Abstract
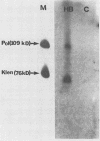
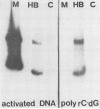
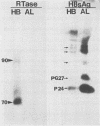
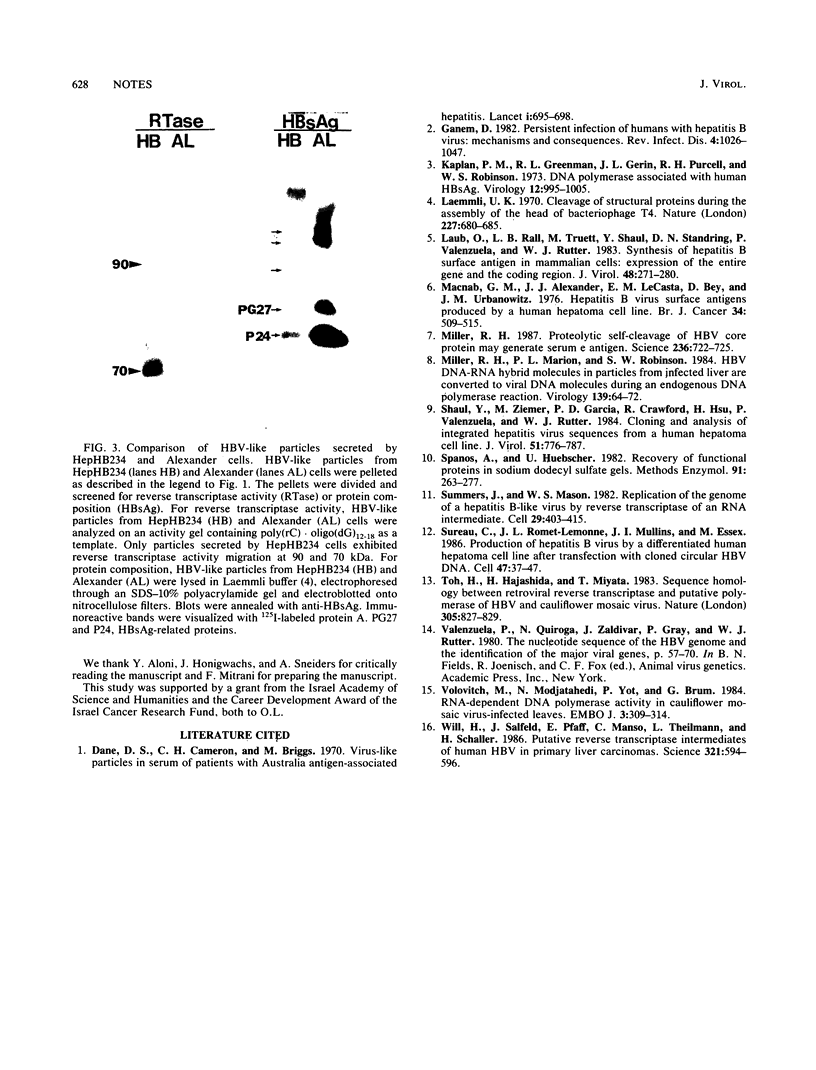
Recent studies suggest that hepatitis B virus (HBV), despite being a DNA virus, replicates via an RNA intermediate (R. H. Miller, P. L. Marion, and S. W. Robinson, Virology 139:64-72, 1984; J. Summers and W. S. Mason, Cell 29:403-415, 1982). The HBV life cycle is therefore a permuted version of the RNA retroviral life cycle. Sequence homology between retroviral reverse transcriptase and the putative HBV polymerase gene product suggests the presence of an HBV reverse transcriptase (H. Toh, H. Hajashida, and T. Miyata, Nature (London) 305:827-829, 1983). As yet, there has been no direct evidence that reverse transcriptase activity is present in the viral particle. We used activity gel analysis to detect the in situ catalytic activities of DNA polymerases after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Our studies demonstrated that HBV-like particles secreted by a differentiated human hepatoma cell line transfected with genomic HBV DNA contain two major polymerase activities which migrate as approximately 90- and approximately 70-kilodalton (kDa) proteins. This demonstrated, for the first time, that HBV-like particles contain a novel DNA polymerase-reverse transcriptase activity. Furthermore, we propose that the 70-kDa reverse transcriptase may be produced by proteolytic self-cleavage of the 90-kDa precursor protein.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dane D. S., Cameron C. H., Briggs M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet. 1970 Apr 4;1(7649):695–698. doi: 10.1016/s0140-6736(70)90926-8. [DOI] [PubMed] [Google Scholar]
- Ganem D. Persistent infection of humans with hepatitis B virus: mechanisms and consequences. Rev Infect Dis. 1982 Sep-Oct;4(5):1026–1047. doi: 10.1093/clinids/4.5.1026. [DOI] [PubMed] [Google Scholar]
- Kaplan P. M., Greenman R. L., Gerin J. L., Purcell R. H., Robinson W. S. DNA polymerase associated with human hepatitis B antigen. J Virol. 1973 Nov;12(5):995–1005. doi: 10.1128/jvi.12.5.995-1005.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laub O., Rall L. B., Truett M., Shaul Y., Standring D. N., Valenzuela P., Rutter W. J. Synthesis of hepatitis B surface antigen in mammalian cells: expression of the entire gene and the coding region. J Virol. 1983 Oct;48(1):271–280. doi: 10.1128/jvi.48.1.271-280.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNab G. M., Alexander J. J., Lecatsas G., Bey E. M., Urbanowicz J. M. Hepatitis B surface antigen produced by a human hepatoma cell line. Br J Cancer. 1976 Nov;34(5):509–515. doi: 10.1038/bjc.1976.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., Marion P. L., Robinson W. S. Hepatitis B viral DNA-RNA hybrid molecules in particles from infected liver are converted to viral DNA molecules during an endogenous DNA polymerase reaction. Virology. 1984 Nov;139(1):64–72. doi: 10.1016/0042-6822(84)90330-1. [DOI] [PubMed] [Google Scholar]
- Miller R. H. Proteolytic self-cleavage of hepatitis B virus core protein may generate serum e antigen. Science. 1987 May 8;236(4802):722–725. doi: 10.1126/science.3554507. [DOI] [PubMed] [Google Scholar]
- Shaul Y., Ziemer M., Garcia P. D., Crawford R., Hsu H., Valenzuela P., Rutter W. J. Cloning and analysis of integrated hepatitis virus sequences from a human hepatoma cell line. J Virol. 1984 Sep;51(3):776–787. doi: 10.1128/jvi.51.3.776-787.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanos A., Hübscher U. Recovery of functional proteins in sodium dodecyl sulfate gels. Methods Enzymol. 1983;91:263–277. doi: 10.1016/s0076-6879(83)91024-8. [DOI] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Sureau C., Romet-Lemonne J. L., Mullins J. I., Essex M. Production of hepatitis B virus by a differentiated human hepatoma cell line after transfection with cloned circular HBV DNA. Cell. 1986 Oct 10;47(1):37–47. doi: 10.1016/0092-8674(86)90364-8. [DOI] [PubMed] [Google Scholar]
- Toh H., Hayashida H., Miyata T. Sequence homology between retroviral reverse transcriptase and putative polymerases of hepatitis B virus and cauliflower mosaic virus. 1983 Oct 27-Nov 2Nature. 305(5937):827–829. doi: 10.1038/305827a0. [DOI] [PubMed] [Google Scholar]
- Volovitch M., Modjtahedi N., Yot P., Brun G. RNA-dependent DNA polymerase activity in cauliflower mosaic virus-infected plant leaves. EMBO J. 1984 Feb;3(2):309–314. doi: 10.1002/j.1460-2075.1984.tb01801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will H., Salfeld J., Pfaff E., Manso C., Theilmann L., Schaler H. Putative reverse transcriptase intermediates of human hepatitis B virus in primary liver carcinomas. Science. 1986 Feb 7;231(4738):594–596. doi: 10.1126/science.2418501. [DOI] [PubMed] [Google Scholar]