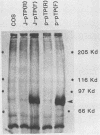
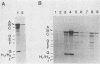
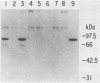
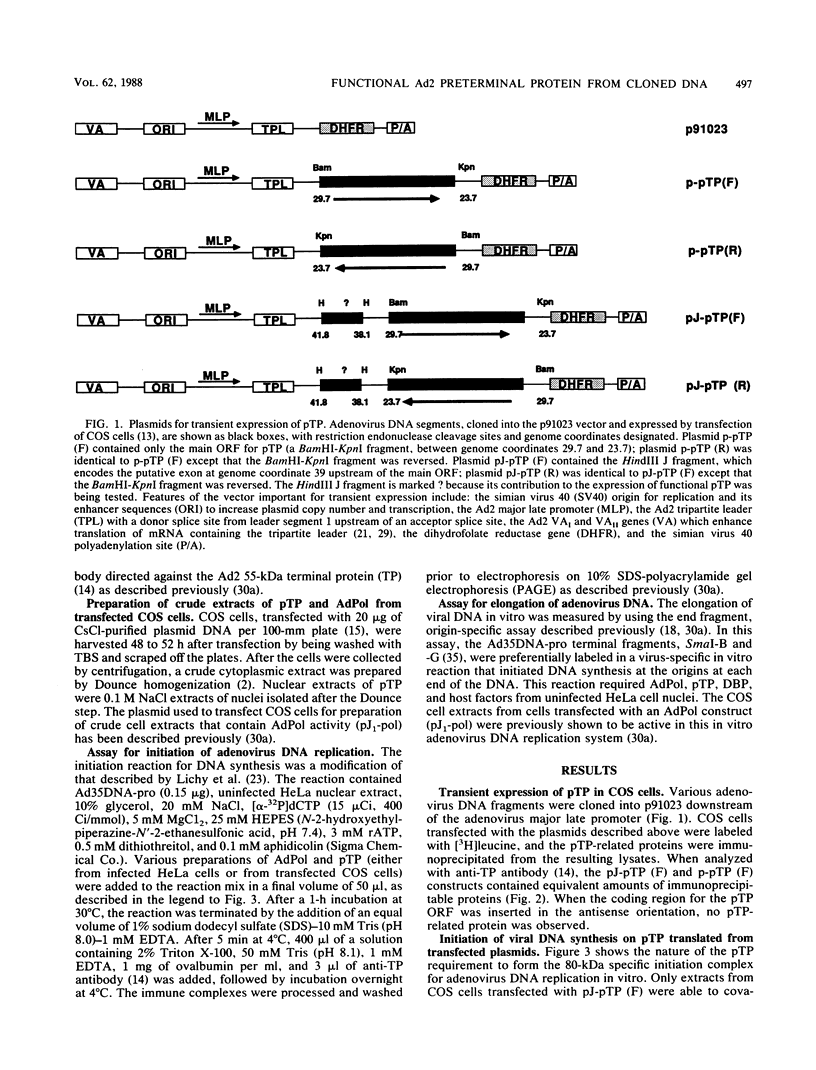
Abstract
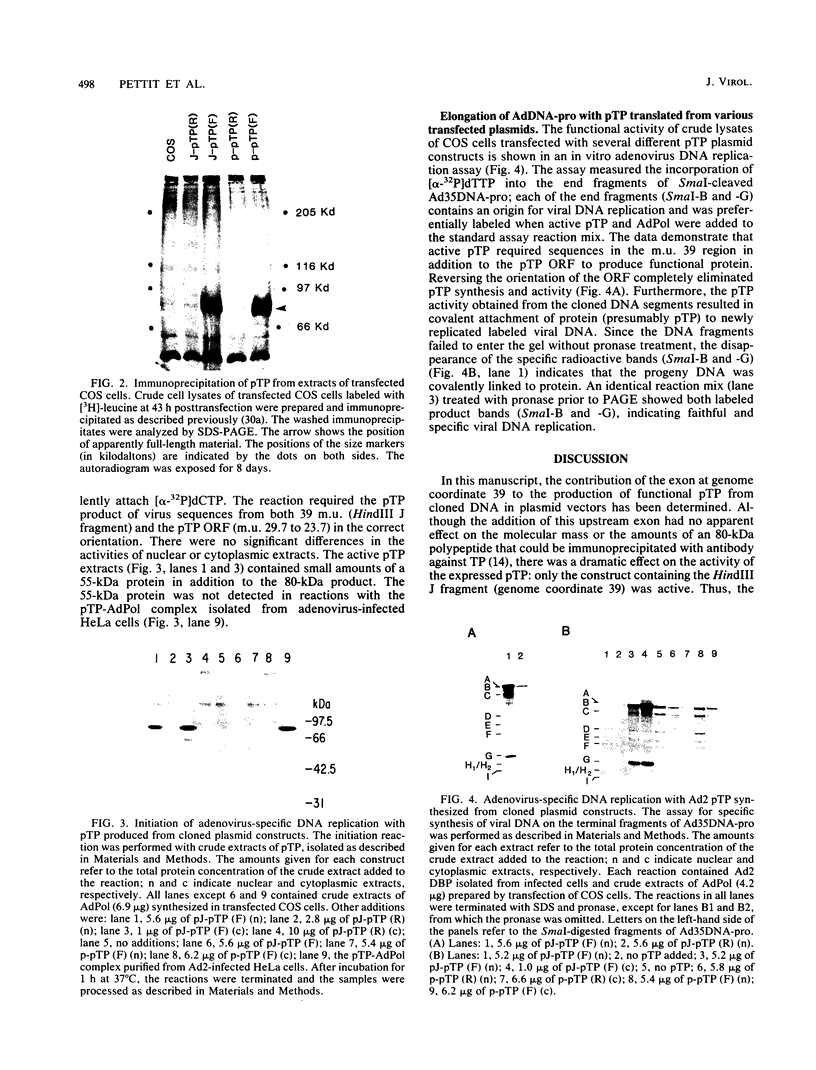
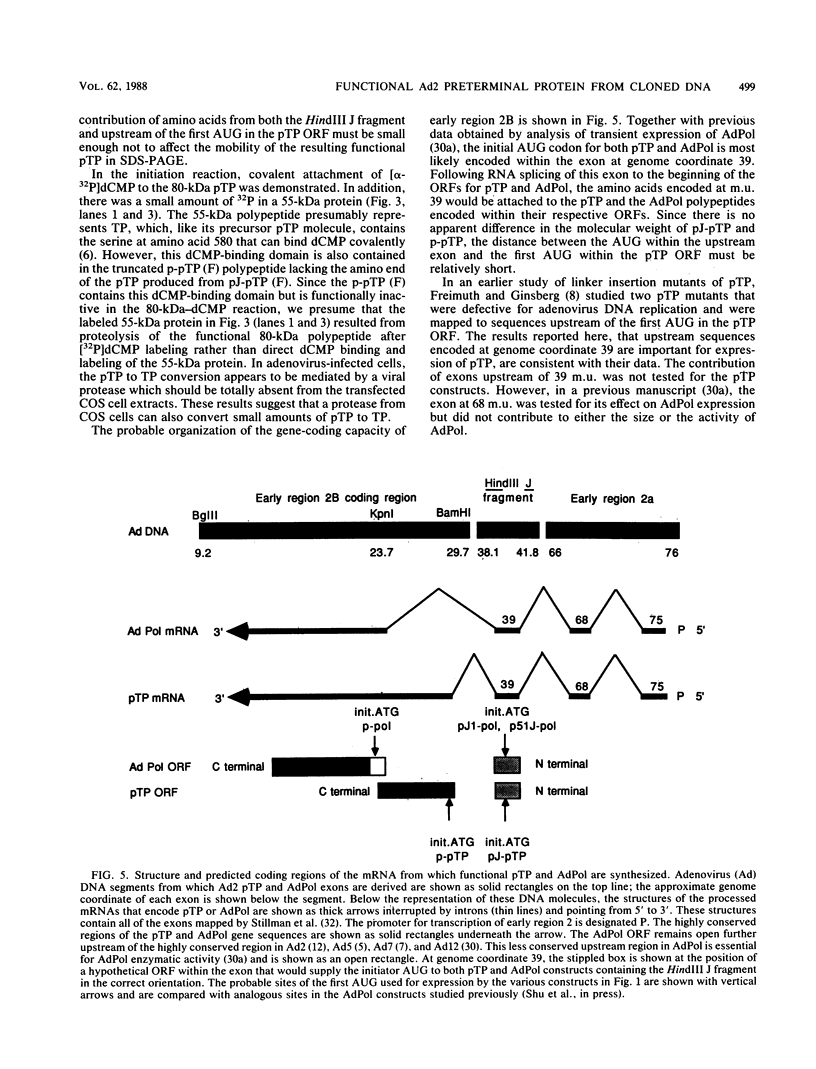
Replication of the DNA genome of human adenovirus serotype 2 requires three virus-encoded proteins. Two of these proteins, the preterminal protein (pTP) and the adenovirus DNA polymerase, are transcribed from a single promoter at early times after virus infection. The mRNAs for these proteins share several exons, including one encoded near adenovirus genome coordinate 39. By using plasmids containing DNA fragments postulated to encode the various exons of pTP mRNA, the contributions of each exon to the synthesis of an active pTP have been measured. Only plasmids that contain both the open reading frame for pTP (genome coordinates 29.4 to 23.9) and the HindIII J fragment that contains the exon at genome coordinate 39 can express functional pTP.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Desiderio S. V., Kelly T. J., Jr Adenovirus DNA replication in vitro: characterization of a protein covalently linked to nascent DNA strands. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5105–5109. doi: 10.1073/pnas.77.9.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Processing of the adenovirus terminal protein. J Virol. 1981 Apr;38(1):272–277. doi: 10.1128/jvi.38.1.272-277.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker B. M., van Ormondt H. The nucleotide sequence of fragment HindIII-C of human adenovirus type 5 DNA (map positions 17.1-31.7). Gene. 1984 Jan;27(1):115–120. doi: 10.1016/0378-1119(84)90244-0. [DOI] [PubMed] [Google Scholar]
- Desiderio S. V., Kelly T. J., Jr Structure of the linkage between adenovirus DNA and the 55,000 molecular weight terminal protein. J Mol Biol. 1981 Jan 15;145(2):319–337. doi: 10.1016/0022-2836(81)90208-4. [DOI] [PubMed] [Google Scholar]
- Engler J. A., Hoppe M. S., van Bree M. P. The nucleotide sequence of the genes encoded in early region 2b of human adenovirus type 7. Gene. 1983 Jan-Feb;21(1-2):145–159. doi: 10.1016/0378-1119(83)90156-7. [DOI] [PubMed] [Google Scholar]
- Freimuth P. I., Ginsberg H. S. Codon insertion mutants of the adenovirus terminal protein. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7816–7820. doi: 10.1073/pnas.83.20.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friefeld B. R., Krevolin M. D., Horwitz M. S. Effects of the adenovirus H5ts125 and H5ts107 DNA binding proteins on DNA replication in vitro. Virology. 1983 Jan 30;124(2):380–389. doi: 10.1016/0042-6822(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Friefeld B. R., Lichy J. H., Field J., Gronostajski R. M., Guggenheimer R. A., Krevolin M. D., Nagata K., Hurwitz J., Horwitz M. S. The in vitro replication of adenovirus DNA. Curr Top Microbiol Immunol. 1984;110:221–255. doi: 10.1007/978-3-642-46494-2_8. [DOI] [PubMed] [Google Scholar]
- Friefeld B. R., Lichy J. H., Hurwitz J., Horwitz M. S. Evidence for an altered adenovirus DNA polymerase in cells infected with the mutant H5ts149. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1589–1593. doi: 10.1073/pnas.80.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Green M., Symington J., Brackmann K. H., Cartas M. A., Thornton H., Young L. Immunological and chemical identification of intracellular forms of adenovirus type 2 terminal protein. J Virol. 1981 Nov;40(2):541–550. doi: 10.1128/jvi.40.2.541-550.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J. L., Rose J. K. Conversion of a secretory protein into a transmembrane protein results in its transport to the Golgi complex but not to the cell surface. Cell. 1984 Jul;37(3):779–787. doi: 10.1016/0092-8674(84)90413-6. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hardwick J. M., Shaw K. E., Wills J. W., Hunter E. Amino-terminal deletion mutants of the Rous sarcoma virus glycoprotein do not block signal peptide cleavage but can block intracellular transport. J Cell Biol. 1986 Sep;103(3):829–838. doi: 10.1083/jcb.103.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S., Ariga H. Multiple rounds of adenovirus DNA synthesis in vitro. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1476–1480. doi: 10.1073/pnas.78.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan L. M., Ariga H., Hurwitz J., Horwitz M. S. Complementation of the temperature-sensitive defect in H5ts125 adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5534–5538. doi: 10.1073/pnas.76.11.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J. Identification of the components necessary for adenovirus translational control and their utilization in cDNA expression vectors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):689–693. doi: 10.1073/pnas.82.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Murtha P. Translational control mediated by eucaryotic initiation factor-2 is restricted to specific mRNAs in transfected cells. Mol Cell Biol. 1987 Apr;7(4):1568–1571. doi: 10.1128/mcb.7.4.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Field J., Horwitz M. S., Hurwitz J. Separation of the adenovirus terminal protein precursor from its associated DNA polymerase: role of both proteins in the initiation of adenovirus DNA replication. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5225–5229. doi: 10.1073/pnas.79.17.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Horwitz M. S., Hurwitz J. Formation of a covalent complex between the 80,000-dalton adenovirus terminal protein and 5'-dCMP in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2678–2682. doi: 10.1073/pnas.78.5.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Nagata K., Friefeld B. R., Enomoto T., Field J., Guggenheimer R. A., Ikeda J. E., Horwitz M. S., Hurwitz J. Isolation of proteins involved in the replication of adenoviral DNA in vitro. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):731–740. doi: 10.1101/sqb.1983.047.01.084. [DOI] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Hurwitz J. Adenovirus DNA replication in vitro: synthesis of full-length DNA with purified proteins. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4266–4270. doi: 10.1073/pnas.80.14.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijn G. J., van Driel W., van der Vliet P. C. Nuclear factor III, a novel sequence-specific DNA-binding protein from HeLa cells stimulating adenovirus DNA replication. Nature. 1986 Aug 14;322(6080):656–659. doi: 10.1038/322656a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld P. J., O'Neill E. A., Wides R. J., Kelly T. J. Sequence-specific interactions between cellular DNA-binding proteins and the adenovirus origin of DNA replication. Mol Cell Biol. 1987 Feb;7(2):875–886. doi: 10.1128/mcb.7.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Weinberger C., Shenk T. Adenovirus VAI RNA facilitates the initiation of translation in virus-infected cells. Cell. 1984 May;37(1):291–298. doi: 10.1016/0092-8674(84)90325-8. [DOI] [PubMed] [Google Scholar]
- Shu L. M., Hong J. S., Wei Y. F., Engler J. A. Nucleotide sequence of the genes encoded in early region 2b of human adenovirus type 12. Gene. 1986;46(2-3):187–195. doi: 10.1016/0378-1119(86)90403-8. [DOI] [PubMed] [Google Scholar]
- Shu L. M., Horwitz M. S., Engler J. A. Expression of enzymatically active adenovirus DNA polymerase from cloned DNA requires sequences upstream of the main open reading frame. Virology. 1987 Dec;161(2):520–526. doi: 10.1016/0042-6822(87)90146-2. [DOI] [PubMed] [Google Scholar]
- Stillman B. W. Adenovirus DNA replication in vitro: a protein linked to the 5' end of nascent DNA strands. J Virol. 1981 Jan;37(1):139–147. doi: 10.1128/jvi.37.1.139-147.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W., Lewis J. B., Chow L. T., Mathews M. B., Smart J. E. Identification of the gene and mRNA for the adenovirus terminal protein precursor. Cell. 1981 Feb;23(2):497–508. doi: 10.1016/0092-8674(81)90145-8. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Tamanoi F., Mathews M. B. Purification of an adenovirus-coded DNA polymerase that is required for initiation of DNA replication. Cell. 1982 Dec;31(3 Pt 2):613–623. doi: 10.1016/0092-8674(82)90317-8. [DOI] [PubMed] [Google Scholar]
- Tamanoi F., Stillman B. W. The origin of adenovirus DNA replication. Curr Top Microbiol Immunol. 1984;109:75–87. doi: 10.1007/978-3-642-69460-8_3. [DOI] [PubMed] [Google Scholar]
- Valderrama-Leon G., Flomenberg P., Horwitz M. S. Restriction endonuclease mapping of adenovirus 35, a type isolated from immunocompromised hosts. J Virol. 1985 Nov;56(2):647–650. doi: 10.1128/jvi.56.2.647-650.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]