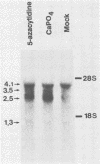
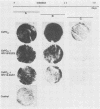
Abstract
Parvovirus H-1 has been shown to suppress spontaneous and chemically or virally induced tumorigenesis in hamsters. In human cell culture systems propagation of H-1 is restricted to transformed cells, which are killed by H-1 infection, in contrast to normal diploid cells, which are nonpermissive for H-1. By analyzing the permissiveness of a variety of human cells for H-1, it was determined that the majority of tested transformed or immortalized cells which were permissive for H-1 contained the DNA of oncogenic viruses (human papillomavirus, simian virus 40, adenovirus, hepatitis B virus, Epstein-Barr virus, and human T-cell lymphotropic virus type I). Of six transformed cell lines negative for persisting tumor virus DNA, only two were permissive for H-1, while two were semipermissive and two were nonpermissive. Thus, persistence and expression of tumor virus functions appears to promote full permissiveness for H-1 in human cells. However, neither expression of genes of specific viral genomes nor the transformed state of apparently virus-free cells alone was sufficient to render human cells permissive for H-1. Therefore, the effect of tumor virus functions on H-1 in transformed cells seems to be indirect, probably mediated by cellular factors which are induced or switched off during the transformation process. It appears that similar factors are induced or switched off by 5-azacytidine or calcium phosphate, both known inducers of cellular gene expression.
Full text
PDF


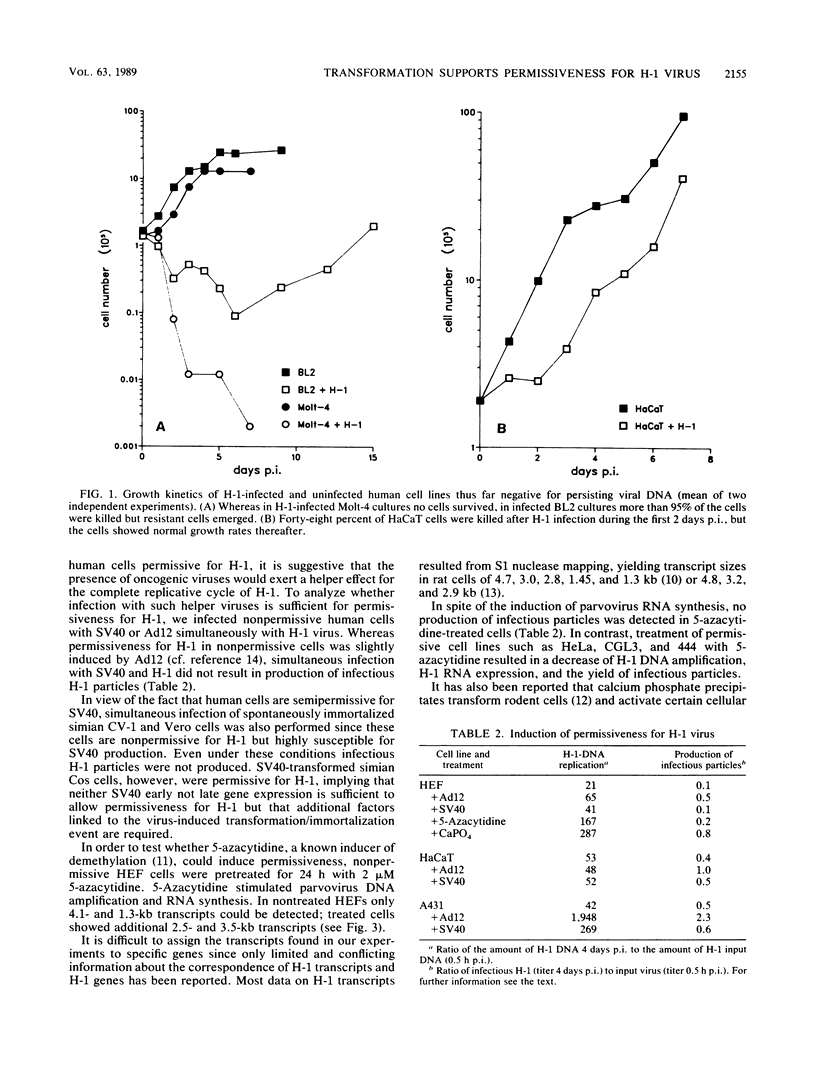
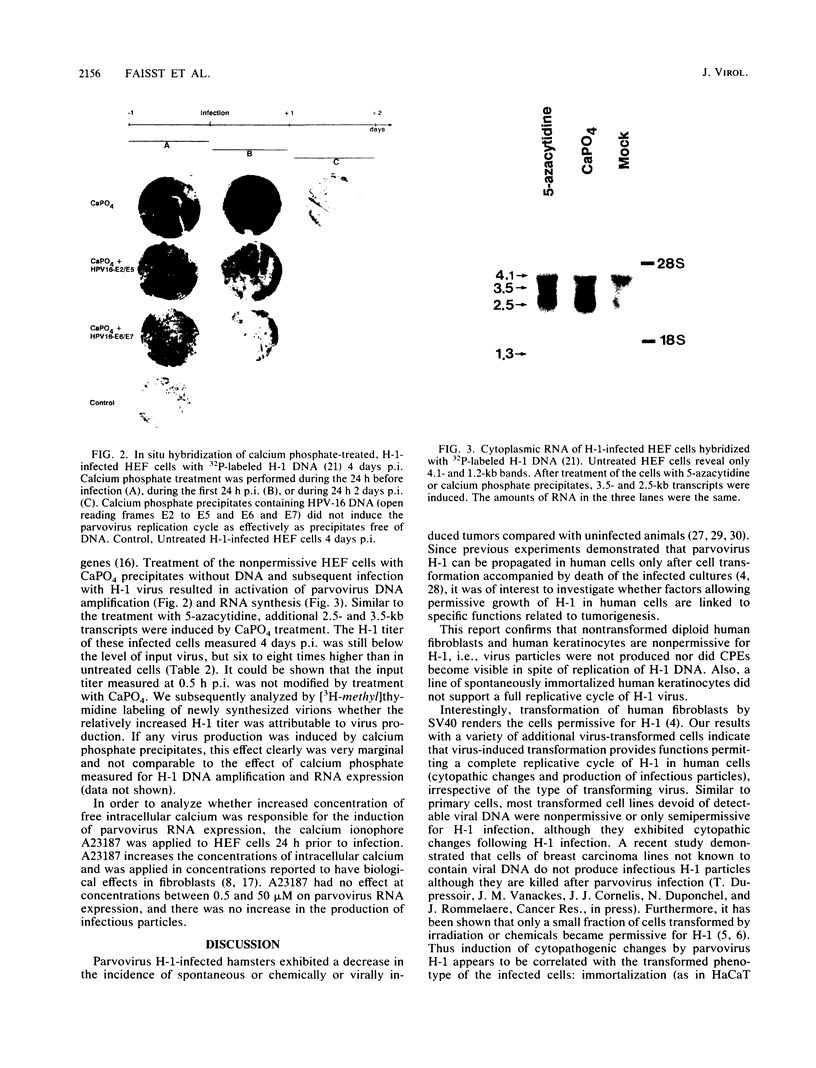


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., Fusenig N. E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988 Mar;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürkle A., Meyer T., Hilz H., zur Hausen H. Enhancement of N-methyl-N'-nitro-N-nitrosoguanidine-induced DNA amplification in a Simian virus 40-transformed Chinese hamster cell line by 3-aminobenzamide. Cancer Res. 1987 Jul 15;47(14):3632–3636. [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. Q., de Foresta F., Hertoghs J., Avalosse B. L., Cornelis J. J., Rommelaere J. Selective killing of simian virus 40-transformed human fibroblasts by parvovirus H-1. Cancer Res. 1986 Jul;46(7):3574–3579. [PubMed] [Google Scholar]
- Cornelis J. J., Becquart P., Duponchel N., Salomé N., Avalosse B. L., Namba M., Rommelaere J. Transformation of human fibroblasts by ionizing radiation, a chemical carcinogen, or simian virus 40 correlates with an increase in susceptibility to the autonomous parvoviruses H-1 virus and minute virus of mice. J Virol. 1988 May;62(5):1679–1686. doi: 10.1128/jvi.62.5.1679-1686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond I. A., Lee A. S., Resendez E., Jr, Steinhardt R. A. Depletion of intracellular calcium stores by calcium ionophore A23187 induces the genes for glucose-regulated proteins in hamster fibroblasts. J Biol Chem. 1987 Sep 15;262(26):12801–12805. [PubMed] [Google Scholar]
- Dürst M., Dzarlieva-Petrusevska R. T., Boukamp P., Fusenig N. E., Gissmann L. Molecular and cytogenetic analysis of immortalized human primary keratinocytes obtained after transfection with human papillomavirus type 16 DNA. Oncogene. 1987;1(3):251–256. [PubMed] [Google Scholar]
- Green M. R., Lebovitz R. M., Roeder R. G. Expression of the autonomous parvovirus H1 genome: evidence for a single transcriptional unit and multiple spliced polyadenylated transcripts. Cell. 1979 Aug;17(4):967–977. doi: 10.1016/0092-8674(79)90336-2. [DOI] [PubMed] [Google Scholar]
- Jones P. A. Altering gene expression with 5-azacytidine. Cell. 1985 Mar;40(3):485–486. doi: 10.1016/0092-8674(85)90192-8. [DOI] [PubMed] [Google Scholar]
- Kerbel R. S., Waghorne C., Man M. S., Elliott B., Breitman M. L. Alteration of the tumorigenic and metastatic properties of neoplastic cells is associated with the process of calcium phosphate-mediated DNA transfection. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1263–1267. doi: 10.1073/pnas.84.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz R. M., Roeder R. G. Parvovirus H-1 expression: mapping of the abundant cytoplasmic transcripts and identification of promoter sites and overlapping transcription units. J Virol. 1986 May;58(2):271–280. doi: 10.1128/jvi.58.2.271-280.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledinko N., Hopkins S., Toolan H. Relationship between potentiation of H-1 growth by human adenovirus 12 and inhibition of the 'helper' adenovirus by H-1. J Gen Virol. 1969 Jul;5(1):19–31. doi: 10.1099/0022-1317-5-1-19. [DOI] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G., Clements G. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975 Jul;22(4):276–284. [PubMed] [Google Scholar]
- Pine R., Levy D. E., Reich N., Darnell J. E., Jr Transcriptional stimulation by CaPO4-DNA precipitates. Nucleic Acids Res. 1988 Feb 25;16(4):1371–1378. doi: 10.1093/nar/16.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed P. W., Lardy H. A. A23187: a divalent cation ionophore. J Biol Chem. 1972 Nov 10;247(21):6970–6977. [PubMed] [Google Scholar]
- Rhode S. L., 3rd Construction of a genetic switch for inducible trans-activation of gene expression in eucaryotic cells. J Virol. 1987 May;61(5):1448–1456. doi: 10.1128/jvi.61.5.1448-1456.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. I. Kinetics in a parasynchronous cell system. J Virol. 1973 Jun;11(6):856–861. doi: 10.1128/jvi.11.6.856-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Richard S. M. Characterization of the trans-activation-responsive element of the parvovirus H-1 P38 promoter. J Virol. 1987 Sep;61(9):2807–2815. doi: 10.1128/jvi.61.9.2807-2815.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rösl F., Dürst M., zur Hausen H. Selective suppression of human papillomavirus transcription in non-tumorigenic cells by 5-azacytidine. EMBO J. 1988 May;7(5):1321–1328. doi: 10.1002/j.1460-2075.1988.tb02947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEIN H. M., ENDERS J. F. Multiplication and cytopathogenicity of Simian vacuolating virus 40 in cultures of human tissues. Proc Soc Exp Biol Med. 1962 Mar;109:495–500. doi: 10.3181/00379727-109-27246. [DOI] [PubMed] [Google Scholar]
- Schlehofer J. R., Ehrbar M., zur Hausen H. Vaccinia virus, herpes simplex virus, and carcinogens induce DNA amplification in a human cell line and support replication of a helpervirus dependent parvovirus. Virology. 1986 Jul 15;152(1):110–117. doi: 10.1016/0042-6822(86)90376-4. [DOI] [PubMed] [Google Scholar]
- Schlehofer J. R., Heilbronn R., Georg-Fries B., zur Hausen H. Inhibition of initiator-induced SV40 gene amplification in SV40-transformed Chinese hamster cells by infection with a defective parvovirus. Int J Cancer. 1983 Nov 15;32(5):591–595. doi: 10.1002/ijc.2910320512. [DOI] [PubMed] [Google Scholar]
- Srivatsan E. S., Benedict W. F., Stanbridge E. J. Implication of chromosome 11 in the suppression of neoplastic expression in human cell hybrids. Cancer Res. 1986 Dec;46(12 Pt 1):6174–6179. [PubMed] [Google Scholar]
- Toolan H. W. Lack of oncogenic effect of the H-viruses for hamsters. Nature. 1967 Jun 3;214(5092):1036–1036. doi: 10.1038/2141036a0. [DOI] [PubMed] [Google Scholar]
- Toolan H. W., Ledinko N. Inhibition by H-1 virus of the incidence of tumors produced by adenovirus 12 in hamsters. Virology. 1968 Jul;35(3):475–478. doi: 10.1016/0042-6822(68)90226-2. [DOI] [PubMed] [Google Scholar]
- Toolan H. W., Rhode S. L., 3rd, Gierthy J. F. Inhibition of 7,12-dimethylbenz(a)anthracene-induced tumors in Syrian hamsters by prior infection with H-1 parvovirus. Cancer Res. 1982 Jul;42(7):2552–2555. [PubMed] [Google Scholar]
- Toolan H., Ledinko N. Growth and cytopathogenicity of H-viruses in human and simian cell cultures. Nature. 1965 Nov 20;208(5012):812–813. doi: 10.1038/208812a0. [DOI] [PubMed] [Google Scholar]
- Yakobson B., Koch T., Winocour E. Replication of adeno-associated virus in synchronized cells without the addition of a helper virus. J Virol. 1987 Apr;61(4):972–981. doi: 10.1128/jvi.61.4.972-981.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalkinoglu A. O., Heilbronn R., Bürkle A., Schlehofer J. R., zur Hausen H. DNA amplification of adeno-associated virus as a response to cellular genotoxic stress. Cancer Res. 1988 Jun 1;48(11):3123–3129. [PubMed] [Google Scholar]