Abstract
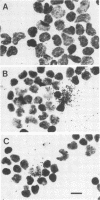
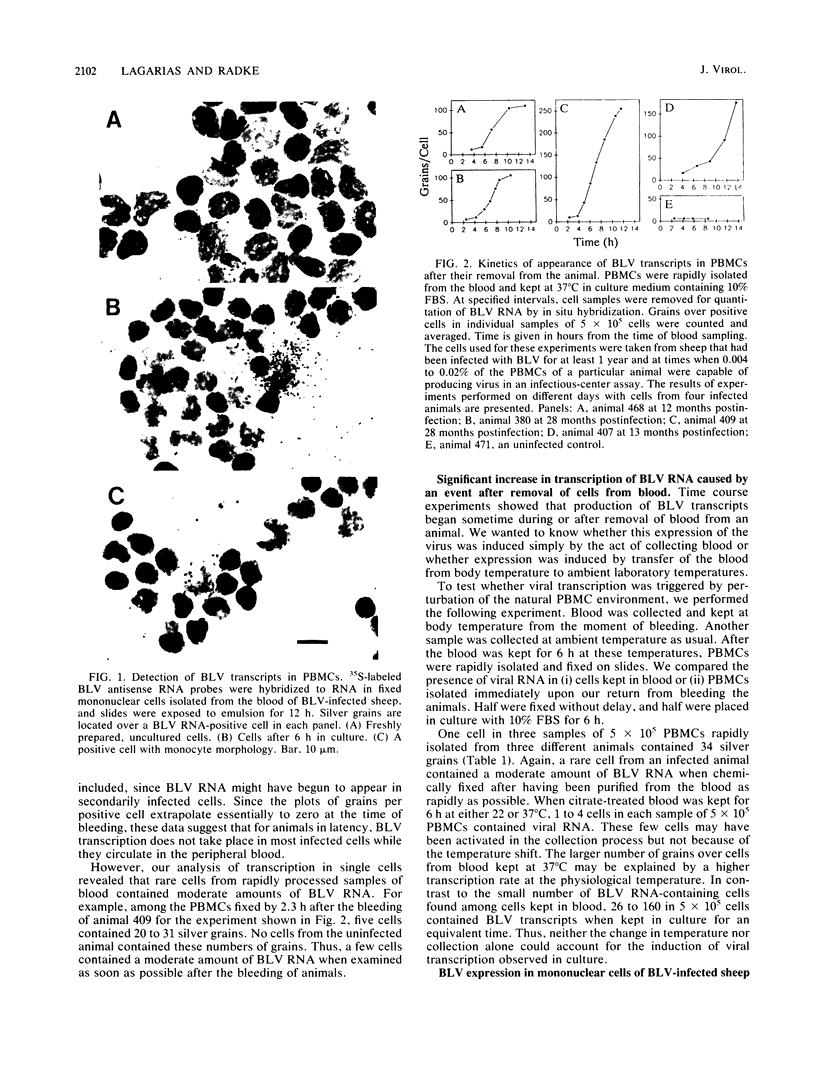
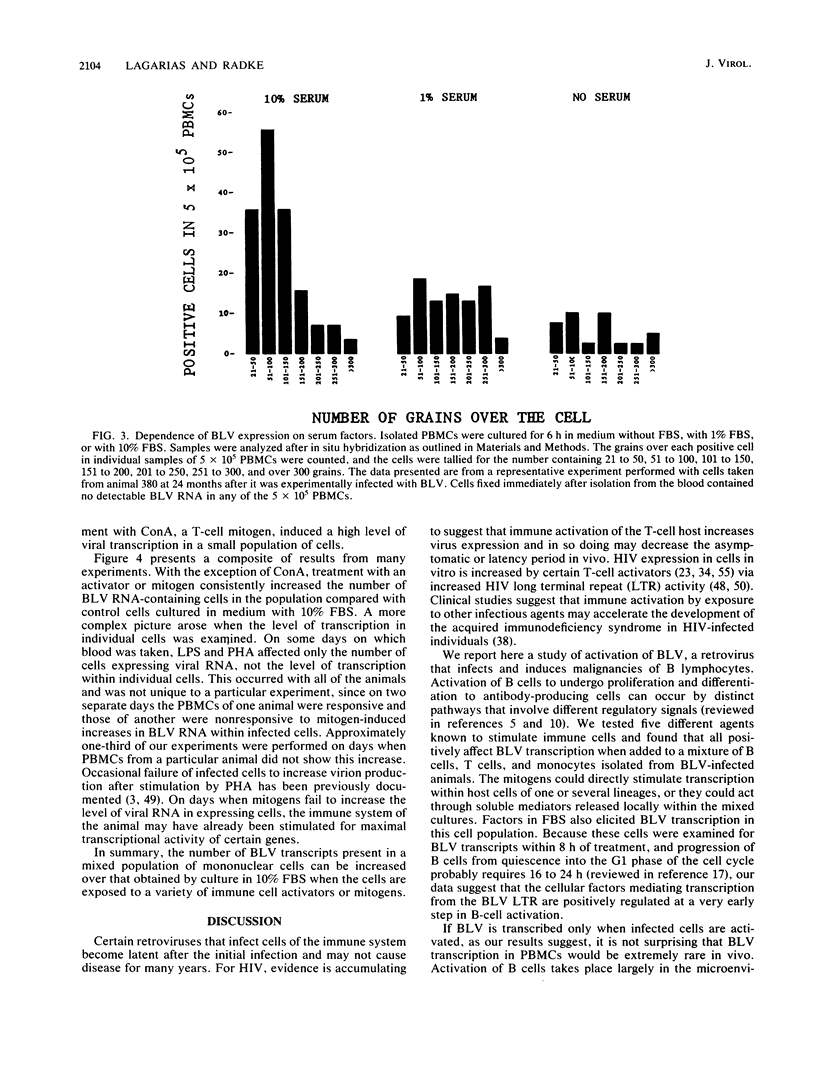
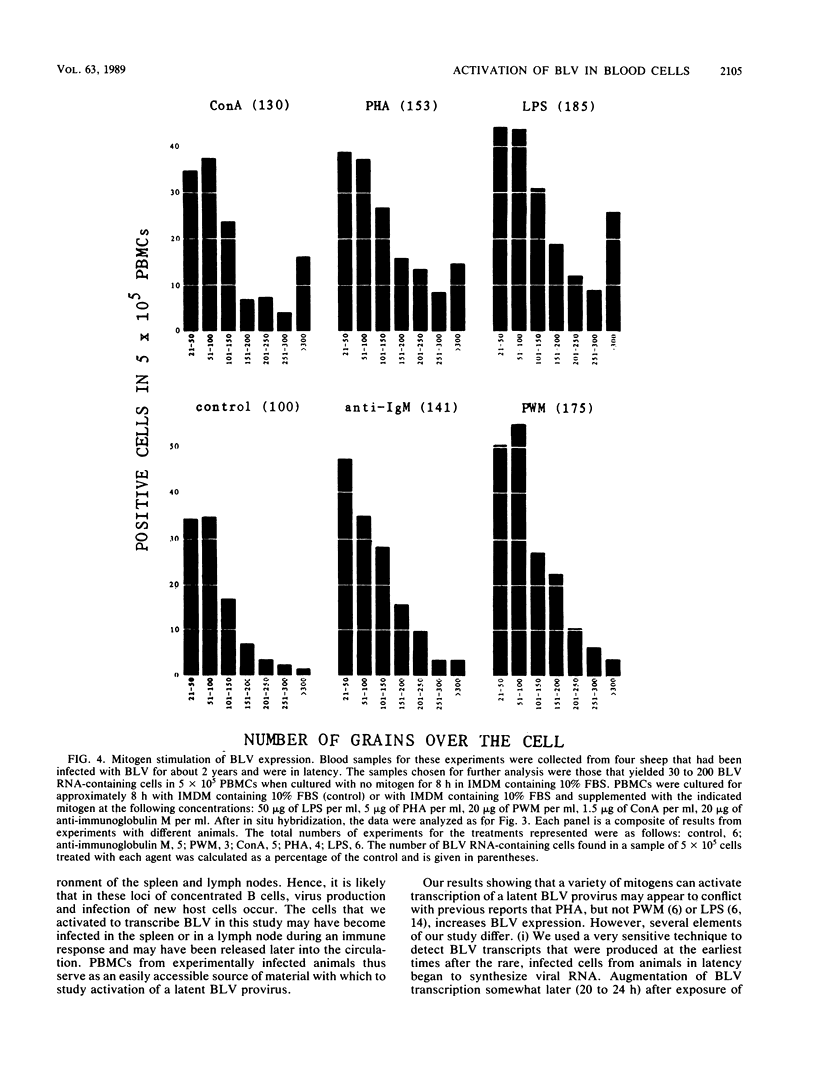
Infection by bovine leukemia virus (BLV) is characterized by a long latency period, after which some individuals develop B-cell tumors. The behavior of BLV and related retroviruses during the latency period between initial infection and subsequent tumorigenesis is poorly understood. We used in situ hybridization to detect BLV transcripts in individual peripheral blood mononuclear cells from experimentally infected, asymptomatic sheep with latent infections. Viral RNA was not found in most peripheral blood cells that had been isolated as rapidly as possible from circulating blood, but it was present in rare cells. BLV RNA transcripts increased in a biphasic manner within a few hours after the blood cells were placed in culture. Exposure to fetal bovine serum was identified as the principal cause of this transcriptional activation, which occurred in fewer than 1 in 1,000 cells. Agents known to activate immune cells polyclonally caused a further increase in the number of cells containing BLV RNA within 8 h. In some cases, the numbers of viral transcripts within individual cells also increased. Thus, BLV is not detectably expressed in most resting lymphocytes circulating in the blood, but its transcription is activated by components of fetal bovine serum and can be augmented by molecules that mimic activation of immune cells. This activation, which might occur in lymphoid tissue during an immune response, may lead to the synthesis of viral regulatory proteins that are thought to initiate tumorigenesis through host cell genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman R., Harrich D., Garcia J. A., Gaynor R. B. Human T-cell leukemia virus types I and II exhibit different DNase I protection patterns. J Virol. 1988 Apr;62(4):1339–1346. doi: 10.1128/jvi.62.4.1339-1346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier T., Mamoun R., Guillemain B., Duplan J. F., Parodi A. L. Bovine leukemia virus (BLV) specific RNA in infected cells. Ann Rech Vet. 1978;9(4):643–649. [PubMed] [Google Scholar]
- Baliga V., Ferrer J. F. Expression of the bovine leukemia virus and its internal antigen in blood lymphocytes. Proc Soc Exp Biol Med. 1977 Nov;156(2):388–391. doi: 10.3181/00379727-156-39942. [DOI] [PubMed] [Google Scholar]
- Burny A., Cleuter Y., Kettmann R., Mammerickx M., Marbaix G., Portetelle D., Van den Broeke A., Willems L., Thomas R. Bovine leukaemia: facts and hypotheses derived from the study of an infectious cancer. Cancer Surv. 1987;6(1):139–159. [PubMed] [Google Scholar]
- Cambier J. C., Ransom J. T. Molecular mechanisms of transmembrane signaling in B lymphocytes. Annu Rev Immunol. 1987;5:175–199. doi: 10.1146/annurev.iy.05.040187.001135. [DOI] [PubMed] [Google Scholar]
- Chatterjee R., Gupta P., Kashmiri S. V., Ferrer J. F. Phytohemagglutinin activation of the transcription of the bovine leukemia virus genome requires de novo protein synthesis. J Virol. 1985 Jun;54(3):860–863. doi: 10.1128/jvi.54.3.860-863.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I. S., Slamon D. J., Rosenblatt J. D., Shah N. P., Quan S. G., Wachsman W. The x gene is essential for HTLV replication. Science. 1985 Jul 5;229(4708):54–58. doi: 10.1126/science.2990037. [DOI] [PubMed] [Google Scholar]
- Couez D., Deschamps J., Kettmann R., Stephens R. M., Gilden R. V., Burny A. Nucleotide sequence analysis of the long terminal repeat of integrated bovine leukemia provirus DNA and of adjacent viral and host sequences. J Virol. 1984 Feb;49(2):615–620. doi: 10.1128/jvi.49.2.615-620.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- DeFranco A. L. Molecular aspects of B-lymphocyte activation. Annu Rev Cell Biol. 1987;3:143–178. doi: 10.1146/annurev.cb.03.110187.001043. [DOI] [PubMed] [Google Scholar]
- Derse D. Bovine leukemia virus transcription is controlled by a virus-encoded trans-acting factor and by cis-acting response elements. J Virol. 1987 Aug;61(8):2462–2471. doi: 10.1128/jvi.61.8.2462-2471.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derse D. trans-acting regulation of bovine leukemia virus mRNA processing. J Virol. 1988 Apr;62(4):1115–1119. doi: 10.1128/jvi.62.4.1115-1119.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps J., Kettmann R., Burny A. Experiments with cloned complete tumor-derived bovine leukemia virus information prove that the virus is totally exogenous to its target animal species. J Virol. 1981 Nov;40(2):605–609. doi: 10.1128/jvi.40.2.605-609.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djilali S., Parodi A. L., Levy D. Bovine leukemia virus replicates in sheep B lymphocytes under a T cell released factor. Eur J Cancer Clin Oncol. 1987 Jan;23(1):81–85. doi: 10.1016/0277-5379(87)90423-8. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Franchini G., Wong-Staal F., Gallo R. C. Human T-cell leukemia virus (HTLV-I) transcripts in fresh and cultured cells of patients with adult T-cell leukemia. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6207–6211. doi: 10.1073/pnas.81.19.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves D. C., Ferrer J. F. In vitro transmission and propagation of the bovine leukemia virus in monolayer cell cultures. Cancer Res. 1976 Nov;36(11 Pt 1):4152–4159. [PubMed] [Google Scholar]
- Greene W. C., Leonard W. J., Wano Y., Svetlik P. B., Peffer N. J., Sodroski J. G., Rosen C. A., Goh W. C., Haseltine W. A. Trans-activator gene of HTLV-II induces IL-2 receptor and IL-2 cellular gene expression. Science. 1986 May 16;232(4752):877–880. doi: 10.1126/science.3010456. [DOI] [PubMed] [Google Scholar]
- Gupta P., Ferrer J. F. Expression of bovine leukemia virus genome is blocked by a nonimmunoglobulin protein in plasma from infected cattle. Science. 1982 Jan 22;215(4531):405–407. doi: 10.1126/science.6276975. [DOI] [PubMed] [Google Scholar]
- Gupta P., Kashmiri S. V., Ferrer J. F. Transcriptional control of the bovine leukemia virus genome: role and characterization of a non-immunoglobulin plasma protein from bovine leukemia virus-infected cattle. J Virol. 1984 Apr;50(1):267–270. doi: 10.1128/jvi.50.1.267-270.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Nakashima H., Kobayashi N., Yamamoto N. Tumor promoter, TPA, enhances replication of HTLV-III/LAV. Virology. 1986 Oct 30;154(2):249–258. doi: 10.1016/0042-6822(86)90451-4. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka M., Inoue J., Yoshida M., Seiki M. Post-transcriptional regulator (rex) of HTLV-1 initiates expression of viral structural proteins but suppresses expression of regulatory proteins. EMBO J. 1988 Feb;7(2):519–523. doi: 10.1002/j.1460-2075.1988.tb02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Seiki M., Taniguchi T., Tsuru S., Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986 Nov;5(11):2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Yoshida M., Seiki M. Transcriptional (p40x) and post-transcriptional (p27x-III) regulators are required for the expression and replication of human T-cell leukemia virus type I genes. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3653–3657. doi: 10.1073/pnas.84.11.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Deschamps J., Cleuter Y., Couez D., Burny A., Marbaix G. Leukemogenesis by bovine leukemia virus: proviral DNA integration and lack of RNA expression of viral long terminal repeat and 3' proximate cellular sequences. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2465–2469. doi: 10.1073/pnas.79.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Marbaix G., Cleuter Y., Portetelle D., Mammerickx M., Burny A. Genomic integration of bovine leukemia provirus and lack of viral RNA expression in the target cells of cattle with different responses to BLV infection. Leuk Res. 1980;4(6):509–519. doi: 10.1016/0145-2126(80)90062-4. [DOI] [PubMed] [Google Scholar]
- Kiyokawa T., Seiki M., Iwashita S., Imagawa K., Shimizu F., Yoshida M. p27x-III and p21x-III, proteins encoded by the pX sequence of human T-cell leukemia virus type I. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8359–8363. doi: 10.1073/pnas.82.24.8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Quantitative analysis of in situ hybridization methods for the detection of actin gene expression. Nucleic Acids Res. 1985 Mar 11;13(5):1777–1799. doi: 10.1093/nar/13.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K., Nabel G. J. HTLV-1 transactivator induces interleukin-2 receptor expression through an NF-kappa B-like factor. Nature. 1988 Jun 23;333(6175):776–778. doi: 10.1038/333776a0. [DOI] [PubMed] [Google Scholar]
- Maruyama M., Shibuya H., Harada H., Hatakeyama M., Seiki M., Fujita T., Inoue J., Yoshida M., Taniguchi T. Evidence for aberrant activation of the interleukin-2 autocrine loop by HTLV-1-encoded p40x and T3/Ti complex triggering. Cell. 1987 Jan 30;48(2):343–350. doi: 10.1016/0092-8674(87)90437-5. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Mawle A., Cort S. P., Nicholson J. K., Cross G. D., Scheppler-Campbell J. A., Hicks D., Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985 Nov;135(5):3151–3162. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Nyborg J. K., Dynan W. S., Chen I. S., Wachsman W. Binding of host-cell factors to DNA sequences in the long terminal repeat of human T-cell leukemia virus type I: implications for viral gene expression. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1457–1461. doi: 10.1073/pnas.85.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn T. C., Piot P., McCormick J. B., Feinsod F. M., Taelman H., Kapita B., Stevens W., Fauci A. S. Serologic and immunologic studies in patients with AIDS in North America and Africa. The potential role of infectious agents as cofactors in human immunodeficiency virus infection. JAMA. 1987 May 15;257(19):2617–2621. [PubMed] [Google Scholar]
- Rice N. R., Simek S. L., Dubois G. C., Showalter S. D., Gilden R. V., Stephens R. M. Expression of the bovine leukemia virus X region in virus-infected cells. J Virol. 1987 May;61(5):1577–1585. doi: 10.1128/jvi.61.5.1577-1585.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Willems L., Kettmann R., Campbell K., Zaya R., Burny A., Haseltine W. A. The 3' region of bovine leukemia virus genome encodes a trans-activator protein. EMBO J. 1986 Oct;5(10):2585–2589. doi: 10.1002/j.1460-2075.1986.tb04538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N., Tsuzuku-Kawamura J., Nagayoshi-Aida M., Shimizu F., Imagawa K., Ikawa Y. Identification and some biochemical properties of the major XBL gene product of bovine leukemia virus. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7879–7883. doi: 10.1073/pnas.82.23.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Ogawa Y., Tsuzuku-Kawamura J., Ikawa Y. Bovine leukemia virus: unique structural features of its long terminal repeats and its evolutionary relationship to human T-cell leukemia virus. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4741–4745. doi: 10.1073/pnas.81.15.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Tsuzuku-Kawamura J., Ohishi K., Ogawa Y., Ikawa Y. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc Natl Acad Sci U S A. 1985 Feb;82(3):677–681. doi: 10.1073/pnas.82.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenborn E. T., Mierendorf R. C., Jr A novel transcription property of SP6 and T7 RNA polymerases: dependence on template structure. Nucleic Acids Res. 1985 Sep 11;13(17):6223–6236. doi: 10.1093/nar/13.17.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Siekevitz M., Josephs S. F., Dukovich M., Peffer N., Wong-Staal F., Greene W. C. Activation of the HIV-1 LTR by T cell mitogens and the trans-activator protein of HTLV-I. Science. 1987 Dec 11;238(4833):1575–1578. doi: 10.1126/science.2825351. [DOI] [PubMed] [Google Scholar]
- Stock N. D., Ferrer J. F. Replicating C-type virus in phytohemagglutinin-treated buffy-coat cultures of bovine origin. J Natl Cancer Inst. 1972 Apr;48(4):985–996. [PubMed] [Google Scholar]
- Tong-Starksen S. E., Luciw P. A., Peterlin B. M. Human immunodeficiency virus long terminal repeat responds to T-cell activation signals. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6845–6849. doi: 10.1073/pnas.84.19.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama K., Onuma M., Izawa H. Effect of platelet-derived factor on expression of bovine leukemia virus genome. Arch Virol. 1987;96(1-2):89–96. doi: 10.1007/BF01310992. [DOI] [PubMed] [Google Scholar]
- Yoshida M. Expression of the HTLV-1 genome and its association with a unique T-cell malignancy. Biochim Biophys Acta. 1987 Jul 8;907(2):145–161. doi: 10.1016/0304-419x(87)90003-5. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Seiki M. Recent advances in the molecular biology of HTLV-1: trans-activation of viral and cellular genes. Annu Rev Immunol. 1987;5:541–559. doi: 10.1146/annurev.iy.05.040187.002545. [DOI] [PubMed] [Google Scholar]
- Zagury D., Bernard J., Leonard R., Cheynier R., Feldman M., Sarin P. S., Gallo R. C. Long-term cultures of HTLV-III--infected T cells: a model of cytopathology of T-cell depletion in AIDS. Science. 1986 Feb 21;231(4740):850–853. doi: 10.1126/science.2418502. [DOI] [PubMed] [Google Scholar]