Abstract
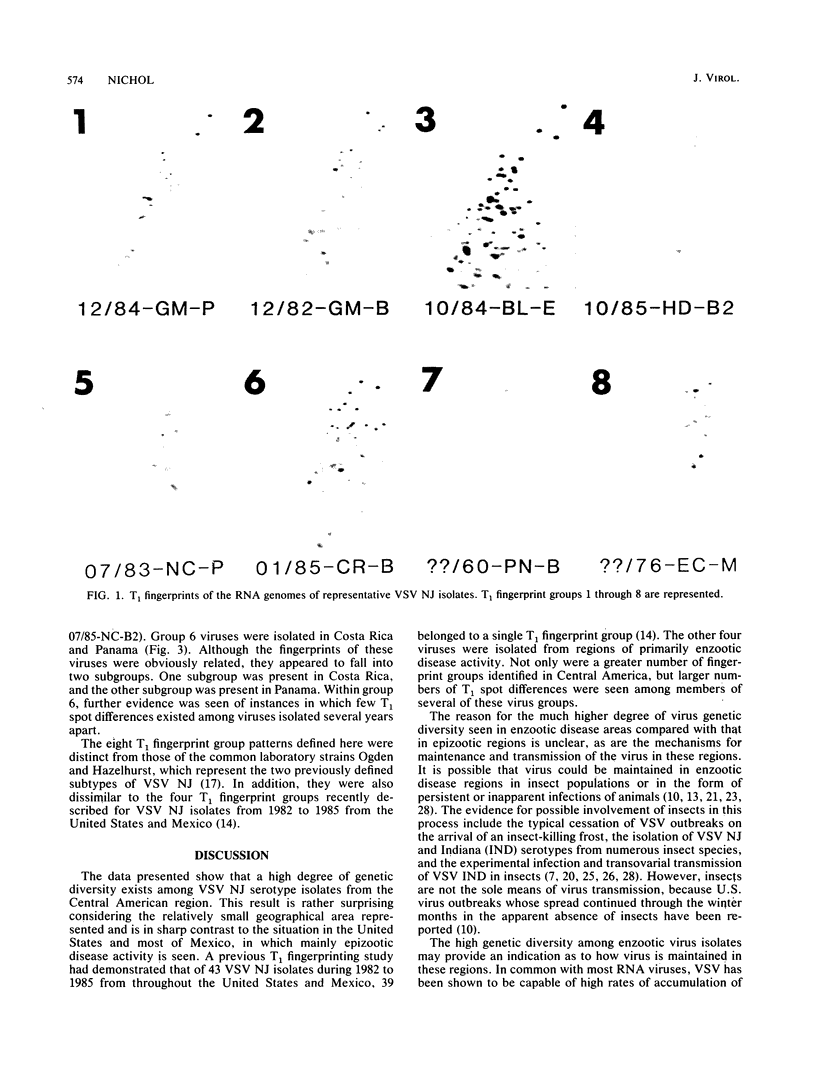
The RNA genomes of 43 vesicular stomatitis virus (VSV) isolates of the New Jersey (NJ) serotype were T1-ribonuclease fingerprinted to compare the extent of genetic diversity of virus from regions of epizootic and enzootic disease activity. Forty of these viruses were obtained from Central America during 1982 to 1985. The other three were older isolates, including a 1970 isolate from Culex nigripalpus mosquitos in Guatemala, a 1960 bovine isolate from Panama, and a 1976 isolate from mosquitos (Mansonia indubitans) in Ecuador. The data indicate that extensive genetic diversity exists among virus isolates from this predominantly enzootic disease zone. Six distinct T1 fingerprint groups were identified for the Central American VSV NJ isolates from 1982 to 1985. The 1960 VSV NJ isolate from Panama and the 1976 isolate from Ecuador formed two additional distinct fingerprint groups. This finding is in sharp contrast to the relatively close genetic relationship existing among VSV NJ isolates obtained from predominantly epizootic disease areas of the United States and Mexico during the same period (S. T. Nichol, J. Virol. 61:1029-1036, 1987). In this previous study, RNA genome T1 fingerprint differences were observed among isolates from different epizootics; however, the isolates were all clearly members of one large T1 fingerprint group. The eight T1 fingerprint groups described here for Central American and Ecuadorian viruses are distinct from those characterized earlier for virus isolates from the United States and Mexico and for the common laboratory virus strains Ogden and Hazelhurst. Despite being isolated 14 years earlier, the 1970 insect isolate from Guatemala is clearly a member of one of the 1982 to 1985 Central American virus fingerprint groups. This indicates that although virus genetic diversity in the region is extensive, under certain natural conditions particular virus genotypes can be relatively stably maintained for an extended period. The implications of these findings for the evolution of VSV NJ and epizootiology of the disease are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson R. P., Young J. F., Palese P. Oligonucleotide mapping: evaluation of its sensitivity by computer-simulation. Nucleic Acids Res. 1982 Jan 11;10(1):237–246. doi: 10.1093/nar/10.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand C., Palese P. Sequential passage of influenza virus in embryonated eggs or tissue culture: emergence of mutants. Virology. 1980 Dec;107(2):424–433. doi: 10.1016/0042-6822(80)90309-8. [DOI] [PubMed] [Google Scholar]
- Calisher C. H., Gutierrez E., Francy D. B., Alava A., Muth D. J., Lazuick J. S. Identification of hitherto unrecognized arboviruses from Ecuador: members of serogroups B, C, Bunyamwera, Patois, and Minatitlan. Am J Trop Med Hyg. 1983 Jul;32(4):877–885. doi: 10.4269/ajtmh.1983.32.877. [DOI] [PubMed] [Google Scholar]
- Clewley J. P., Bishop D. H., Kang C. Y., Coffin J., Schnitzlein W. M., Reichmann M. E., Shope R. E. Oligonucleotide fingerprints of RNA species obtained from rhabdoviruses belonging to the vesicular stomatitis virus subgroup. J Virol. 1977 Jul;23(1):152–166. doi: 10.1128/jvi.23.1.152-166.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRIS D. H., HANSON R. P., DICKE R. J., ROBERTS R. H. Experimental transmission of vesicular stomatitis virus by Diptera. J Infect Dis. 1955 Mar-Apr;96(2):184–192. doi: 10.1093/infdis/96.2.184. [DOI] [PubMed] [Google Scholar]
- Gordon I. Sample size estimation in occupational mortality studies with use of confidence interval theory. Am J Epidemiol. 1987 Jan;125(1):158–162. doi: 10.1093/oxfordjournals.aje.a114499. [DOI] [PubMed] [Google Scholar]
- HANSON R. P. The natural history of vesicular stomatitis. Bacteriol Rev. 1952 Sep;16(3):179–204. doi: 10.1128/br.16.3.179-204.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Grabau E. A., Jones C. L., Semler B. L. Evolution of multiple genome mutations during long-term persistent infection by vesicular stomatitis virus. Cell. 1979 Mar;16(3):495–504. doi: 10.1016/0092-8674(79)90024-2. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Nichol S. T. Molecular epizootiology and evolution of vesicular stomatitis virus New Jersey. J Virol. 1987 Apr;61(4):1029–1036. doi: 10.1128/jvi.61.4.1029-1036.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol S. T., O'Hara P. J., Holland J. J., Perrault J. Structure and origin of a novel class of defective interfering particle of vesicular stomatitis virus. Nucleic Acids Res. 1984 Mar 26;12(6):2775–2790. doi: 10.1093/nar/12.6.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara P. J., Horodyski F. M., Nichol S. T., Holland J. J. Vesicular stomatitis virus mutants resistant to defective-interfering particles accumulate stable 5'-terminal and fewer 3'-terminal mutations in a stepwise manner. J Virol. 1984 Mar;49(3):793–798. doi: 10.1128/jvi.49.3.793-798.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann M. E., Schnitzlein W. M., Bishop D. H., Lazzerini R. A., Beatrice S. T., Wagner R. R. Classification of the New Jersey serotype of vesicular stomatitis virus into two subtypes. J Virol. 1978 Jan;25(1):446–449. doi: 10.1128/jvi.25.1.446-449.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands K., Grabau E., Spindler K., Jones C., Semler B., Holland J. Virus protein changes and RNA termini alterations evolving during persistent infection. Cell. 1980 Apr;19(4):871–880. doi: 10.1016/0092-8674(80)90078-1. [DOI] [PubMed] [Google Scholar]
- SORENSEN D. K., CHOW T. L., KOWALCZYK T., HANSON R. P., BRANDLY C. A. Persistence in cattle of serum-neutralizing antibodies of vesicular stomatitis virus. Am J Vet Res. 1958 Jan;19(70):74–77. [PubMed] [Google Scholar]
- Schnitzlein W. M., Reichmann M. E. Characterization of New Jersey vesicular stomatitis virus isolates from horses and black flies during the 1982 outbreak in Colorado. Virology. 1985 Apr 30;142(2):426–431. doi: 10.1016/0042-6822(85)90352-6. [DOI] [PubMed] [Google Scholar]
- Shelokov A., Peralta P. H. Vesicular stomatitis virus, Indiana type: an arbovirus infection of tropical sandflies and humans? Am J Epidemiol. 1967 Jul;86(1):149–157. doi: 10.1093/oxfordjournals.aje.a120720. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Horodyski F. M., Holland J. J. High multiplicities of infection favor rapid and random evolution of vesicular stomatitis virus. Virology. 1982 May;119(1):96–108. doi: 10.1016/0042-6822(82)90068-x. [DOI] [PubMed] [Google Scholar]
- Steinhauer D. A., Holland J. J. Direct method for quantitation of extreme polymerase error frequencies at selected single base sites in viral RNA. J Virol. 1986 Jan;57(1):219–228. doi: 10.1128/jvi.57.1.219-228.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudia W. D., Fields B. N., Calisher C. H. The isolation of vesiculay stomatitis virus (Indiana strain) and other viruses from mosquitoes in New Mexico, 1965. Am J Epidemiol. 1967 Nov;86(3):598–602. doi: 10.1093/oxfordjournals.aje.a120769. [DOI] [PubMed] [Google Scholar]
- Tesh R. B., Chaniotis B. N., Johnson K. M. Vesicular stomatitis virus (Indiana serotype): transovarial transmission by phlebotomine sandlies. Science. 1972 Mar 31;175(4029):1477–1479. doi: 10.1126/science.175.4029.1477. [DOI] [PubMed] [Google Scholar]
- Walton T. E., Webb P. A., Kramer W. L., Smith G. C., Davis T., Holbrook F. R., Moore C. G., Schiefer T. J., Jones R. H., Janney G. C. Epizootic vesicular stomatitis in Colorado, 1982: epidemiologic and entomologic studies. Am J Trop Med Hyg. 1987 Jan;36(1):166–176. doi: 10.4269/ajtmh.1987.36.166. [DOI] [PubMed] [Google Scholar]
- Wilusz J., Youngner J. S., Keene J. D. Base mutations in the terminal noncoding regions of the genome of vesicular stomatitis virus isolated from persistent infections of L cells. Virology. 1985 Jan 30;140(2):249–256. doi: 10.1016/0042-6822(85)90363-0. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Jones E. V., Kelly M., Frielle D. W. Generation and amplification of temperature-sensitive mutants during serial undiluted passages of vesicular stomatitis virus. Virology. 1981 Jan 15;108(1):87–97. doi: 10.1016/0042-6822(81)90529-8. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Preble O. T., Jones E. V. Persistent infection of L cells with vesicular stomatitis virus: evolution of virus populations. J Virol. 1978 Oct;28(1):6–12. doi: 10.1128/jvi.28.1.6-13.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]