Abstract
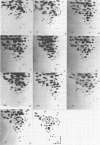
The RNA genomes of 26 isolates of pepper mild mottle virus were compared by their RNase T1 fingerprints. Twenty-three isolates came from epidemic outbreaks in greenhouse-grown peppers in Almería (southeastern Spain) from 1983 to 1987; three other isolates, from 1980, came from Sicily (Italy) and Zaragoza (central Spain). The 26 fingerprints can be classified into 10 different types; nucleotide substitution rates show them to be very similar. Cluster and cladistic analyses group types corresponding to the Almería isolates separate from those of 1980. Intraannual and interannual nucleotide differences were estimated. An evolutionary model for pepper mild mottle virus built on these data indicates a highly stable population, maintaining its diversity through time, with a main prevailing haplotype from which closely related variants arise that do not replace it. This high stability could be due to strong functional constraints on variation, as suggested by the high proportion of invariant versus polymorphic sites in fingerprints.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAWDEN F. C. Reversible, host-induced, changes in a strain of tobacco mosaic virus. Nature. 1956 Feb 18;177(4503):302–304. doi: 10.1038/177302a0. [DOI] [PubMed] [Google Scholar]
- Blok J., Mackenzie A., Guy P., Gibbs A. Nucleotide sequence comparisons of turnip yellow mosaic virus isolates from Australia and Europe. Arch Virol. 1987;97(3-4):283–295. doi: 10.1007/BF01314427. [DOI] [PubMed] [Google Scholar]
- Buonagurio D. A., Nakada S., Fitch W. M., Palese P. Epidemiology of influenza C virus in man: multiple evolutionary lineages and low rate of change. Virology. 1986 Aug;153(1):12–21. doi: 10.1016/0042-6822(86)90003-6. [DOI] [PubMed] [Google Scholar]
- Buonagurio D. A., Nakada S., Parvin J. D., Krystal M., Palese P., Fitch W. M. Evolution of human influenza A viruses over 50 years: rapid, uniform rate of change in NS gene. Science. 1986 May 23;232(4753):980–982. doi: 10.1126/science.2939560. [DOI] [PubMed] [Google Scholar]
- Domingo E., Dávila M., Ortín J. Nucleotide sequence heterogeneity of the RNA from a natural population of foot-and-mouth-disease virus. Gene. 1980 Nov;11(3-4):333–346. doi: 10.1016/0378-1119(80)90073-6. [DOI] [PubMed] [Google Scholar]
- Domingo E., Martínez-Salas E., Sobrino F., de la Torre J. C., Portela A., Ortín J., López-Galindez C., Pérez-Breña P., Villanueva N., Nájera R. The quasispecies (extremely heterogeneous) nature of viral RNA genome populations: biological relevance--a review. Gene. 1985;40(1):1–8. doi: 10.1016/0378-1119(85)90017-4. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Nichol S. T. Molecular epizootiology and evolution of vesicular stomatitis virus New Jersey. J Virol. 1987 Apr;61(4):1029–1036. doi: 10.1128/jvi.61.4.1029-1036.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccone M. E., Kaplan G., Giavedoni L., Domingo E., Palma E. L. VP1 of serotype C foot-and-mouth disease viruses: long-term conservation of sequences. J Virol. 1988 Apr;62(4):1469–1473. doi: 10.1128/jvi.62.4.1469-1473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reanney D. C. The evolution of RNA viruses. Annu Rev Microbiol. 1982;36:47–73. doi: 10.1146/annurev.mi.36.100182.000403. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Inglis S. C. The mutation rate and variability of eukaryotic viruses: an analytical review. J Gen Virol. 1987 Nov;68(Pt 11):2729–2740. doi: 10.1099/0022-1317-68-11-2729. [DOI] [PubMed] [Google Scholar]
- Sobrino F., Palma E. L., Beck E., Dávila M., de la Torre J. C., Negro P., Villanueva N., Ortín J., Domingo E. Fixation of mutations in the viral genome during an outbreak of foot-and-mouth disease: heterogeneity and rate variations. Gene. 1986;50(1-3):149–159. doi: 10.1016/0378-1119(86)90320-3. [DOI] [PubMed] [Google Scholar]
- Takeda N., Miyamura K., Ogino T., Natori K., Yamazaki S., Sakurai N., Nakazono N., Ishii K., Kono R. Evolution of enterovirus type 70: oligonucleotide mapping analysis of RNA genome. Virology. 1984 Apr 30;134(2):375–388. doi: 10.1016/0042-6822(84)90305-2. [DOI] [PubMed] [Google Scholar]
- Yamashita M., Krystal M., Fitch W. M., Palese P. Influenza B virus evolution: co-circulating lineages and comparison of evolutionary pattern with those of influenza A and C viruses. Virology. 1988 Mar;163(1):112–122. doi: 10.1016/0042-6822(88)90238-3. [DOI] [PubMed] [Google Scholar]