Abstract
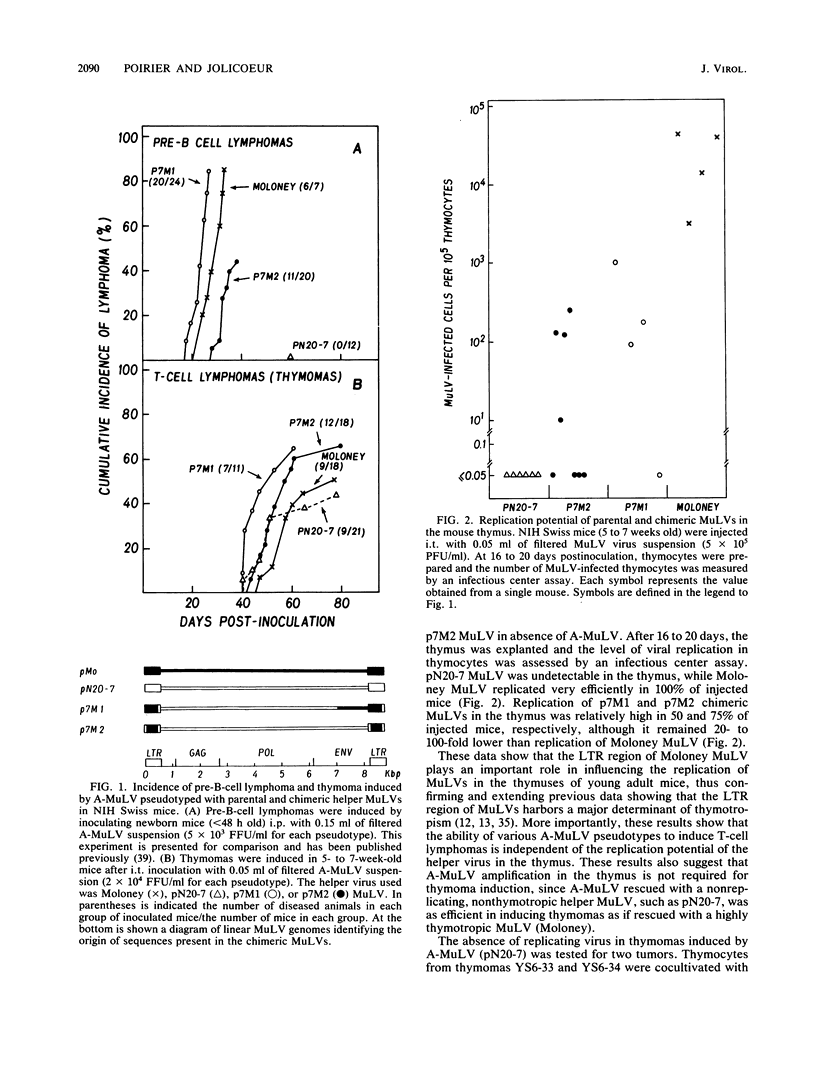
Abelson murine leukemia virus (A-MuLV) can induce pre-B- or T-cell lymphomas (thymomas) in mice depending on the route and time of injection. Previous studies have shown that the choice of the helper virus used to rescue A-MuLV greatly influences its ability to induce pre-B-cell lymphomas. In this study, we investigated the role of the helper virus in A-MuLV-induced thymomas. A-MuLV rescued with the helper Moloney MuLV, BALB/c endogenous N-tropic MuLV, and two chimeric MuLVs derived from these two parents were injected intrathymically in young adult NIH Swiss mice. All four A-MuLV pseudotypes were found to be equally efficient in the induction of thymomas, whereas drastic differences were observed in their pre-B-cell lymphomagenic potential. Thymoma induction by A-MuLV was independent of the replication potential of the helper virus in the thymus, and no helper proviral sequences could be detected in the majority of thymomas induced by A-MuLV rescued with parental BALB/c endogenous or chimeric MuLVs. In the thymomas in which helper proviruses were present, none of them were found integrated in the Ahi-1 region, a common proviral integration site found in A-MuLV-induced pre-B-cell lymphomas (Y. Poirer, C. Kozak, and P. Jolicoeur, J. Virol. 62:3985-3992, 1988). In addition, helper-free stocks of A-MuLV were found to be as lymphomagneic as other pseudotypes in inducing thymomas after intrathymic inoculation, in contrast to their inability to induce pre-B-cell lymphomas when injected intraperitoneally in newborn mice. Restriction enzyme analysis revealed one to three A-MuLV proviruses in each thymoma, indicating the oligoclonality of these tumors. Analysis of the immunoglobulin and T-cell receptor loci confirmed that the major population of cells of these primary thymomas belongs to the T-cell lineage. Together, these results indicate that the helper virus has no effect in the induction of A-MuLV-induced T-cell lymphomas, in contrast to its important role in the induction of A-MuLV-induced pre-B-cell lymphomas. Our data also revealed distinct biological requirements for transformation of these two target cells by v-abl.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Rabstein L. S. Lymphosarcoma: virus-induced thymic-independent disease in mice. Cancer Res. 1970 Aug;30(8):2213–2222. [PubMed] [Google Scholar]
- Alt F., Rosenberg N., Lewis S., Thomas E., Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981 Dec;27(2 Pt 1):381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Boss M., Greaves M., Teich N. Abelson virus-transformed haematopoietic cell lines with pre-B-cell characteristics. Nature. 1979 Apr 5;278(5704):551–553. doi: 10.1038/278551a0. [DOI] [PubMed] [Google Scholar]
- Caccia N., Kronenberg M., Saxe D., Haars R., Bruns G. A., Goverman J., Malissen M., Willard H., Yoshikai Y., Simon M. The T cell receptor beta chain genes are located on chromosome 6 in mice and chromosome 7 in humans. Cell. 1984 Jul;37(3):1091–1099. doi: 10.1016/0092-8674(84)90443-4. [DOI] [PubMed] [Google Scholar]
- Cook W. D., Balaton A. M. T-cell receptor and immunoglobulin genes are rearranged together in Abelson virus-transformed pre-B and pre-T cells. Mol Cell Biol. 1987 Jan;7(1):266–272. doi: 10.1128/mcb.7.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D. Rapid thymomas induced by Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1982 May;79(9):2917–2921. doi: 10.1073/pnas.79.9.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D. Thymocyte subsets transformed by Abelson murine leukemia virus. Mol Cell Biol. 1985 Feb;5(2):390–397. doi: 10.1128/mcb.5.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran L. M., Adams J. M., Dunn A. R., Cory S. Murine T lymphomas in which the cellular myc oncogene has been activated by retroviral insertion. Cell. 1984 May;37(1):113–122. doi: 10.1016/0092-8674(84)90306-4. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M., Kemp D. J. Somatic rearrangements forming active immunoglobulin mu genes in B and T lymphoid cell lines. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4943–4947. doi: 10.1073/pnas.77.8.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuypers H. T., Selten G., Quint W., Zijlstra M., Maandag E. R., Boelens W., van Wezenbeek P., Melief C., Berns A. Murine leukemia virus-induced T-cell lymphomagenesis: integration of proviruses in a distinct chromosomal region. Cell. 1984 May;37(1):141–150. doi: 10.1016/0092-8674(84)90309-x. [DOI] [PubMed] [Google Scholar]
- D'Andrea E., Saggioro D., Fleissner E., Chieco-Bianchi L. Abelson murine leukemia virus-induced thymic lymphomas: transformation of a primitive lymphoid precursor. J Natl Cancer Inst. 1987 Jul;79(1):189–195. [PubMed] [Google Scholar]
- DesGroseillers L., Rassart E., Jolicoeur P. Thymotropism of murine leukemia virus is conferred by its long terminal repeat. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4203–4207. doi: 10.1073/pnas.80.14.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. H., Morrey J. D. Tissue-specific replication of Friend and Moloney murine leukemia viruses in infected mice. J Virol. 1987 May;61(5):1350–1357. doi: 10.1128/jvi.61.5.1350-1357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Forster A., Hobart M., Hengartner H., Rabbitts T. H. An immunoglobulin heavy-chain gene is altered in two T-cell clones. Nature. 1980 Aug 28;286(5776):897–899. doi: 10.1038/286897a0. [DOI] [PubMed] [Google Scholar]
- Garman R. D., Doherty P. J., Raulet D. H. Diversity, rearrangement, and expression of murine T cell gamma genes. Cell. 1986 Jun 6;45(5):733–742. doi: 10.1016/0092-8674(86)90787-7. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Gilboa E., Witte O. N., Baltimore D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980 Dec;22(3):777–785. doi: 10.1016/0092-8674(80)90554-1. [DOI] [PubMed] [Google Scholar]
- Green P. L., Kaehler D. A., Risser R. Cell transformation and tumor induction by Abelson murine leukemia virus in the absence of helper virus. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5932–5936. doi: 10.1073/pnas.84.16.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. L., Kaehler D. A., Risser R. Clonal dominance and progression in Abelson murine leukemia virus lymphomagenesis. J Virol. 1987 Jul;61(7):2192–2197. doi: 10.1128/jvi.61.7.2192-2197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayday A. C., Saito H., Gillies S. D., Kranz D. M., Tanigawa G., Eisen H. N., Tonegawa S. Structure, organization, and somatic rearrangement of T cell gamma genes. Cell. 1985 Feb;40(2):259–269. doi: 10.1016/0092-8674(85)90140-0. [DOI] [PubMed] [Google Scholar]
- Herr W., Schwartz D., Gilbert W. Isolation and mapping of cDNA hybridization probes specific for ecotropic and nonecotropic murine leukemia proviruses. Virology. 1983 Feb;125(1):139–154. doi: 10.1016/0042-6822(83)90070-3. [DOI] [PubMed] [Google Scholar]
- Iwamoto A., Rupp F., Ohashi P. S., Walker C. L., Pircher H., Joho R., Hengartner H., Mak T. W. T cell-specific gamma genes in C57BL/10 mice. Sequence and expression of new constant and variable region genes. J Exp Med. 1986 May 1;163(5):1203–1212. doi: 10.1084/jem.163.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz D. M., Saito H., Heller M., Takagaki Y., Haas W., Eisen H. N., Tonegawa S. Limited diversity of the rearranged T-cell gamma gene. 1985 Feb 28-Mar 6Nature. 313(6005):752–755. doi: 10.1038/313752a0. [DOI] [PubMed] [Google Scholar]
- Kurosawa Y., von Boehmer H., Haas W., Sakano H., Trauneker A., Tonegawa S. Identification of D segments of immunoglobulin heavy-chain genes and their rearrangement in T lymphocytes. Nature. 1981 Apr 16;290(5807):565–570. doi: 10.1038/290565a0. [DOI] [PubMed] [Google Scholar]
- Lemay G., Jolicoeur P. Rearrangement of a DNA sequence homologous to a cell-virus junction fragment in several Moloney murine leukemia virus-induced rat thymomas. Proc Natl Acad Sci U S A. 1984 Jan;81(1):38–42. doi: 10.1073/pnas.81.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S., Rosenberg N., Alt F., Baltimore D. Continuing kappa-gene rearrangement in a cell line transformed by Abelson murine leukemia virus. Cell. 1982 Oct;30(3):807–816. doi: 10.1016/0092-8674(82)90285-9. [DOI] [PubMed] [Google Scholar]
- Max E. E., Maizel J. V., Jr, Leder P. The nucleotide sequence of a 5.5-kilobase DNA segment containing the mouse kappa immunoglobulin J and C region genes. J Biol Chem. 1981 May 25;256(10):5116–5120. [PubMed] [Google Scholar]
- Pillemer E., Whitlock C., Weissman I. L. Transformation-associated proteins in murine B-cell lymphomas that are distinct from Abelson virus gene products. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4434–4438. doi: 10.1073/pnas.81.14.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
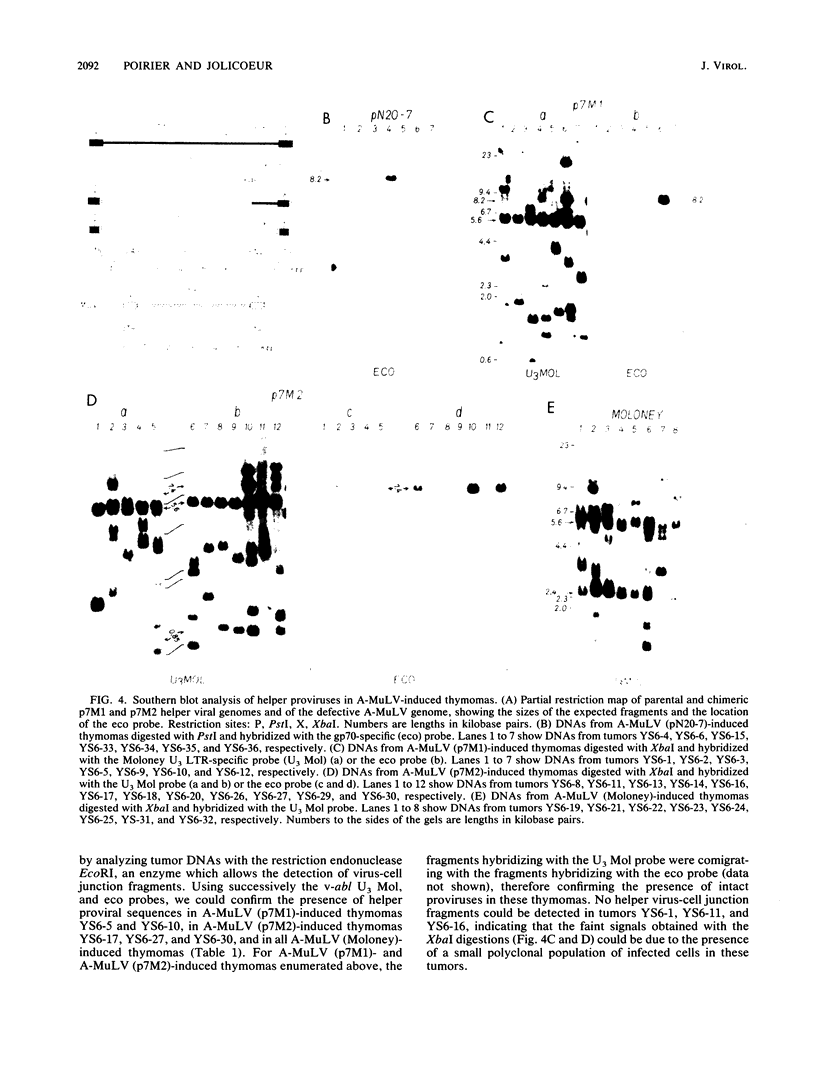
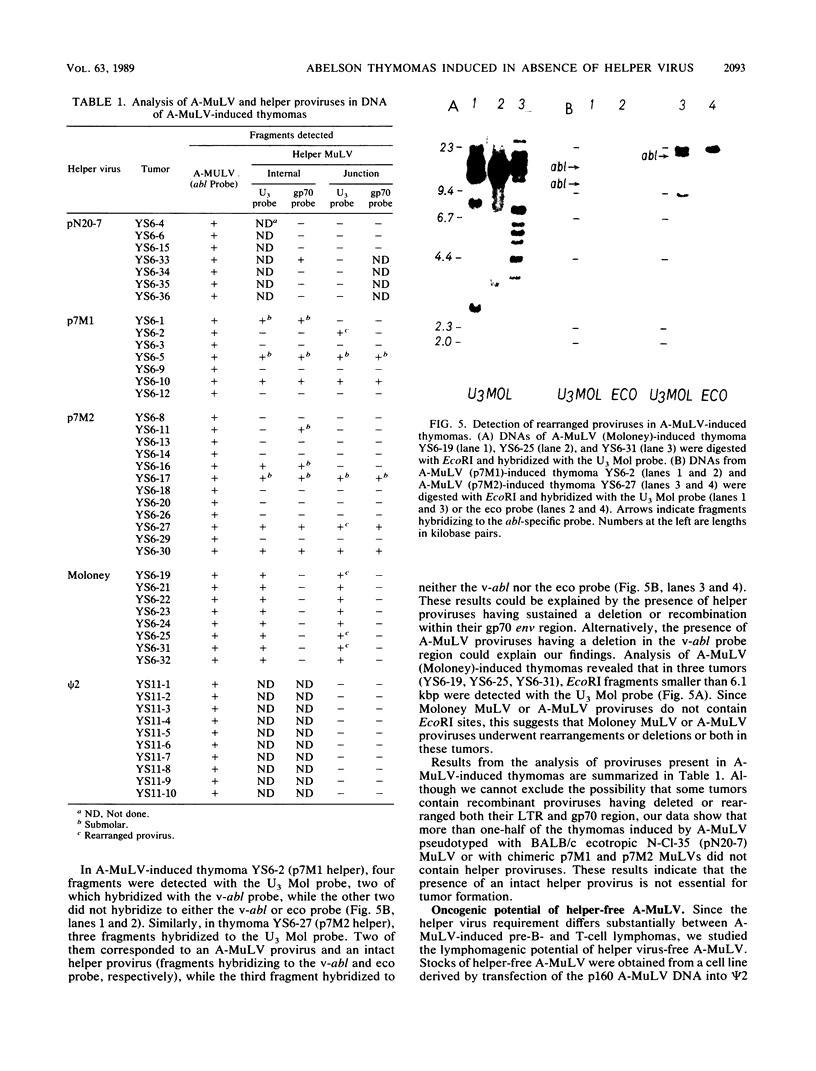
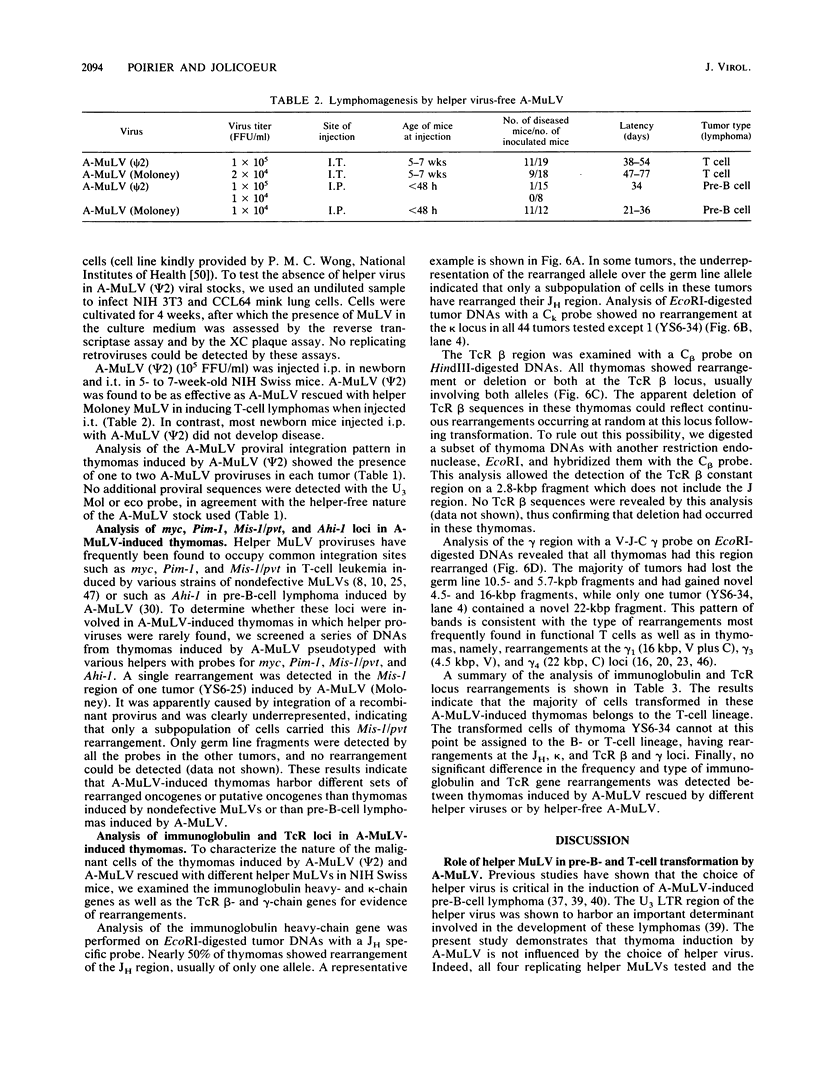
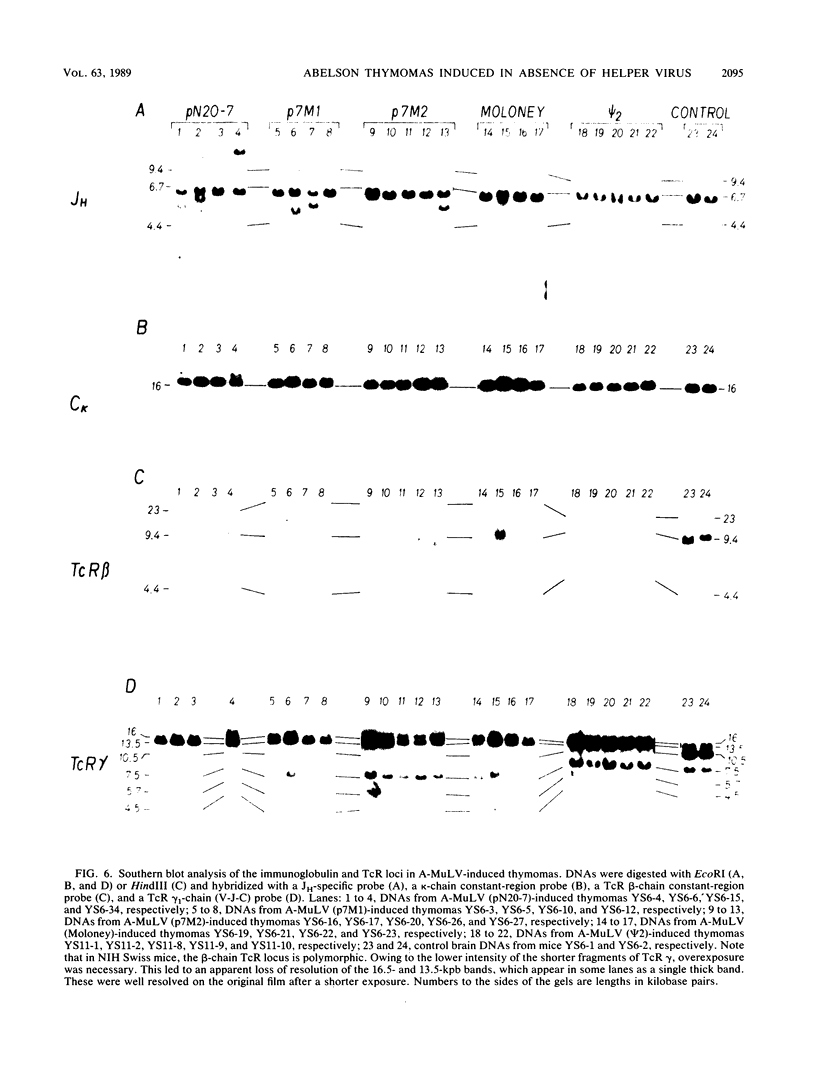
- Poirier Y., Kozak C., Jolicoeur P. Identification of a common helper provirus integration site in Abelson murine leukemia virus-induced lymphoma DNA. J Virol. 1988 Nov;62(11):3985–3992. doi: 10.1128/jvi.62.11.3985-3992.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassart E., DesGroseillers L., Jolicoeur P. Molecular cloning of B- and N-tropic endogenous BALB/c murine leukemia virus circular DNA intermediates: isolation and characterization of infectious recombinant clones. J Virol. 1981 Jul;39(1):162–171. doi: 10.1128/jvi.39.1.162-171.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Kaehler D., Lamph W. W. Different genes control the susceptibility of mice to Moloney or Abelson murine leukemia viruses. J Virol. 1985 Sep;55(3):547–553. doi: 10.1128/jvi.55.3.547-553.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R. The pathogenesis of Abelson virus lymphomas of the mouse. Biochim Biophys Acta. 1982 Jun 28;651(4):213–244. doi: 10.1016/0304-419x(82)90013-0. [DOI] [PubMed] [Google Scholar]
- Rodland K. D., Brown A. M., Magun B. E. Individual mouse VL30 elements transferred to rat cells by viral pseudotypes retain their responsiveness to activators of protein kinase C. Mol Cell Biol. 1987 Jun;7(6):2296–2298. doi: 10.1128/mcb.7.6.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Haseltine W. A., Lenz J., Ruprecht R., Cloyd M. W. Tissue selectivity of murine leukemia virus infection is determined by long terminal repeat sequences. J Virol. 1985 Sep;55(3):862–866. doi: 10.1128/jvi.55.3.862-866.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N. Abelson leukemia virus. Curr Top Microbiol Immunol. 1982;101:95–126. doi: 10.1007/978-3-642-68654-2_5. [DOI] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. The effect of helper virus on Abelson virus-induced transformation of lymphoid cells. J Exp Med. 1978 Apr 1;147(4):1126–1141. doi: 10.1084/jem.147.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Savard P., DesGroseillers L., Rassart E., Poirier Y., Jolicoeur P. Important role of the long terminal repeat of the helper Moloney murine leukemia virus in Abelson virus-induced lymphoma. J Virol. 1987 Oct;61(10):3266–3275. doi: 10.1128/jvi.61.10.3266-3275.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D. Effect of pseudotype on Abelson virus and Kirsten sarcoma virus-induced leukemia. J Exp Med. 1978 Apr 1;147(4):1044–1053. doi: 10.1084/jem.147.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Scott M. L., Davis M. M., Feinberg M. B. Transformation of T-lymphoid cells by Abelson murine leukemia virus. J Virol. 1986 Aug;59(2):434–443. doi: 10.1128/jvi.59.2.434-443.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siden E. J., Baltimore D., Clark D., Rosenberg N. E. Immunoglobulin synthesis by lymphoid cells transformed in vitro by Abelson murine leukemia virus. Cell. 1979 Feb;16(2):389–396. doi: 10.1016/0092-8674(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Traunecker A., Kiefer M., Dembić Z., Steinmetz M., Karjalainen K. Rearrangements of T cell receptor loci can be found only rarely in B lymphoid cells. Eur J Immunol. 1986 Apr;16(4):430–434. doi: 10.1002/eji.1830160420. [DOI] [PubMed] [Google Scholar]
- Traunecker A., Oliveri F., Allen N., Karjalainen K. Normal T cell development is possible without 'functional' gamma chain genes. EMBO J. 1986 Jul;5(7):1589–1593. doi: 10.1002/j.1460-2075.1986.tb04400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve L., Rassart E., Jolicoeur P., Graham M., Adams J. M. Proviral integration site Mis-1 in rat thymomas corresponds to the pvt-1 translocation breakpoint in murine plasmacytomas. Mol Cell Biol. 1986 May;6(5):1834–1837. doi: 10.1128/mcb.6.5.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N. Molecular and cellular biology of Abelson virus transformation. Curr Top Microbiol Immunol. 1983;103:127–146. doi: 10.1007/978-3-642-68943-7_6. [DOI] [PubMed] [Google Scholar]
- Wolff L., Ruscetti S. Malignant transformation of erythroid cells in vivo by introduction of a nonreplicating retrovirus vector. Science. 1985 Jun 28;228(4707):1549–1552. doi: 10.1126/science.2990034. [DOI] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Raefsky E., Eaves C. J., Nienhuis A. W. Blast colonies containing hemopoietic progenitor cells can give rise to Abelson virus (A-MuLV)-transformed cell lines. Exp Hematol. 1988 Jan;16(1):5–11. [PubMed] [Google Scholar]