Abstract
The increased expression of epidermal growth factor receptor induced by tumor necrosis factor α renders pancreatic cancer cells more susceptible to antibody-dependent cellular cytotoxicity by a mAb specific for this receptor. Laboratory studies with athymic mice bearing xenografts of human pancreatic cancer cells demonstrated a cytokine-induced ability of the mAb to cause significant tumor regression. In a phase I/II clinical trial, 26 patients with unresectable pancreatic cancer were enrolled into three cohorts receiving variable amounts of the antibody together with a constant amount of tumor necrosis factor α. With increasing doses of antibody, the growth of the primary tumor was significantly inhibited. This was reflected by a longer median survival, with one complete remission lasting for 3 years obtained with the highest dose of antibody employed. Thus, a combination of the cytokine, tumor necrosis factor α, with a mAb to the epidermal growth factor receptor offers a potentially useful approach for the treatment of pancreatic cancer.
Pancreatic cancer accounts for about 25,000 deaths per year in the United States (1) and 50,000 in Europe, excluding the former USSR, and the mortality rates are increasing (2). Because symptoms are minor in early stages and often overlooked, therapy evaluation takes place at an advanced stage of the disease where available treatments are almost ineffective (3). Therefore the chances of a 5-year-survival for a patient suffering from pancreatic cancer are about 0.4% (4). Even when the diagnosis is made at an early stage (such as T1N0M0 in the International Union Against Cancer classification), which occurs in only 2% of all pancreatic cancer patients, radical resection does not improve 5-year survival rate above 37% (5). Studies of resectable tumors, which account for less than 22% of all pancreatic cancers, revealed 5-year survival rates of 17–30% (1).
Pancreatic cancer is characterized by genetic (6) and regulatory alterations including several receptor tyrosine kinases (7), in particular the epidermal growth factor receptor (EGFR) (8). Although growth factor receptors represent an attractive target for antibodies (9), application to cancer therapy has so far had only limited success. Accordingly, we focused on regulatory mechanisms influencing the target antigen density on pancreatic cancer cells. As we recently reported, tumor necrosis factor α (TNF-α) has a strong ability to increase EGFR expression via the p55 TNF receptor (TNF-R I) (10, 11). This observation opens new possibilities in immunotherapy, because immune effector mechanisms such as ADCC depend essentially on the antigen density on target cells (12).
Here we describe that, in contrast to other cytokines, TNF-α enhances anti-tumor effects, especially antibody-dependent cellular cytotoxicity (ADCC), of an antibody to the EGFR in pancreatic cancer. Immunotherapy of xenografted mice with an EGFR-specific mAb suggested a critical threshold of antigen density on target cells for tumor response (unpublished results). This promising therapeutic effect of an EGFR-specific mAb in combination with TNF-α is evaluated in the context of a clinical phase I/II trial.
MATERIALS AND METHODS
Cells and Culture Conditions.
Human pancreatic cancer cells from the line 818-4, previously established in our laboratory, were cultured as described (13).
Cytokines and Other Reagents.
The specific biological activities of the cytokines as stated by the suppliers were 2 × 107 units/mg for recombinant human interferon γ (IFN-γ) and 5 × 107 units/mg (G. R. Adolf, Bender, Vienna, Austria), 2.7 × 107 units/mg (Eurocetus, Amsterdam), and 7 × 107 units/mg (Knoll, Ludwigshafen, Germany) for recombinant human TNF-α; the latter preparation was used in the clinical trial. The anti-EGFR mAb 425 (14) used in preclinical experiments was generously provided by U. Rodeck (Wistar Institute, Philadelphia). The clinical trial was performed with the mAb EMD55900, identical with mAb 425, from Merck.
Northern Blot Analysis and DNA Probes.
Preparation of total cellular RNA and Northern blot hybridizations were performed as described (11, 15). In addition to ethidium bromide staining, autoradiographic signals obtained with the TNF-RI probe served as control for equal loading of the gel slots. The subcloned entire 1.0-kb EcoRI insert of a human TNF-RI cDNA clone was made available by Immunex (Seattle). A recombinant pBluescriptSK plasmid containing a 640-bp NotI/BglII fragment of the human TNF-RII cDNA (kindly donated by Immunex) was linearized with NotI.
ADCC Assay.
The ADCC mediated by splenocytes obtained from BALB/c nu/nu mice in the presence of mAb 425 was tested with pretreated (1,000 units/ml TNF-α plus 10 units/ml IFN-γ for 72 h) and untreated 818-4 cells by using a modification of the method described (16). Target cells labeled with Na251CrO4 (Amersham) were added to wells of U-bottomed tissue culture microtiter plates and incubated with mAb 425 at final concentrations of 0.4 ng/ml to 4 μg/ml at 4°C for 2 h. Target cells were then washed and exposed to a suspension of effector cells at an effector to target cell ratio of 100:1. After further incubation for 6 h at 37°C in an atmosphere of 5% CO2, the plates were centrifuged and the radioactivity of the supernatant fluid was measured in a γ-counter. Maximal 51Cr release was measured after incubation with 1% SDS, and the percentage of lyses was calculated as follows: % cytotoxicity = (test or control release − spontaneous release)/(maximal release − spontaneous release) × 100. The ADCC was obtained by subtracting the cytotoxicity of the control from that of the test sample.
Xenografts.
The 818-4 cells in the amount of 1.8 × 107 cells per mouse were inoculated subcutaneously into BALB/c nu/nu mice. Ten days later, when solid tumors were palpable, intraperitoneal injections were started as follows: (i) normal saline, (ii) 250 μg mAb 425, (iii) cytokines, (iv) cytokines plus 250 μg mAb 425, and (v) cytokines plus 155 μg F(ab′)2 425. Cytokines used were 105 units TNF-α plus 10 units IFN-γ per mouse, with 10–17 mice per group. This compares to about 0.1 mg/kg body weight for TNF-α and 10 mg/kg for mAb 425. Two cycles of therapy were performed. In the first cycle, treatment with the antibody or its fragment was started 2 days after cytokine injections to allow EGFR up-regulation.
TNF-α and mAb 425 in a Phase I/II Clinical Trial.
This study was designed as a monocenter, nonrandomized trial in patients with unresectable pancreatic cancer. Diagnosis was achieved by endoscopic retrograde cholangiopancreatography or computed tomography (CT)-scan and/or cytology or histology. Patients who had cytostatic therapy, immunotherapy, or radiotherapy within 4 weeks before, prior exposure to murine Igs, or a Karnofsky index below 70% were excluded. A total of 26 patients were enrolled sequentially into three cohorts with 5, 7, and 14 patients in cohorts A, B, and C, respectively. The mAb EMD55900 was administered in a dose-escalating manner together with a constant amount of TNF-α, 125 μg/m2 body surface area. TNF-α was given on days 1, 2, 3, 5, 8, 9, and 11. In cohort A, 80 mg mAb was given at day 4 followed by daily infusions of 40 mg until day 12. In cohorts B and C, the doses of mAb were twice those of the preceding cohort—i.e., 160 mg (B) and 320 mg (C) initially, followed by 80 mg and 160 mg, respectively. The tumor size imaging was done by CT-scan and ultrasound prior to therapy and during weeks 3, 11, and 19. The results were analyzed by independent radiologists.
Statistical Methods.
All statistical tests used for the clinical trial were performed two-sided at the 5% level. Analyses of demographic data and size in CT-scans of the primary tumor between the cohorts were done with the χ2 test (sex) or ANOVA (age, height, weight, and tumor size). Regarding the evaluation of the primary tumor size after therapy, the last value was carried forward in case of discontinuation. Analysis of percent increase was performed by regression analysis. The on-study survival curves were analyzed with the log-rank test.
RESULTS
Cytokine and Antibody Treatment of Xenografts.
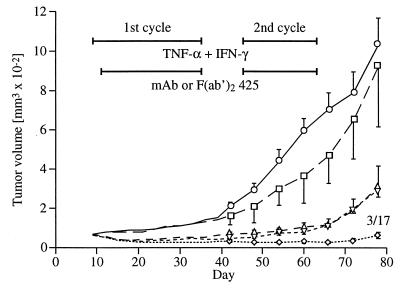
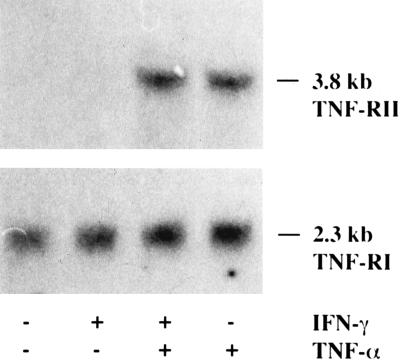
The 818-4 cells were strongly tumorigenic in nude mice and developed solid subcutaneous tumors. The mAb 425 alone had no effect on tumor growth compared with controls, whereas cytokines alone caused an initial decrease followed by a slow progression (Fig. 1). Significant tumor regression, which correlates with the stimulated ADCC (Table 1), was effected by the combined therapy of cytokines and mAb 425, achieving a complete remission in 3 of 17 mice during the second cycle of treatment. In contrast to the intact mAb, the supplementary use of the F(ab′)2 fragment did not show any greater effect than cytokines alone. TNF-R I and TNF-R II (Fig. 2) as well as EGFR (data not shown) mRNA expression were not altered by treating cells with IFN-γ in vitro. Thus, the human studies were performed with TNF-α but without additional IFN-γ. TNF-α from three different sources proved to be biologically equivalent concerning antitumor effects in vivo as well as EGFR modulation in vitro (data not shown).
Figure 1.
Treatment of nude mice bearing 818-4 with daily intraperitoneal injections of normal saline (□), mAb 425 (○), cytokines (▵), cytokines plus mAb 425 (⋄), and cytokines plus F(ab′)2 425 (▿) for the indicated periods. Error bars represent the standard errors and, if complete remission occurred under treatment, numbers of cases per group are assigned as fractions.
Table 1.
ADCC against pretreated pancreatic cancer cells
| Cytotoxicity, % of total cells
|
||
|---|---|---|
| − Cytokines | + Cytokines | |
| − mAb 425 | 1.0 ± 0.6 | 10.1 ± 0.3 |
| + mAb 425 | 4.1 ± 0.4 | 19.5 ± 1.6 |
818-4 cells were treated with 1,000 units/ml TNF-α and 10 units/ml IFN-γ for 72 h and compared to controls. ADCC was enhanced by cytokines plus mAb 425, when compared to either substance alone with an overall cytotoxicity of almost 20% at an effector to target ratio of 100:1.
Figure 2.
Effects of 1,000 units/ml TNF-α and 10 units/ml IFN-γ after a 72-h treatment period on the TNF-RI and TNF-RII mRNA levels in the pancreatic cancer cell line 818-4.
TNF-α and Antibody in a Phase I/II Clinical Trial.
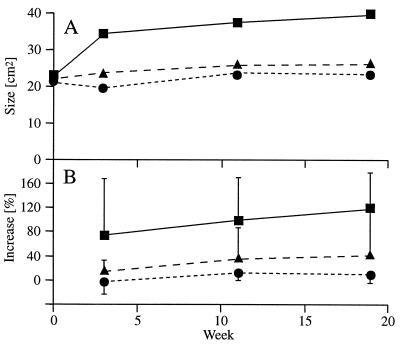
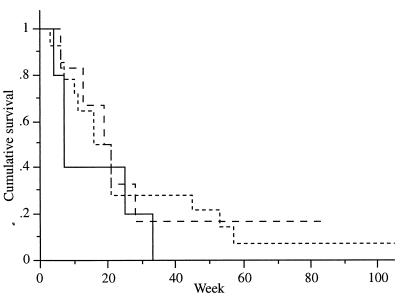
Twenty-six patients suffering from pancreatic cancer were enrolled; these consisted of 6 females and 20 males aged 44–72 years. Although patients were recruited sequentially and not randomized, no significant differences between the three cohorts were observed concerning demographic data as well as primary tumor size. The increase of the primary tumor size was significantly curbed in cohorts B and C compared with cohort A (Fig. 3, P < 0.01). This is reflected by a tendency to a longer median survival in cohorts B (18.6 weeks) and C (17.1 weeks) compared with cohort A (5.9 weeks) (Fig. 4).
Figure 3.
Assessment of mean primary tumor size by maximum cross section in CT scans (A) and mean percentage increase of primary tumor size (B) of cohort A (▪), B (•) and C (▴).
Figure 4.
Cumulative survivals of cohort A (—), B (– –) and C (- -) plotted vs. time.
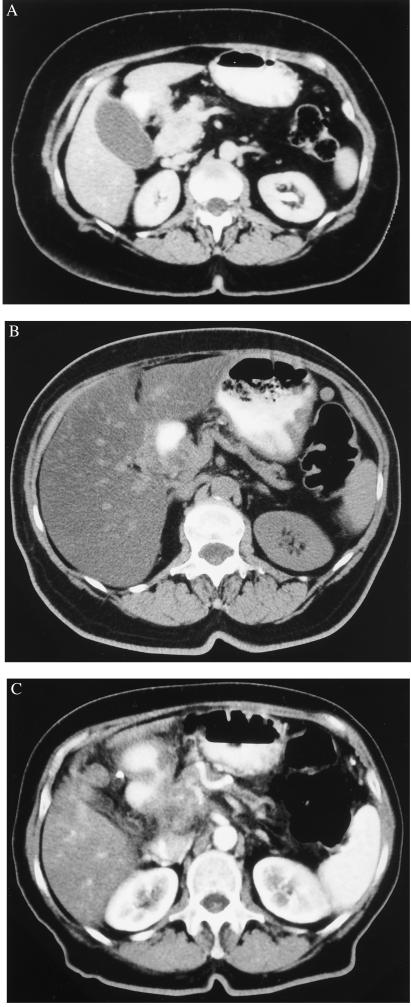
One complete remission, lasting for 3 years, was obtained with the maximal dose of EMD 55900 used. This female patient entered the study at the age of 66 years when jaundice and weight loss led to the diagnosis of a pancreatic head cancer. CT scans clearly indicated fatty tissue invasion and highly suspicious duodenal wall infiltration without metastatic lesions (Fig. 5A). The diagnosis was confirmed by endoscopic retrograde cholangiopancreatography and cytological examination of the fluid obtained by peritoneal lavage when a biliodigestive anastomosis with gastroenterostomy was performed. On the basis of CT scans, a partial remission was apparent at week 11, whereas further follow-up showed a dramatic decrease with a complete remission as stated by two independent radiologists (Fig. 5B). Tumor relapse was detected 3 years after diagnosis (Fig. 5C), and liver metastases were confirmed at autopsy, when the patient died after another 4 months.
Figure 5.
CT scans of the patient with pancreatic head cancer before (A), and 1 (B) or 3 (C) years after therapy.
Immune Response and Side Effects.
Human anti-mouse antibodies (HAMA) were seen in 9 of 26 patients with no correlation to the antibody dose used. This result had no apparent influence on tumor suppression, as the one patient with tumor remission also developed low levels of HAMA. Well-known side effects related to TNF-α, such as fever and chills, were observed in all patients, and additional symptoms such as nausea, vomiting, hypotension, muscle pain, and cramps occurred frequently but did not affect the therapy schedule. One patient with a history of coronary artery disease experienced a myocardial infarction during therapy and subsequently died of cardiac failure.
DISCUSSION
A sufficient antigen expression as a prerequisite for ADCC has been evaluated with mAb against human tumor cell lines (12, 17). Growth inhibition was observed previously in nude mice xenografted with melanoma cells when treated with mAb and paralleled ADCC activity in vitro with cytotoxic effects depending on a maximum of antibody density on tumor cells (12). Enhanced EGFR expression is a common phenomenon in malignant growth, especially in pancreatic cancer (18). To establish a cellular basis for an antibody therapy, we focused on regulatory mechanisms influencing the EGFR expression instead of using antibodies with different epitope specificity. Among cytokines, including granulocyte/macrophage colony-stimulating hormone (GM-CSF), interleukin 3 (IL-3), and IFN-γ, TNF-α proved to be most competent in up-regulating the EGFR. GM-CSF and IL-3 caused a rather weak increase in EGFR protein expression whereas IFN-γ had no effect (data not shown). Recent studies revealed the TNF-α-induced EGFR expression to be a consequence of a signal pathway subsequent to TNF-R I activation, whereas the activation of TNF-R II leads to a secretion of one of its ligands TGF-α (11). Although TNF-α up regulates the receptor and its ligand (i.e., EGFR and TGF-α) in an autocrine pathway, a moderate antiproliferative effect rather than growth stimulation was seen in pancreatic cancer cells, in contrast to a growth-promoting effect observed in autologous fibroblasts (13). This result corresponds to a response seen in other human normal diploid fibroblasts where mitogenic effects have been related to a stimulated EGFR expression (19).
In xenografted mice treated with mAb 425, the tumor response correlated with the EGFR protein expression (20), suggesting a critical threshold antigen density for tumoricidal effects that exceeds TNF-α-induced expression in normal cells such as fibroblasts, which have 4 × 104 molecules/cell (19). Considering possible cytotoxic effects for normal cells, this antigen number is still less than half that of the EGFR expression seen in untreated 818-4 cells with only minor susceptibility for ADCC and therefore implies that normal cells are insensitive to ADCC. Furthermore, TNF-α has no regulating effect on normal cultured hepatocytes tested as an example of EGFR expression in normal cells (data not shown). The therapy of the pancreatic cancer cell 818-4 xenografts in nude mice with cytokines and mAb 425 as demonstrated here caused remarkable tumor regression up to complete remission. Furthermore, as a fact relevant to clinical application, these effects are independent of the source of TNF-α used (data not shown).
Corresponding results were also obtained in human glioma cell lines, where the EGFR protein expression could be doubled to a maximum number of 2.5 × 105 receptors/cell (21). In cells exposed to radiolabeled mAb 425, antiproliferative effects were significantly raised by TNF-α, but no consistent antigen density threshold was found. In accord with a continuously rising antiproliferative effect with increasing labeled antibody binding, a response was seen with a number as low as 3.9 × 104 receptors/cell without TNF-α treatment. This does not ensure a selective effect for malignant cells with an overexpression of the EGFR as a cellular-guided immunotherapy does.
The findings with immunotherapy in mice provided the incentive for a clinical phase I/II trial with patients with unresectable pancreatic cancer. The mAb 425 (EMD 55900) was administered in a dose-escalating manner together with a constant amount of TNF-α in regard to the body surface area. HAMA were occasionally seen in about one-third of patients with no correlation to the antibody dose used or therapeutic effect observed. There has been no limiting side effect associated with this therapy. Although the number of patients studied so far has been limited (phase I/II trial) and only one cycle of the combined therapy in respect to elicited HAMA response has been given, mAb-dependent inhibition has been observed in cancers notoriously resistant to treatment. Growth of the primary tumor size was significantly restricted in cohorts with medium (B) or high (C) doses of mAb 425 (EMD 55900) and was accompanied by a longer median survival. The median survival time was more than twice as long in cohorts B and C compared with cohort A, with no difference between cohorts B and C. The reported clinical trial is not based on a randomized patient selection. Therefore, although the demographic data and the size of the primary tumor at the start of therapy were not significantly different in the three groups, statements about tumor progression and patient survival have to be considered with caution. Nevertheless, the one complete remission with the maximum antibody dose, in addition to reduced tumor growth and prolonged survival, are encouraging and provide the basis for future testing in a randomized study design.
Several physiological barriers, including heterogeneous blood supply, elevated interstitial pressure, and large transport distance, have been discussed concerning the failure of a necessary antibody accumulation in tumor tissue (22). Even when antibodies invade the tumor, up to 80% were detected in the extracellular fluid of which up to two-thirds were bound to shed tumor antigen in examined melanoma xenografts (23). These antigen–antibody complexes compete with tumor-bound antigen and thereby are demolishing the cellular defense. In a therapeutic situation in metastatic disease, where single cells enter blood or lymph vessels before invading tissue at a distant site, they are exposed to a sufficient quantity of antibody and effector cells without morphological obstacles. In a model where human melanoma cell lines, strongly expressing the EGFR because of an extra copy of chromosome 7, are growing in scid mice, the mAb 425 suppressed the appearance of metastases whereas the primary tumor was not affected (24). This finding has been related to an Fc-dependent immune response because the F(ab′)2 fragment lacked any effect.
Regarding these concerns, the efficacy of a mAb in a study with an adjuvant design can be considered. Although in vitro and in vivo experiments with colorectal tumor cells have been most promising, only limited evidence for a therapeutic benefit in humans was obtained using the mAb 17-1A in patients with advanced malignancies (25). In the context of an adjuvant study design, the antibody has been faced with a situation where only minor tumor involvement or micrometastases were present (26). Interestingly the mAb 17-A was unable to protect against a local recurrence, whereas it showed a clear benefit in the prevention of distant metastases.
In contrast to earlier studies, where therapy of pancreatic cancer with either TNF-α (27, 28) or mAb (29, 30) alone has been disappointing, combination of TNF-α with a mAb has provided an potential immunotherapy of pancreatic cancer, which is related to a modulation of antigen expression. A sufficient antigen density on tumor cells is a prerequisite for antibody therapy as shown here where the mAb alone has been ineffective in vivo. Further studies are needed to evaluate the introduced therapy in distinct clinical situations such as in adjuvant therapy after tumor resection where the 5-year survival rates are still unacceptably poor. Moreover, chimeric antibodies may overcome serious limitations when therapeutic effects disappear upon HAMA development.
Acknowledgments
We thank Andreas Kessler for his assistance regarding the treatment and follow-up of the patients in the clinical trial. This work was supported by the Bundesministerium für Bildung und Forschung, Grants 01 KV 9529 and 01 GB 9502.
ABBREVIATIONS
- ADCC
antibody-dependent cellular cytotoxicity
- EGFR
epidermal growth factor receptor
- TNF-α
tumor necrosis factor α
- TNF-R (I or II)
tumor necrosis factor receptors
- IFN-γ
interferon γ
- CT
computed tomography
- HAMA
human anti-mouse antibodies
References
- 1.Warshaw A L, Fernàndez-del Castillo C. N Engl J Med. 1992;326:455–456. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez E, La Vecchia C, Porta M, Negri E, Luchini F, Levi F. Int J Cancer. 1994;57:786–792. doi: 10.1002/ijc.2910570605. [DOI] [PubMed] [Google Scholar]
- 3.Wagener, D. J. T., Punt, C. J. A. & Wilke, H. (1994) Ann. Oncol. 5 (Suppl. 3), S87–S90. [DOI] [PubMed]
- 4.Gudjonsson B. Cancer. 1987;60:2284–2303. doi: 10.1002/1097-0142(19871101)60:9<2284::aid-cncr2820600930>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Tsuchiya R, Noda T, Harada N, Miyamoto T, Tomioka T, Yamamoto K, Yamaguchi T, Izawa K, Tsunoda T, Yoshino R, Eto T. Ann Surg. 1986;203:77–81. doi: 10.1097/00000658-198601000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schutte M, Kern S E. In: The Molecular Genetics of Pancreatic Adenocarcinoma. Neoptolemos J, Lemoine N, editors. London: Blackwell; 1995. pp. 115–132. [Google Scholar]
- 7.Friess H, Büchler M W, Korc M. In: Growth Factors and Growth Factor Receptors in Pancreatic Cancer. Neoptolemos J, Lemoine N, editors. London: Blackwell; 1995. pp. 51–60. [Google Scholar]
- 8.Lemoine N R, Leung H Y, Barton C M, Hughes C M, Klöppel G, Gullick W J. Int J Pancreatol. 1993;14:69–70. [Google Scholar]
- 9.Mendelsohn J. Semin Cancer Biol. 1990;1:339–344. [PubMed] [Google Scholar]
- 10.Schmiegel W, Roeder C, Schmielau J, Rodeck U, Kalthoff H. Proc Natl Acad Sci USA. 1993;90:863–867. doi: 10.1073/pnas.90.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalthoff H, Roeder C, Brockhaus M, Thiele H-G, Schmiegel W. J Biol Chem. 1993;268:2762–2766. [PubMed] [Google Scholar]
- 12.Herlyn D, Powe J, Ross A H, Herlyn M, Koprowski H. J Immunol. 1985;134:1300–1304. [PubMed] [Google Scholar]
- 13.Schmiegel W H, Caesar J, Kalthoff H, Greten H, Schreiber H W, Thiele H-G. Pancreas. 1988;3:180–188. doi: 10.1097/00006676-198804000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Murthy U, Basu A, Rodeck U, Herlyn M, Ross A H, Das M. Arch Biochem Biophys. 1987;252:549–560. doi: 10.1016/0003-9861(87)90062-2. [DOI] [PubMed] [Google Scholar]
- 15.Kalthoff H, Roeder C, Humburg I, Thiele H-G, Greten H, Schmiegel W. Oncogene. 1991;6:1015–1021. [PubMed] [Google Scholar]
- 16.Engers H D. Methods Enzymol. 1986;132:437–445. doi: 10.1016/s0076-6879(86)32029-9. [DOI] [PubMed] [Google Scholar]
- 17.Capone P, Papsidero L, Chu T. J Natl Cancer Inst. 1984;72:673–677. [PubMed] [Google Scholar]
- 18.Lemoine N R, Hughes C M, Barton C M, Poulsom R, Jeffery R E, Klöppel G, Hall P A, Gullick W J. J Pathol. 1992;166:7–12. doi: 10.1002/path.1711660103. [DOI] [PubMed] [Google Scholar]
- 19.Palombella V J, Yamashiro D J, Maxfield F R, Decker S J, Vilcek J. J Biol Chem. 1987;262:1950–1954. [PubMed] [Google Scholar]
- 20.Schmiegel W, Schmielau J, Heringlake S, Klöppel G, Rodeck U, Greten H, Roeder C, Kalthoff H. Digestion. 1990;46:176. (abstr.). [Google Scholar]
- 21.Adachi K, Belser P, Bender H, Li D, Rodeck U, Benveniste E N, Woo D, Schmiegel W H, Herlyn D. Cancer Immunol Immunother. 1992;34:370–376. doi: 10.1007/BF01741746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain, R. K. (1990) Cancer Res. 50 (Suppl.), 814s–819s. [PubMed]
- 23.Lin K, Nagy J A, Xu H, Shockley T R, Yarmush M L, Dvorak H F. Cancer Res. 1994;54:2269–2277. [PubMed] [Google Scholar]
- 24.Mueller B M, Romerdahl C A, Trent J M, Reisfeld R A. Cancer Res. 1991;51:2193–2198. [PubMed] [Google Scholar]
- 25.Sears H F, Herlyn D, Steplewski Z, Koprowski H. Cancer Res. 1985;45:5910–5913. [PubMed] [Google Scholar]
- 26.Riethmüller G, Schneider-Gädicke E, Schlimok G, Schmiegel W, Raab R, Höffken K, Gruber R, Pichlmaier H, Hirche H, Pichlmaier R, Buggisch P, Witte J German Cancer Aid 17–1A Study Group. Lancet. 1994;343:1177–1183. doi: 10.1016/s0140-6736(94)92398-1. [DOI] [PubMed] [Google Scholar]
- 27.Brown T D, Goodman P, Flemming T, Macdonald J S, Hersh E M, Braun T J. J Immunother. 1991;10:376–378. doi: 10.1097/00002371-199110000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Abbruzzese J L, Levin B, Ajani J A, Faintuch J S, Pazdur R, Saks S, Edwards C, Gutterman J U. J Biol Response Modif. 1990;9:522–527. [PubMed] [Google Scholar]
- 29.Tempero M A, Pour P M, Uchida E, Herlyn D, Steplewski Z. Hybridoma. 1986;5:S133–S138. [PubMed] [Google Scholar]
- 30.Büchler M, Friess H, Schultheiss K-H, Gebhardt C, Kübel R, Muhrer K-H, Winkelmann M, Wagener T, Klapdor R, Kaul M, Müller G, Schulz G, Beger H G. Cancer. 1991;68:1507–1512. doi: 10.1002/1097-0142(19911001)68:7<1507::aid-cncr2820680707>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]