Abstract
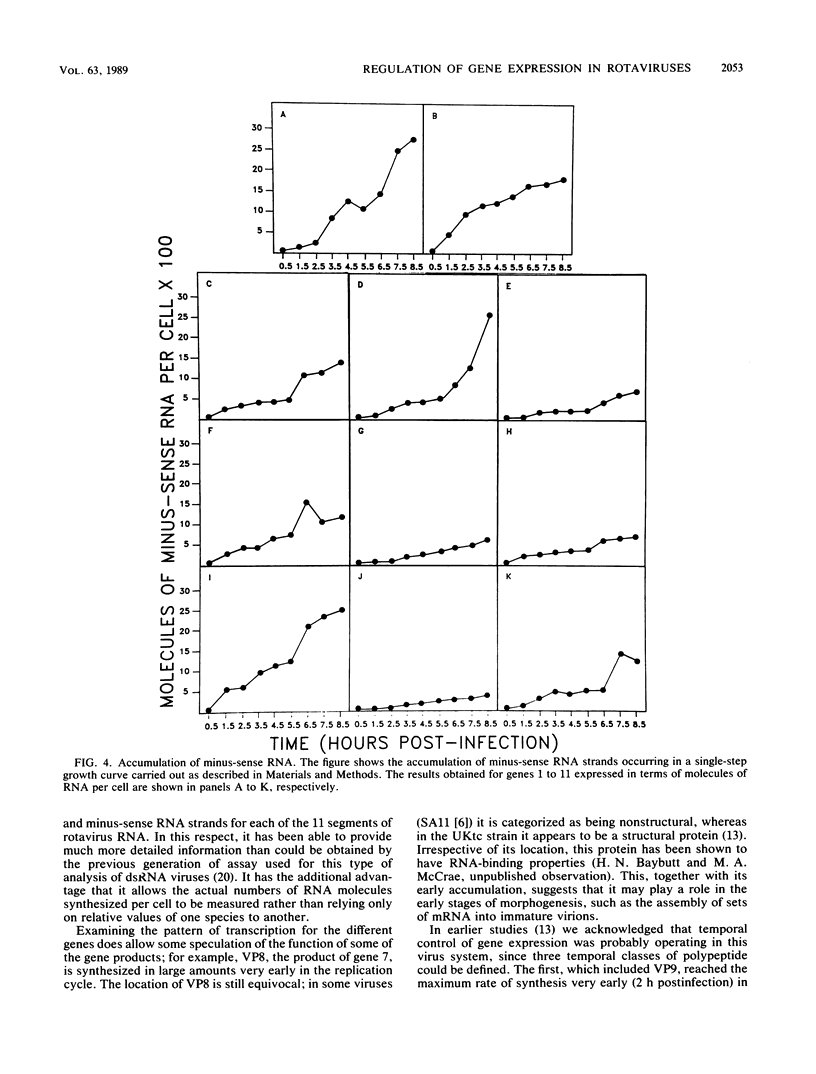
A sensitive and quantitative solution hybridization assay recently developed in this laboratory has been applied to the study of the regulation of viral gene expression in rotavirus-infected cells. Measurement of the cumulative level of viral plus-strand (mRNA) synthesis at hourly intervals throughout the growth cycle has provided evidence for both quantitative and qualitative regulation of transcription. Qualitative control was found only when cycloheximide was used to block protein synthesis in infected cells, when transcription of four of the viral genes (genes 5, 6, 7, and 9) was independent of protein synthesis. Quantitative regulation was demonstrated by the accumulation of mRNA to much higher levels for some of the viral genes (e.g., genes 2 and 7) relative to others (e.g., genes 4 and 6). In addition to quantitative control at the level of transcription, measurement of the relative molar amounts of the various viral proteins at 6.5 h postinfection showed that their levels did not directly reflect those of their encoding RNAs, indicating the existence of translational control of gene expression in the rotavirus system. Analysis of the levels of minus strand synthesized for each viral gene showed that they were not all accumulated to the same level. The significance of this result in the light of the presumed similarities in replication strategy to that of the mammalian reoviruses is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acs G., Klett H., Schonberg M., Christman J., Levin D. H., Silverstein S. C. Mechanism of reovirus double-stranded ribonucleic acid synthesis in vivo and in vitro. J Virol. 1971 Nov;8(5):684–689. doi: 10.1128/jvi.8.5.684-689.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A. A cure for a killer--but how to deliver it? Nature. 1979 Mar 29;278(5703):389–391. doi: 10.1038/278389a0. [DOI] [PubMed] [Google Scholar]
- Ahmed R., Graham A. F. Persistent infections in L cells with temperature-sensitive mutants of reovirus. J Virol. 1977 Aug;23(2):250–262. doi: 10.1128/jvi.23.2.250-262.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baybutt H. N., McCrae M. A. The molecular biology of rotaviruses. VII. Detailed structural analysis of gene 10 of bovine rotavirus. Virus Res. 1984 Oct;1(7):533–541. doi: 10.1016/0168-1702(84)90011-x. [DOI] [PubMed] [Google Scholar]
- Bishop R. F., Davidson G. P., Holmes I. H., Ruck B. J. Detection of a new virus by electron microscopy of faecal extracts from children with acute gastroenteritis. Lancet. 1974 Feb 2;1(7849):149–151. doi: 10.1016/s0140-6736(74)92440-4. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Palmer E. L., Obijeski J. F. Rotaviruses: a review. Curr Top Microbiol Immunol. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- Flewett T. H., Bryden A. S., Davies H., Woode G. N., Bridger J. C., Derrick J. M. Relation between viruses from acute gastroenteritis of children and newborn calves. Lancet. 1974 Jul 13;2(7872):61–63. doi: 10.1016/s0140-6736(74)91631-6. [DOI] [PubMed] [Google Scholar]
- Johnson M. A., McCrae M. A. A rapid and sensitive solution hybridisation assay for the quantitative determination of specific viral RNA sequences. J Virol Methods. 1988 Dec;22(2-3):247–254. doi: 10.1016/0166-0934(88)90107-3. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Flores J., Hoshino Y., Glass R. I., Midthun K., Gorziglia M., Chanock R. M. Rotavirus: the major etiologic agent of severe infantile diarrhea may be controllable by a "Jennerian" approach to vaccination. J Infect Dis. 1986 May;153(5):815–822. doi: 10.1093/infdis/153.5.815. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lau R. Y., Van Alstyne D., Berckmans R., Graham A. F. Synthesis of reovirus-specific polypeptides in cells pretreated with cycloheximide. J Virol. 1975 Sep;16(3):470–478. doi: 10.1128/jvi.16.3.470-478.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae M. A., Faulkner-Valle G. P. Molecular biology of rotaviruses. I. Characterization of basic growth parameters and pattern of macromolecular synthesis. J Virol. 1981 Aug;39(2):490–496. doi: 10.1128/jvi.39.2.490-496.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae M. A., McCorquodale J. G. Molecular biology of rotaviruses. IV. Molecular cloning of the bovine rotavirus genome. J Virol. 1982 Dec;44(3):1076–1079. doi: 10.1128/jvi.44.3.1076-1079.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae M. A., McCorquodale J. G. The molecular biology of rotaviruses. II. Identification of the protein-coding assignments of calf rotavirus genome RNA species. Virology. 1982 Mar;117(2):435–443. doi: 10.1016/0042-6822(82)90482-2. [DOI] [PubMed] [Google Scholar]
- Nayak D. P., Chambers T. M., Akkina R. K. Defective-interfering (DI) RNAs of influenza viruses: origin, structure, expression, and interference. Curr Top Microbiol Immunol. 1985;114:103–151. doi: 10.1007/978-3-642-70227-3_3. [DOI] [PubMed] [Google Scholar]
- Nayak D. P. Defective virus RNA synthesis and production of incomplete influenza virus in chick embryo cells. J Gen Virol. 1972 Jan;14(1):63–67. doi: 10.1099/0022-1317-14-1-63. [DOI] [PubMed] [Google Scholar]
- Newman J. F., Brown F., Bridger J. C., Woode G. N. Characterisation of a rotavirus.20b. Nature. 1975 Dec 18;258(5536):631–633. doi: 10.1038/258631a0. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Graham A. F. Appearance of defective virions in clones of reovirus. J Virol. 1970 Nov;6(5):693–694. doi: 10.1128/jvi.6.5.693-694.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama M., Millward S., Graham A. F. Control of transcription of the reovirus genome. Nucleic Acids Res. 1974 Mar;1(3):373–385. doi: 10.1093/nar/1.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K. I., Joklik W. K. Hybridization and sedimentation studies on "early" and "late" vaccinia messenger RNA. J Mol Biol. 1967 Aug 14;27(3):395–419. doi: 10.1016/0022-2836(67)90047-2. [DOI] [PubMed] [Google Scholar]
- Paoletti E., Grady L. J. Transcriptional complexity of vaccinia virus in vivo and in vitro. J Virol. 1977 Sep;23(3):608–615. doi: 10.1128/jvi.23.3.608-615.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedley S., Bridger J. C., Brown J. F., McCrae M. A. Molecular characterization of rotaviruses with distinct group antigens. J Gen Virol. 1983 Oct;64(Pt 10):2093–2101. doi: 10.1099/0022-1317-64-10-2093. [DOI] [PubMed] [Google Scholar]
- Pons M. W. The genome of incomplete influenza virus. Virology. 1980 Jan 15;100(1):43–52. doi: 10.1016/0042-6822(80)90550-4. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Biochemical and biophysical characteristics of diarrhea viruses of human and calf origin. J Virol. 1975 Nov;16(5):1229–1235. doi: 10.1128/jvi.16.5.1229-1235.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuerch A. R., Matsuhisa T., Joklik W. K. Temperature-sensitive mutants of reovirus. VI. Mutant ts 447 and ts 556 particles that lack either one or two genome RNA segments. Intervirology. 1974;3(1-2):36–46. doi: 10.1159/000149740. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Christman J. K., Acs G. The reovirus replicative cycle. Annu Rev Biochem. 1976;45:375–408. doi: 10.1146/annurev.bi.45.070176.002111. [DOI] [PubMed] [Google Scholar]
- Todd D., McNulty M. S. Characterization of pig rotavirus RNA. J Gen Virol. 1976 Oct;33(1):147–150. doi: 10.1099/0022-1317-33-1-147. [DOI] [PubMed] [Google Scholar]
- Woode G. N., Bridger J. C., Jones J. M., Flewett T. H., Davies H. A., Davis H. A., White G. B. Morphological and antigenic relationships between viruses (rotaviruses) from acute gastroenteritis of children, calves, piglets, mice, and foals. Infect Immun. 1976 Sep;14(3):804–810. doi: 10.1128/iai.14.3.804-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]