase in bovine endothelial cells but increases the release of bioactive nitric oxide by inhibiting superoxide anion production. Proc Natl Acad Sci USA. 1996;93:4108–4113. doi: 10.1073/pnas.93.9.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White CR, Shelton J, Chen SJ, Darley-Usmar V, Allen L, Nabors C, Sanders PW, Chen YF, Oparil S. Estrogen restores endothelial cell function in experimental models of vascular injury. Circulation. 1997;96:1624–1630. doi: 10.1161/01.cir.96.5.1624. [DOI] [PubMed] [Google Scholar]
- 13.Gerhard M, Walsh BW, Tawakol A, Haley EA, Creager SJ, Seely EW, Ganz P, Creager MAI. Estradiol therapy combined with progesterone and endothelium-dependent vasodilation in postmenopausal women. Circulation. 1998;98:1158–1163. doi: 10.1161/01.cir.98.12.1158. [DOI] [PubMed] [Google Scholar]
- 14.Koh KK, Cardillo C, Bui MN, Hathaway L, Csako G, Waclawiw MA, Panza JA, Cannon RO. Vascular effects of estrogen and cholesterol-lowering therapies in hypercholesterolemic postmenopausal women. Circulation. 1999;99:354–360. doi: 10.1161/01.cir.99.3.354. [DOI] [PubMed] [Google Scholar]
- 15.Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res. 1994;74:349–353. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- 16.Niebauer J, Cooke JP. Cardiovascular effects of exercise: Role of endothelial shear stress. J Am Coll Cardiol. 1996;28:1652–1660. doi: 10.1016/S0735-1097(96)00393-2. [DOI] [PubMed] [Google Scholar]
- 17.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Rand WM, Udelson JE, Karas RH. Peripheral vascular endothelial function testing as a noninvasive indicator of coronary artery disease. J Am Coll Cardiol. 2001;38:1843–1849. doi: 10.1016/s0735-1097(01)01657-6. [DOI] [PubMed] [Google Scholar]
- 18.Koller-Strametz J, Matulla B, Wolzt M, Muller M, Enttlicher J, Eichler HG, Schmetterer L. Role of nitric oxide in exercised-induced vasodilation in man. Life Sci. 1998;62:1035–1042. doi: 10.1016/s0024-3205(98)00024-1. [DOI] [PubMed] [Google Scholar]
- 19.Blair SN, Kohl HW, Paffenbarger RS, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 20.Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, Blair SN. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA. 2007;298:2507–2516. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulati M, Pandey DK, Arnsdorf MF, Lauderdale DS, Thisted RA, Wicklund RH, Al-Hani AJ, Black HR. Exercise capacity and the risk of death in women. The St. James Take Heart Project. Circulation. 2003;108:1554–1559. doi: 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- 22.Li TY, Rana JS, Manson JE, Willett WC, Stampfer MJ, Colditz GA, Rexrode KM, Hu FB. Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation. 2006;113:499–506. doi: 10.1161/CIRCULATIONAHA.105.574087. [DOI] [PMC free article] [PubMed] [Google Scholar]