Abstract
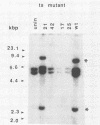
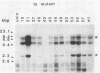
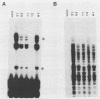
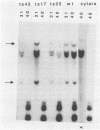
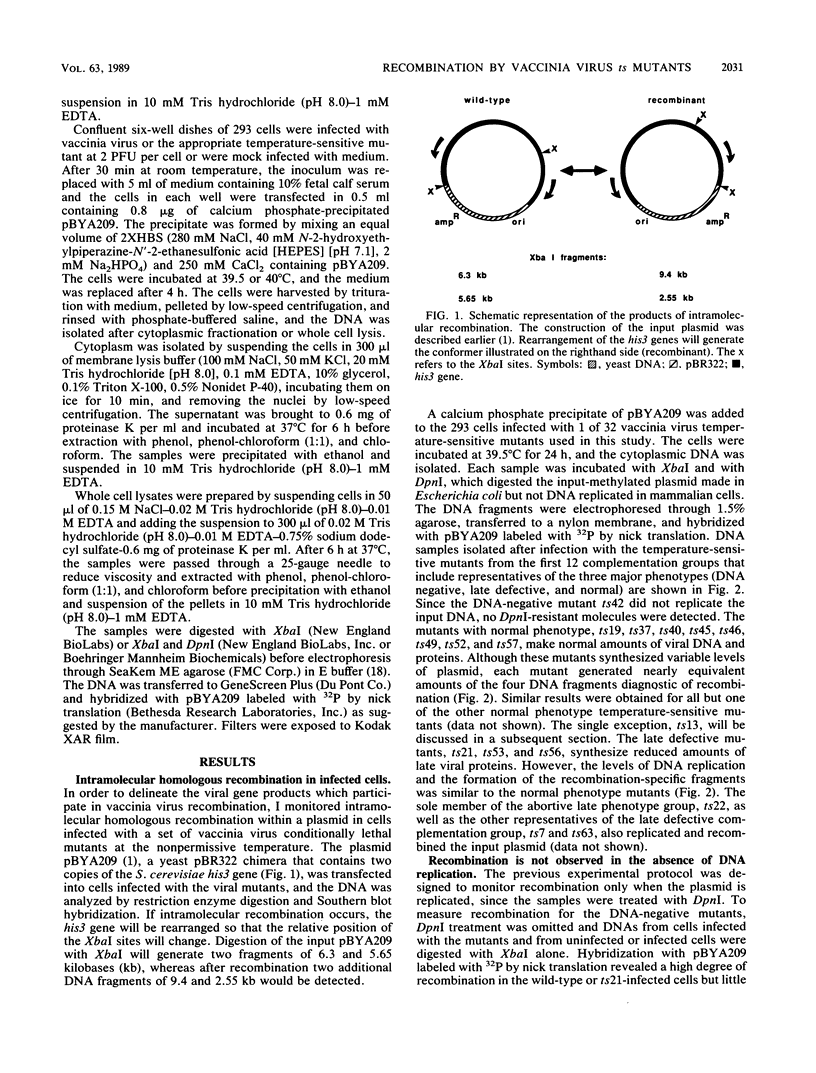
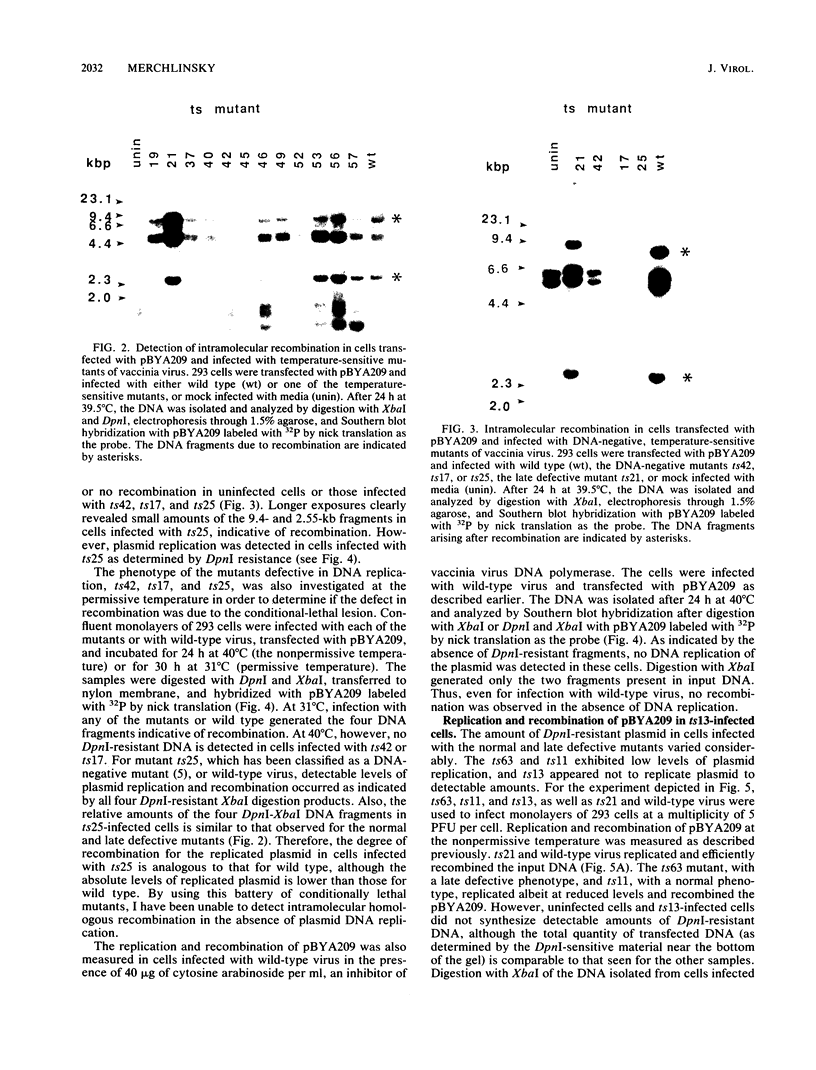
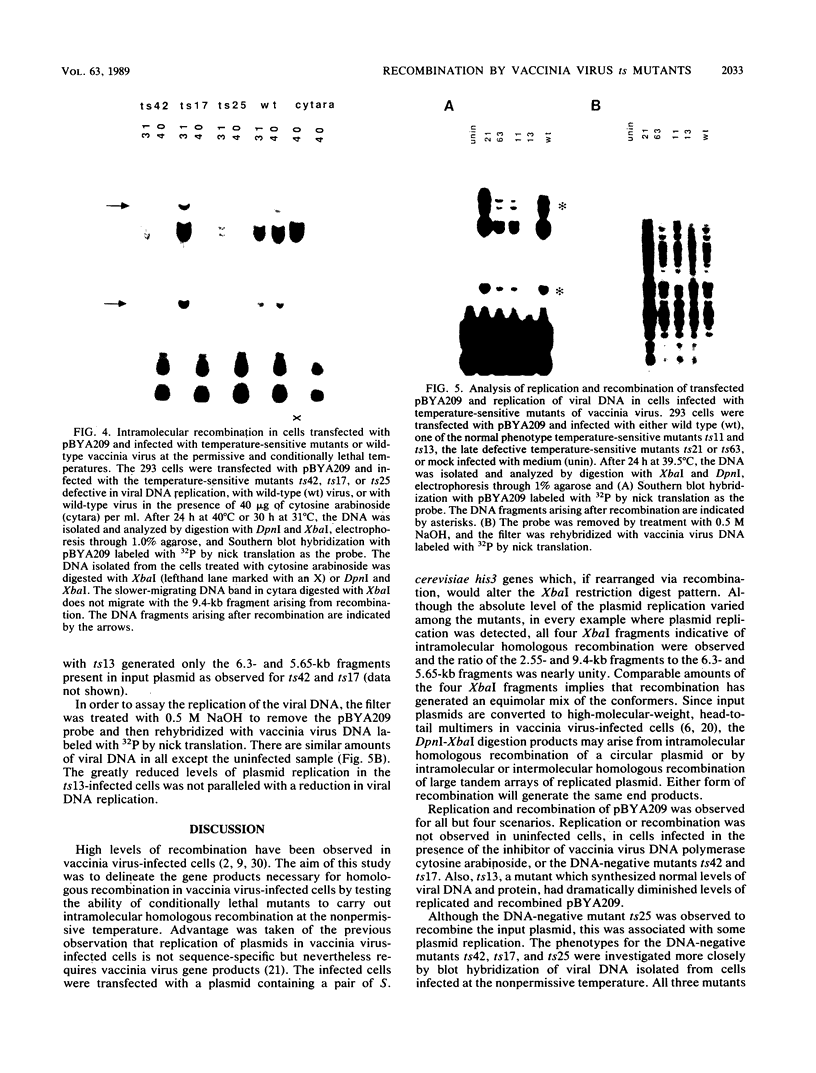
I have used a plasmid containing two copies of the Saccharmyces cerevisiae his3 gene to study intramolecular homologous recombination in vaccina virus-infected cells. Recombination of the plasmid was monitored by restriction enzyme digestion and Southern blot hybridization in cells infected with representatives from each of 32 complementation groups of temperature-sensitive mutants ts42 and ts17 did not replicate nor detectably recombine the input plasmid. All except one of the mutants that synthesized normal amounts of viral DNA and protein replicated and recombined the plasmid in a manner indistinguishable from wild-type virus. The remaining mutant, ts13, only poorly replicated and recombined the input plasmid. Thus, the processes of replication and recombination could not be separated by using this battery of mutants. Viral mutants defective in late protein synthesis were unable to resolve the vaccinia virus concatemer junction in plasmids but carried out intramolecular homologous recombination with plasmids as efficiently as did wild-type virus at the conditionally lethal temperature. This result distinguishes homologous recombination, which requires early gene products, from resolution of concatemer junctions, which requires additional late gene products.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn B. Y., Dornfeld K. J., Fagrelius T. J., Livingston D. M. Effect of limited homology on gene conversion in a Saccharomyces cerevisiae plasmid recombination system. Mol Cell Biol. 1988 Jun;8(6):2442–2448. doi: 10.1128/mcb.8.6.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L. A. High-frequency homologous recombination in vaccinia virus DNA. J Virol. 1987 Jun;61(6):1788–1795. doi: 10.1128/jvi.61.6.1788-1795.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroudy B. M., Venkatesan S., Moss B. Incompletely base-paired flip-flop terminal loops link the two DNA strands of the vaccinia virus genome into one uninterrupted polynucleotide chain. Cell. 1982 Feb;28(2):315–324. doi: 10.1016/0092-8674(82)90349-x. [DOI] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981 Aug;113(1):224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A., Spizz G. Isolation, characterization, and physical mapping of temperature-sensitive mutants of vaccinia virus. Virology. 1983 Jul 30;128(2):429–443. doi: 10.1016/0042-6822(83)90268-4. [DOI] [PubMed] [Google Scholar]
- DeLange A. M., McFadden G. Sequence-nonspecific replication of transfected plasmid DNA in poxvirus-infected cells. Proc Natl Acad Sci U S A. 1986 Feb;83(3):614–618. doi: 10.1073/pnas.83.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J., Rovinsky M. Marker rescue of temperature-sensitive mutations of vaccinia virus WR: correlation of genetic and physical maps. J Virol. 1983 Nov;48(2):419–428. doi: 10.1128/jvi.48.2.419-428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito J. J., Cabradilla C. D., Nakano J. H., Obijeski J. F. Intragenomic sequence transposition in monkeypox virus. Virology. 1981 Mar;109(2):231–243. doi: 10.1016/0042-6822(81)90495-5. [DOI] [PubMed] [Google Scholar]
- Evans D. H., Stuart D., McFadden G. High levels of genetic recombination among cotransfected plasmid DNAs in poxvirus-infected mammalian cells. J Virol. 1988 Feb;62(2):367–375. doi: 10.1128/jvi.62.2.367-375.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENNER F., COMBEN B. M. [Genetic studies with mammalian poxviruses. I. Demonstration of recombination between two strains of vaccina virus]. Virology. 1958 Jun;5(3):530–548. doi: 10.1016/0042-6822(58)90043-6. [DOI] [PubMed] [Google Scholar]
- FENNER F. Genetic studies with mammalian poxviruses. II. Recombination between two strains of vaccinia virus in single HeLa cells. Virology. 1959 Aug;8:499–507. doi: 10.1016/0042-6822(59)90051-0. [DOI] [PubMed] [Google Scholar]
- Formosa T., Alberts B. M. DNA synthesis dependent on genetic recombination: characterization of a reaction catalyzed by purified bacteriophage T4 proteins. Cell. 1986 Dec 5;47(5):793–806. doi: 10.1016/0092-8674(86)90522-2. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hruby D. E., Ball L. A. Mapping and identification of the vaccinia virus thymidine kinase gene. J Virol. 1982 Aug;43(2):403–409. doi: 10.1128/jvi.43.2.403-409.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. V., Moss B. Mapping of the vaccinia virus DNA polymerase gene by marker rescue and cell-free translation of selected RNA. J Virol. 1984 Jan;49(1):72–77. doi: 10.1128/jvi.49.1.72-77.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwal G. J., Moss B. Analysis of a large cluster of nonessential genes deleted from a vaccinia virus terminal transposition mutant. Virology. 1988 Dec;167(2):524–537. [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden G., Dales S. Biogenesis of poxviruses: mirror-image deletions in vaccinia virus DNA. Cell. 1979 Sep;18(1):101–108. doi: 10.1016/0092-8674(79)90358-1. [DOI] [PubMed] [Google Scholar]
- Merchlinsky M., Moss B. Resolution of linear minichromosomes with hairpin ends from circular plasmids containing vaccinia virus concatemer junctions. Cell. 1986 Jun 20;45(6):879–884. doi: 10.1016/0092-8674(86)90562-3. [DOI] [PubMed] [Google Scholar]
- Merchlinsky M., Moss B. Resolution of vaccinia virus DNA concatemer junctions requires late-gene expression. J Virol. 1989 Apr;63(4):1595–1603. doi: 10.1128/jvi.63.4.1595-1603.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosig G. The essential role of recombination in phage T4 growth. Annu Rev Genet. 1987;21:347–371. doi: 10.1146/annurev.ge.21.120187.002023. [DOI] [PubMed] [Google Scholar]
- Moyer R. W., Graves R. L., Rothe C. T. The white pock (mu) mutants of rabbit poxvirus. III. Terminal DNA sequence duplication and transposition in rabbit poxvirus. Cell. 1980 Nov;22(2 Pt 2):545–553. doi: 10.1016/0092-8674(80)90364-5. [DOI] [PubMed] [Google Scholar]
- Nakano E., Panicali D., Paoletti E. Molecular genetics of vaccinia virus: demonstration of marker rescue. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1593–1596. doi: 10.1073/pnas.79.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup D. J., Ink B. S., Parsons B. L., Hu W., Joklik W. K. Spontaneous deletions and duplications of sequences in the genome of cowpox virus. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6817–6821. doi: 10.1073/pnas.81.21.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabaugh M., Roseman N., Davis R., Mathews C. Vaccinia virus-encoded ribonucleotide reductase: sequence conservation of the gene for the small subunit and its amplification in hydroxyurea-resistant mutants. J Virol. 1988 Feb;62(2):519–527. doi: 10.1128/jvi.62.2.519-527.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyropoulos D. D., Roberts B. E., Panicali D. L., Cohen L. K. Delineation of the viral products of recombination in vaccinia virus-infected cells. J Virol. 1988 Mar;62(3):1046–1054. doi: 10.1128/jvi.62.3.1046-1054.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar P., Condit R. C. Selection for temperature-sensitive mutations in specific vaccinia virus genes: isolation and characterization of a virus mutant which encodes a phosphonoacetic acid-resistant, temperature-sensitive DNA polymerase. Virology. 1983 Jul 30;128(2):444–457. doi: 10.1016/0042-6822(83)90269-6. [DOI] [PubMed] [Google Scholar]
- Tengelsen L. A., Slabaugh M. B., Bibler J. K., Hruby D. E. Nucleotide sequence and molecular genetic analysis of the large subunit of ribonucleotide reductase encoded by vaccinia virus. Virology. 1988 May;164(1):121–131. doi: 10.1016/0042-6822(88)90627-7. [DOI] [PubMed] [Google Scholar]
- Thompson C. L., Condit R. C. Marker rescue mapping of vaccinia virus temperature-sensitive mutants using overlapping cosmid clones representing the entire virus genome. Virology. 1986 Apr 15;150(1):10–20. doi: 10.1016/0042-6822(86)90261-8. [DOI] [PubMed] [Google Scholar]
- Traktman P., Sridhar P., Condit R. C., Roberts B. E. Transcriptional mapping of the DNA polymerase gene of vaccinia virus. J Virol. 1984 Jan;49(1):125–131. doi: 10.1128/jvi.49.1.125-131.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal E. C., Roseman N. A., Hruby D. E. Isolation of vaccinia virus mutants capable of replicating independently of the host cell nucleus. J Virol. 1984 Aug;51(2):359–366. doi: 10.1128/jvi.51.2.359-366.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P. C., Challberg M. D., Nelson N. J., Levine M., Glorioso J. C. Inversion events in the HSV-1 genome are directly mediated by the viral DNA replication machinery and lack sequence specificity. Cell. 1988 Jul 29;54(3):369–381. doi: 10.1016/0092-8674(88)90200-0. [DOI] [PubMed] [Google Scholar]
- Weir J. P., Bajszár G., Moss B. Mapping of the vaccinia virus thymidine kinase gene by marker rescue and by cell-free translation of selected mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1210–1214. doi: 10.1073/pnas.79.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Menna A., Müller H. K., Schümperli D., Boseley P. G., Wyler R. Inverted terminal repeats in rabbit poxvirus and vaccinia virus DNA. J Virol. 1978 Oct;28(1):171–181. doi: 10.1128/jvi.28.1.171-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]