Abstract
A series of eight antiviral compounds complexed with human rhinovirus 14 (HRV-14) were previously shown to displace segments of polypeptide chains in the floor of the "canyon" by as much as 0.45 nm in C-alpha positions from the native conformation (J. Badger, I. Minor, M. J. Kremer, M. A. Oliveira, T. J. Smith, J. P. Griffith, D. M. A. Guerin, S. Krishnaswamy, M. Luo, M. G. Rossman, M. A. McKinlay, G. D. Diana, F. J. Dutko, M. Fancher, R. R. Rueckert, and B. A. Heinz, Proc. Natl. Acad. Sci. USA 85:3304-3308, 1988). Because the canyon is thought to serve as the viral receptor-binding site (M. G. Rossmann, E. Arnold, J. W. Erickson, E. A. Frankenberger, J. P. Griffith, H. J. Hecht, J. E. Johnson, G. Kamer, M. Luo, A. G. Mosser, R. R. Rueckert, B. Sherry, and G. Vriend, Nature [London] 317:145-153, 1985; M. G. Rossmann and R. R. Rueckert, Microbiol. Sci. 4:206-214, 1987), these compounds were assessed for their ability to block adsorption of HRV-14 to HeLa cell membrane receptors. In parallel experiments, the compounds were assessed directly for antiviral activity in an in vitro plaque reduction assay in intact HeLa cells. All eight compounds blocked the adsorption of 50% of HRV-14 at approximately the same concentration required to reduce the number of visible plaques by 50% (MIC). A structurally related compound which was inactive in the plaque reduction assay had no effect on HRV-14 binding. A drug-resistant mutant of HRV-14 (Leu-1188), which was less sensitive to the eight compounds in plaque reduction assays was similarly less sensitive in the adsorption assay. We propose that the conformational changes in the floor of the HRV-14 canyon induced by these compounds substantially decrease adsorption of the virion to its receptor. These results provide further evidence for the role of the HRV canyon in receptor binding.
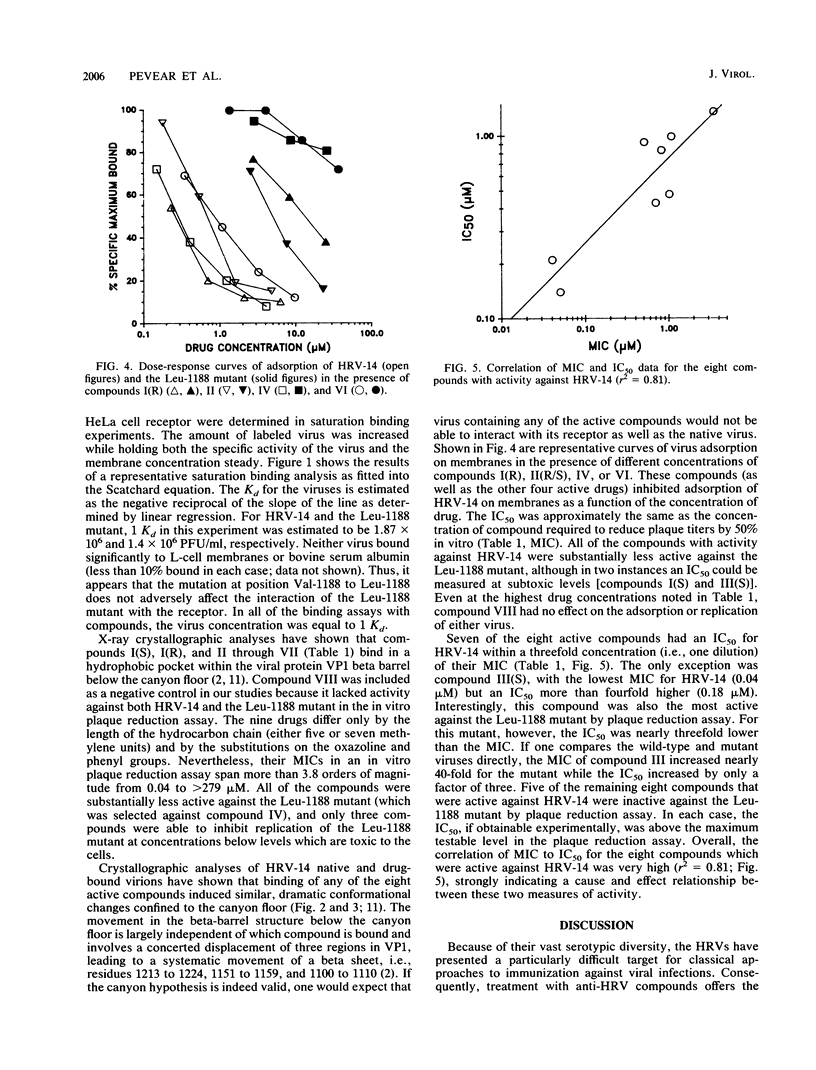
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Colonno R. J. Characterization of human rhinoviruses displaced by an anti-receptor monoclonal antibody. J Virol. 1988 Jul;62(7):2300–2306. doi: 10.1128/jvi.62.7.2300-2306.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger J., Minor I., Kremer M. J., Oliveira M. A., Smith T. J., Griffith J. P., Guerin D. M., Krishnaswamy S., Luo M., Rossmann M. G. Structural analysis of a series of antiviral agents complexed with human rhinovirus 14. Proc Natl Acad Sci U S A. 1988 May;85(10):3304–3308. doi: 10.1073/pnas.85.10.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Callahan P. L., Long W. J. Isolation of a monoclonal antibody that blocks attachment of the major group of human rhinoviruses. J Virol. 1986 Jan;57(1):7–12. doi: 10.1128/jvi.57.1.7-12.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Condra J. H., Mizutani S., Callahan P. L., Davies M. E., Murcko M. A. Evidence for the direct involvement of the rhinovirus canyon in receptor binding. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5449–5453. doi: 10.1073/pnas.85.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. P., Otto M. J., McKinlay M. A. Prevention of rhinovirus and poliovirus uncoating by WIN 51711, a new antiviral drug. Antimicrob Agents Chemother. 1986 Jul;30(1):110–116. doi: 10.1128/aac.30.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Korant B. D. Early interaction of rhinoviruses with host cells. J Virol. 1972 Jan;9(1):29–40. doi: 10.1128/jvi.9.1.29-40.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. J., Fox M. P., Fancher M. J., Kuhrt M. F., Diana G. D., McKinlay M. A. In vitro activity of WIN 51711, a new broad-spectrum antipicornavirus drug. Antimicrob Agents Chemother. 1985 Jun;27(6):883–886. doi: 10.1128/aac.27.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Palmenberg A. C. Conservation of the putative receptor attachment site in picornaviruses. Virology. 1988 Jun;164(2):373–382. doi: 10.1016/0042-6822(88)90550-8. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Reuckert R. R. What does the molecular structure of viruses tell us about viral functions? Microbiol Sci. 1987 Jul;4(7):206–214. [PubMed] [Google Scholar]
- Smith T. J., Kremer M. J., Luo M., Vriend G., Arnold E., Kamer G., Rossmann M. G., McKinlay M. A., Diana G. D., Otto M. J. The site of attachment in human rhinovirus 14 for antiviral agents that inhibit uncoating. Science. 1986 Sep 19;233(4770):1286–1293. doi: 10.1126/science.3018924. [DOI] [PubMed] [Google Scholar]
- Tomassini J. E., Colonno R. J. Isolation of a receptor protein involved in attachment of human rhinoviruses. J Virol. 1986 May;58(2):290–295. doi: 10.1128/jvi.58.2.290-295.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F. H., Lomax N. B. Host range mutants of human rhinovirus in which nonstructural proteins are altered. J Virol. 1983 Nov;48(2):410–418. doi: 10.1128/jvi.48.2.410-418.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeichhardt H., Otto M. J., McKinlay M. A., Willingmann P., Habermehl K. O. Inhibition of poliovirus uncoating by disoxaril (WIN 51711). Virology. 1987 Sep;160(1):281–285. doi: 10.1016/0042-6822(87)90075-4. [DOI] [PubMed] [Google Scholar]