Abstract
Ferritins are the main iron storage proteins found in animals, plants, and bacteria. The capacity to store iron in ferritin is essential for life in mammals, but the mechanism by which cytosolic iron is delivered to ferritin is unknown. Human ferritins expressed in yeast contain little iron. Human Poly r(C)-Binding Protein 1 (PCBP1) increased the amount of iron loaded into ferritin when expressed in yeast. PCBP1 bound to ferritin in vivo, and bound iron and facilitated iron loading into ferritin in vitro. Depletion of PCBP1 in human cells inhibited ferritin iron loading and increased cytosolic iron pools. Thus, PCBP1 can function as a cytosolic iron chaperone in the delivery of iron to ferritin.
Ferritins are iron storage proteins that are ubiquitously expressed in animals, plants, and bacteria. They serve both to sequester excess iron taken up by the cell and to release stored iron to meet the cell's metabolic needs during iron scarcity (1). In animals, ferritin is a cytosolic heteropolymer consisting of 24 subunits of H- and L-isoforms that assemble into a hollow sphere into which iron is deposited. Ferritin H-chains contain the iron-binding and ferroxidase activities that are required for mineralization of the ferritin core. Deletion of the H-ferritin gene is lethal in mice (2) and in flies (3).
In cells, metallochaperones deliver metals to their cognate enzymes and transporters. Although cytosolic copper and nickel chaperones have been described (4-7), no cytosolic iron chaperones have been identified, despite the presence of numerous iron-dependent enzymes in the cytosol. Frataxin, the protein lacking in the neurological disease Friedreich's ataxia, functions as a mitochondrial iron chaperone for iron-sulfur cluster and heme biosynthesis (8, 9).
Fungi are anomalous among eukaryotes in that they do not express ferritins. We expressed human H- and L-ferritins in the yeast Saccharomyces cerevisiae. The peptides assembled into multimeric complexes with properties similar to native human ferritins, but contained only small amounts of iron (fig. S1, A and B). We hypothesized that yeast might also lack the requisite iron chaperones needed for delivery of iron to ferritin and designed a genetic screen to identify human genes that, when expressed in yeast, could increase the amount of iron loaded into ferritin. We introduced an iron-regulated FeRE/HIS3 reporter construct (10) into a yeast strain expressing H- and L-ferritin (Fig. 1A). This construct confers histidine prototrophy to cells when the reporter is bound and transcriptionally activated by Aft1p, the major iron-dependent transcription factor in yeast. Aft1p is activated during periods of cytosolic iron depletion (11), which could occur if substantial amounts of cytosolic iron were diverted into ferritin.
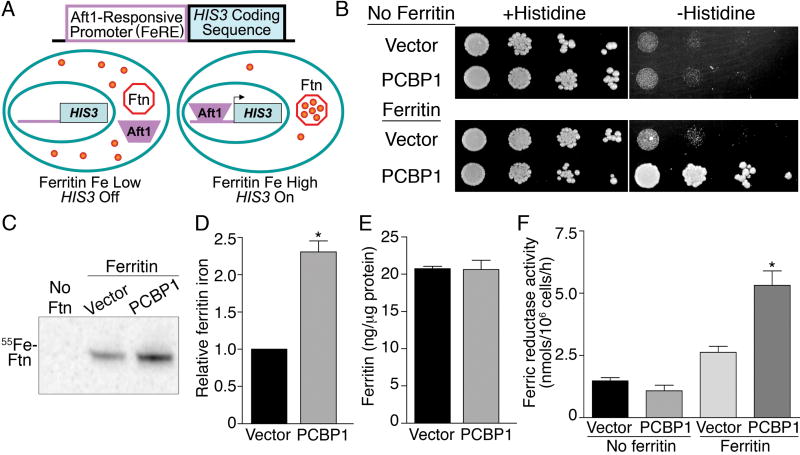
Figure 1.
PCBP1 delivers iron to ferritin in Saccharomyces cerevisiae. (A) Activation of an iron-regulated, Aft1-responsive promoter (FeRE) fused to the HIS3 coding sequence when cytosolic iron is transferred into ferritin. (B) PCBP1-dependent activation of the FeRE/HIS3 reporter in yeast expressing ferritin, but not in yeast without ferritin. Transformed strains were plated in serial dilutions on media with and without histidine. (C and D) PCBP1 increases ferritin mineralization. Strains transformed as in B were grown with 55FeCl3, ferritin was isolated by native gel electrophoresis and iron within ferritin was detected by autoradiography. In D, replicates were normalized to the vector transformed strain (n=5). (E) PCBP1 did not affect ferritin protein levels (n=10). (F) PCBP1-dependent increase in surface ferric reductase activity in yeast expressing ferritin, but not in yeast without ferritin (n=5). *P<0.002. This and subsequent p values were determined using 2-tailed t test.
Yeast cells containing ferritin and the iron-responsive reporter were transformed with a library synthesized from human liver cDNA engineered into a yeast expression vector. Transformants that exhibited growth on plates lacking histidine were selected for further analysis. We isolated multiple copies of Poly r(C)-Binding Protein 1 (PCBP1), as well as other unrelated genes, including H-ferritin. Plasmids containing PCBP1 or the empty vector were retransformed into reporter yeast strains lacking or expressing H- and L-ferritins (Fig. 1B). Expression of PCBP1 did not activate the FeRE/HIS3 reporter in cells lacking ferritin, as indicated by a lack of growth on medium without histidine. But expression of PCBP1 did activate the FeRE/HIS3 reporter in the yeast strain expressing ferritins, resulting in growth on medium lacking histidine. Thus, expression of human PCBP1 activated the iron-responsive reporter only in the presence of ferritin. To confirm that reporter activation was due to delivery of cytosolic iron into ferritin, we directly measured the incorporation of iron into ferritin by growing yeast in the presence of 55FeCl3, isolating ferritin on non-denaturing gels, and measuring the amount of 55Fe in the ferritin heteropolymer (Fig. 1C and D). Significant amounts of iron-containing protein were detected only in cells expressing ferritin, and iron was detected in a single species that co-migrated with the ferritin heteropolymer. Co-expression of PCBP1 in these cells resulted in a 2.3-fold increase in the amount of iron in ferritin. This increase was not due to changes in the overall amount of ferritin (Fig. 1E) or due to changes in the relative ratio of H- and L- chains (fig. S1A). Similarly, the total amount of 55Fe taken up by the cells expressing ferritin alone was not different from the amount taken up by cells expressing both PCBP1 and ferritin (fig. S1C).
The delivery of cytosolic iron to ferritin in the presence of PCBP1 activated the FeRE/HIS3 reporter. We asked whether other proteins expressed during yeast iron deficiency, such as the plasma membrane ferric reductases, were also activated by expression of PCBP1 (Fig. 1F). Ferric reductase activity was low in cells that did not express ferritins, regardless of whether the cells contained pPCBP1 or the empty vector. The ferritin-expressing strain exhibited slightly greater reductase activity than the non-ferritin strain when transformed with vector alone and 4-fold greater reductase activity when the ferritin strain also expressed PCBP1. Thus, when human PCBP1 was expressed in yeast cells containing human ferritins, iron was diverted into ferritin and the cellular iron deficiency response was activated.
PCBP1 is an RNA-binding protein that is ubiquitously expressed in mammalian cells and is located in both the cytosol and the nucleus (12). We tested whether PCBP1 was involved in ferritin iron loading in human cells by depleting cellular PCBP1, loading cells with 55Fe, and measuring the amount of 55Fe loaded into endogenous cytosolic ferritin. Huh7 cells were transfected with PCBP1 or control siRNAs, and partial depletion of PCBP1 mRNA and protein was confirmed (fig. S2). Transfected cells were loaded with 55FeCl3, and ferritin was examined (Fig. 2, A-C). Cells depleted of PCBP1 exhibited a 63% reduction in the amount of 55Fe incorporated into ferritin when compared to control cells at 6, 12, and 24 hrs. This reduction in ferritin iron loading was not due to lowered levels of ferritin protein, because these levels did not change significantly when PCBP1 was depleted (Fig. 2B). The reduction in ferritin iron loading was also not due to loss of 55Fe uptake in the PCBP1-depleted cells, because uptake of both 55FeCl3 and 55Fe2-transferrin was equivalent in cells transfected with control or PCBP1 siRNAs (fig. S3). The loss of ferritin iron loading in cells treated with PCBP1 siRNA was not due to off-target effects of the siRNA. Ferritin iron loading was restored in cells co-transfected with a plasmid expressing human PCBP1 containing silent mutations (fig. S4). The ferritin mineralization that remained after PCBP1 depletion might have been due to the activity of residual PCBP1. Alternatively, paralogues of PCBP1, such as PCBP2, which also activated the FeRE/HIS3 reporter in yeast (fig. S5), may contribute to ferritin iron loading.
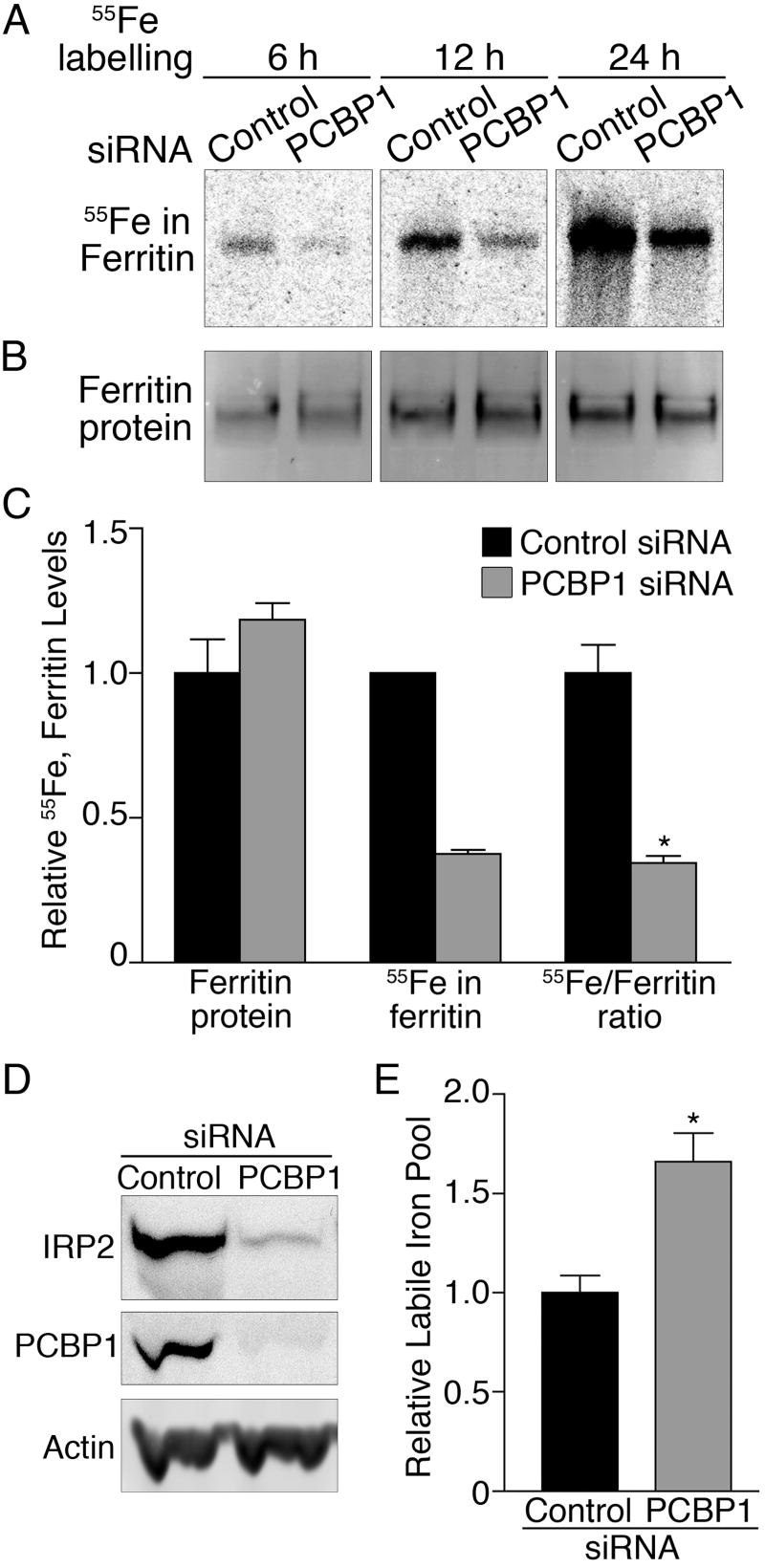
Figure 2.
Depletion of PCBP1 impairs ferritin iron loading in Huh7 cells. Huh7 cells transfected with PCBP1 or control siRNAs were labeled with 55FeCl3 for the indicated times. Ferritin was detected by autoradiography (A), or immunoblotting (B). (C) Relative quantitation of ferritin protein, iron, and iron/protein ratio (n=5). *P<0.0002. (D) Degradation of IRP2 in cells lacking PCBP1. HEK293 cells expressing IRP2 were transfected with PCBP1 and control siRNAs. IRP2, PCBP1, and actin were detected by immunoblotting. (E) Increased labile iron pool in cells lacking PCBP1. PCBP1 was depleted in Huh7 cells, and the relative amounts of the chelatable intracellular iron pool measured (n=6). *P=0.003.
To determine whether the loss of ferritin iron loading during PCBP1 depletion also resulted in an increase in cytosolic iron, we measured the levels of iron regulatory protein 2 (IRP2). The half-life of IRP2 is inversely related to cytosolic iron levels (13), with IRP2 levels increasing when iron is scarce and decreasing when iron is abundant (fig. S6). We transfected HEK293 cells stably overexpressing IRP2 (14) with control and PCBP1 siRNAs and measured the levels of IRP2 (Fig. 2D). Loss of PCBP1 was associated with a decrease in IRP2, consistent with the loss of PCBP1 leading to an increase in the cytosolic iron pool. The relative levels of the chelatable cytosolic iron pool can be measured using fluorescent iron chelators (15), and depletion of PCBP1 in Huh7 cells led to a 67% increase in the chelatable iron pool (Fig. 2E).
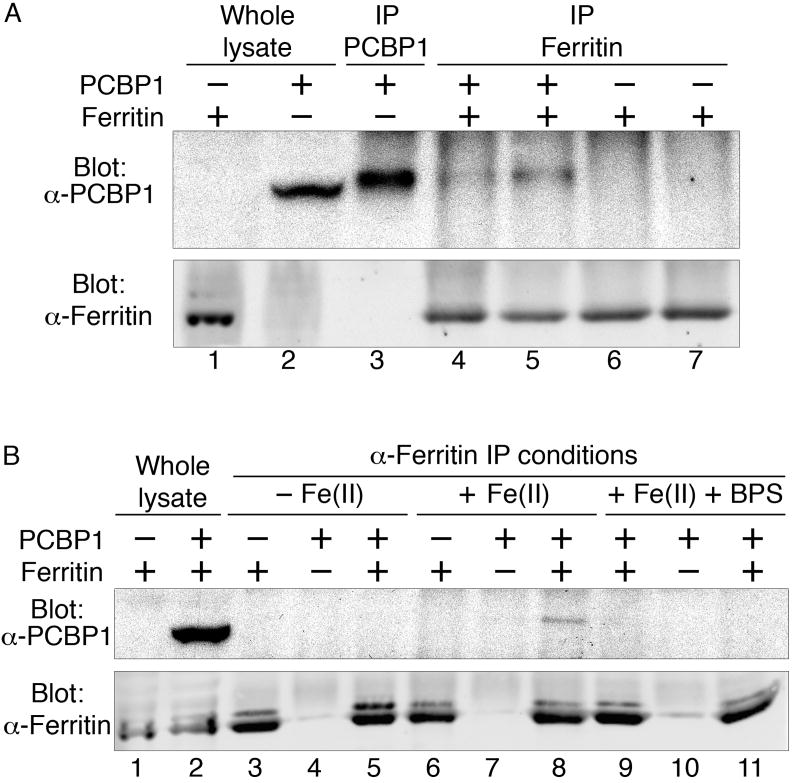
PCBP1 may facilitate ferritin iron loading by directly interacting with ferritin, or by an indirect mechanism that requires other cellular factors. We tested for a direct, in vivo, interaction between ferritin and PCBP1 by co-immunoprecipitation in yeast cells (Fig. 3). PCBP1 co-immunoprecipitated with ferritin in cells expressing PCBP1 and H- and L-ferritin (Fig. 3, A and B). No PCBP1 was detected in immune complexes from cells lacking either PCBP1 or ferritin. When cells expressed both ferritins and PCBP1, PCBP1 was not detected in immunoprecipitates collected in buffer without iron (Fig. 3B), but when ferrous iron was added to the buffer, PCBP1 was detected in anti-ferritin immunoprecipitates (Fig. 3A, 3B). The addition of bathophrenanthroline disulfonate, a ferrous iron chelator, to buffer containing iron blocked the co-immunoprecipitation of PCBP1 with ferritin (Fig. 3B). Thus, PCBP1 physically interacted with ferritin in the presence of iron and might directly bind iron.
Figure 3.
PCBP1 binds to ferritin in vivo. Yeast cells expressing ferritin H- and L-chains, PCBP1, or both were lysed and subjected to immunoprecipitation with anti-PCBP1 or anti-ferritin antibody in buffers containing ferrous iron (A and B), no iron (B), or ferrous iron and a ferrous iron chelator (BPS, B). Immune complexes were detected with antibodies against PCBP1 and ferritin. Note that PCBP1 migrates more rapidly in whole lysates than after IP (A).
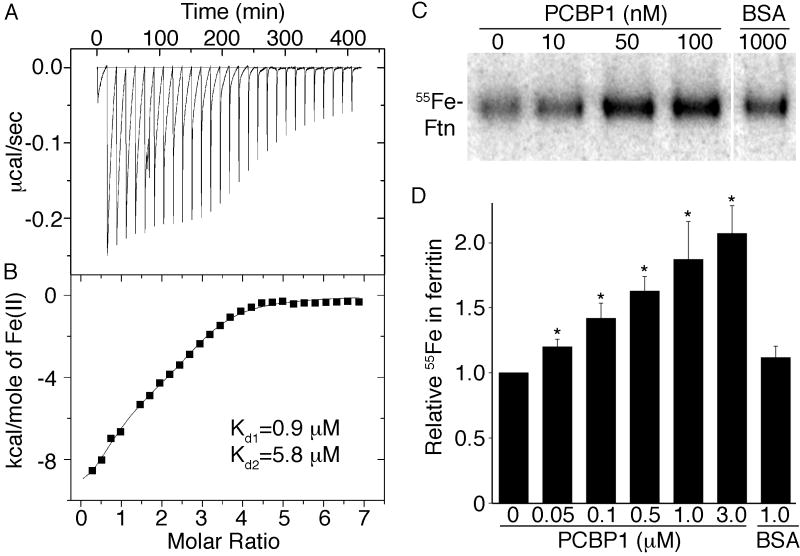
We used isothermal titration calorimetry (ITC) to directly measure interactions between PCBP1 and iron. PCBP1 overexpressed and purified from E. coli was folded with high helical content (fig. S7). Titration of ferrous iron into solutions of PCBP1 under anaerobic conditions produced negative peaks in the raw thermogram, which indicated ferrous iron bound to PCBP1 in an exothermic process (Fig. 4A). An integration of each individual titration peak gave rise to the processed spectrum (Fig. 4B). PCBP1 bound a total of 3 iron atoms with a dissociation constant of 0.9 ± 0.1 μM for the first and an average dissociation constant of 5.8 ± 0.3 μM for the remaining two metal ions.
Figure 4.
PCBP1 binds ferrous iron and increases ferritin iron loading in vitro. (A and B) PCBP1 binds ferrous iron with micromolar affinity by isothermal titration calorimetry (ITC). A, raw ITC spectrum; B, heat evolved per addition of titrant. Metal binding was enthalpically favorable (ΔH E − 9.9 kcal/mol) with an overall favorable ΔG. Binding was entropically unfavorable (ΔS E -10.7 cal/K•mol). (C and D) Increased ferritin mineralization in the presence of PCBP1. Equine apoferritin was incubated with 55Fe(II) and PCBP1 or albumin (BSA). Ferritin iron was detected by autoradiography. (D) Relative 55Fe incorporated into ferritin. Samples were normalized to the sample without added PCBP1 (n=6). *P<0.007.
Although ferritin mineralization occurs in vitro in the presence of only ferrous iron and oxygen, we tested whether PCBP1 could enhance mineralization of ferritin at low iron concentrations (Fig. 4C and D). Addition of purified PCBP1 to solutions of apoferritin and 55Fe(II) increased the amount of 55Fe detected in ferritin in a dose-dependent manner, with mineralization increasing 2-fold at the higher concentrations of PCBP1. Albumin, which binds iron with low affinity, did not significantly alter the amount of iron incorporated into ferritin, indicating that PCBP1 specifically and directly delivered iron for ferritin mineralization in vitro.
Human H-ferritin homopolymers bind ferrous iron with an affinity of 15 μM (16), a concentration far above the levels of “free” ferrous iron thought to be present in cytosol, which raises the likelihood that a specific iron carrier is required for delivery of iron to ferritin. PCBP1 bound 3 atoms of ferrous iron with dissociation constants of 0.9-5.8 μM, which is similar to the binding affinity of yeast frataxin, a mitochondrial iron chaperone that binds 2 ferrous iron atoms with dissociation constants of 2-3 μM (17). Similarly, the cytosolic copper chaperone Atox1 binds copper with a Kd of ∼10 μM (18). PCBP1 bound to ferritin only in the presence of Fe(II). Similarly, the interaction between yeast Atx1p and the copper transporter Ccc2p only occurs in the presence of copper (19).
PCBP1 is a member of a family of four homologous RNA binding proteins belonging to the KH domain superfamily and is widely expressed and highly conserved among mammals. PCBP1 and 2 bind specifically to sequences within multiple cellular or viral mRNA species with a consequent increase in the stability of the messages or alteration of their translation efficiency (12). The bifunctional nature of PCBP1 as both an iron- and a RNA-binding protein is reminiscent of the mammalian iron regulatory protein, IRP1. IRP1 functions as cytosolic aconitase when it contains an iron-sulfur cluster and binds to mRNA transcripts involved in iron homeostasis (such as ferritin) when it does not (13). We propose that PCBP1 acts as a cytosolic iron chaperone, directly binding iron and loading ferritin.
Supplementary Material
Acknowledgments
The authors thank S. Liebhaber for PCBP1 plasmid, protein samples, and helpful discussions; H. Levin for helpful discussions and ferritin plasmids; P. Ponka for salicylaldehyde isonicotinoyl hydrazone; and T. Rouault for helpful discussions, cell lines, and IRP2 antibody. These studies were supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (H. S. and C. C. P.) and by NIH R01 DK068139 (K. Z. B. and T. L. S.).
References
- 1.Harrison PM, Arosio P. Biochim Biophys Acta. 1996 Jul 31;1275:161. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 2.Theil EC, Matzapetakis M, Liu X. J Biol Inorg Chem. 2006 Oct;11:803. doi: 10.1007/s00775-006-0125-6. [DOI] [PubMed] [Google Scholar]
- 3.Missirlis F, et al. Genetics. 2007 Sep;177:89. doi: 10.1534/genetics.107.075150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuchar J, Hausinger RP. Chem Rev. 2004 Feb;104:509. doi: 10.1021/cr020613p. [DOI] [PubMed] [Google Scholar]
- 5.O'Halloran TV, Culotta VC. J Biol Chem. 2000 Aug 18;275:25057. doi: 10.1074/jbc.R000006200. [DOI] [PubMed] [Google Scholar]
- 6.Pufahl RA, et al. Science. 1997;278:853. doi: 10.1126/science.278.5339.853. [DOI] [PubMed] [Google Scholar]
- 7.Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O'Halloran TV. Science. 1999;284:805. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 8.Bulteau AL, et al. Science. 2004 Jul 9;305:242. doi: 10.1126/science.1098991. [DOI] [PubMed] [Google Scholar]
- 9.Pandolfo M. J Neural Transm Suppl. 2006:143. doi: 10.1007/978-3-211-45295-0_22. [DOI] [PubMed] [Google Scholar]
- 10.Dancis A, et al. Cell. 1994;76:393. doi: 10.1016/0092-8674(94)90345-x. [DOI] [PubMed] [Google Scholar]
- 11.Philpott CC, Protchenko O. Eukaryot Cell. 2008 Jan;7:20. doi: 10.1128/EC.00354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Makeyev AV, Liebhaber SA. Rna. 2002 Mar;8:265. doi: 10.1017/s1355838202024627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rouault TA. Nat Chem Biol. 2006 Aug;2:406. doi: 10.1038/nchembio807. [DOI] [PubMed] [Google Scholar]
- 14.Bourdon E, et al. Blood Cells Mol Dis. 2003 Sep-Oct;31:247. doi: 10.1016/s1079-9796(03)00161-x. [DOI] [PubMed] [Google Scholar]
- 15.Epsztejn S, Kakhlon O, Glickstein H, Breuer W, Cabantchik I. Anal Biochem. 1997 May 15;248:31. doi: 10.1006/abio.1997.2126. [DOI] [PubMed] [Google Scholar]
- 16.Bou-Abdallah F, et al. Biochemistry. 2002 Sep 17;41:11184. doi: 10.1021/bi020215g. [DOI] [PubMed] [Google Scholar]
- 17.Cook JD, et al. Biochemistry. 2006 Jun 27;45:7767. doi: 10.1021/bi060424r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wernimont AK, Yatsunyk LA, Rosenzweig AC. J Biol Chem. 2004 Mar 26;279:12269. doi: 10.1074/jbc.M311213200. [DOI] [PubMed] [Google Scholar]
- 19.Banci L, et al. Nat Chem Biol. 2006 Jul;2:367. doi: 10.1038/nchembio797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.