Abstract
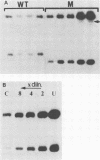
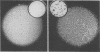
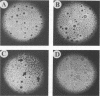
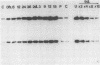
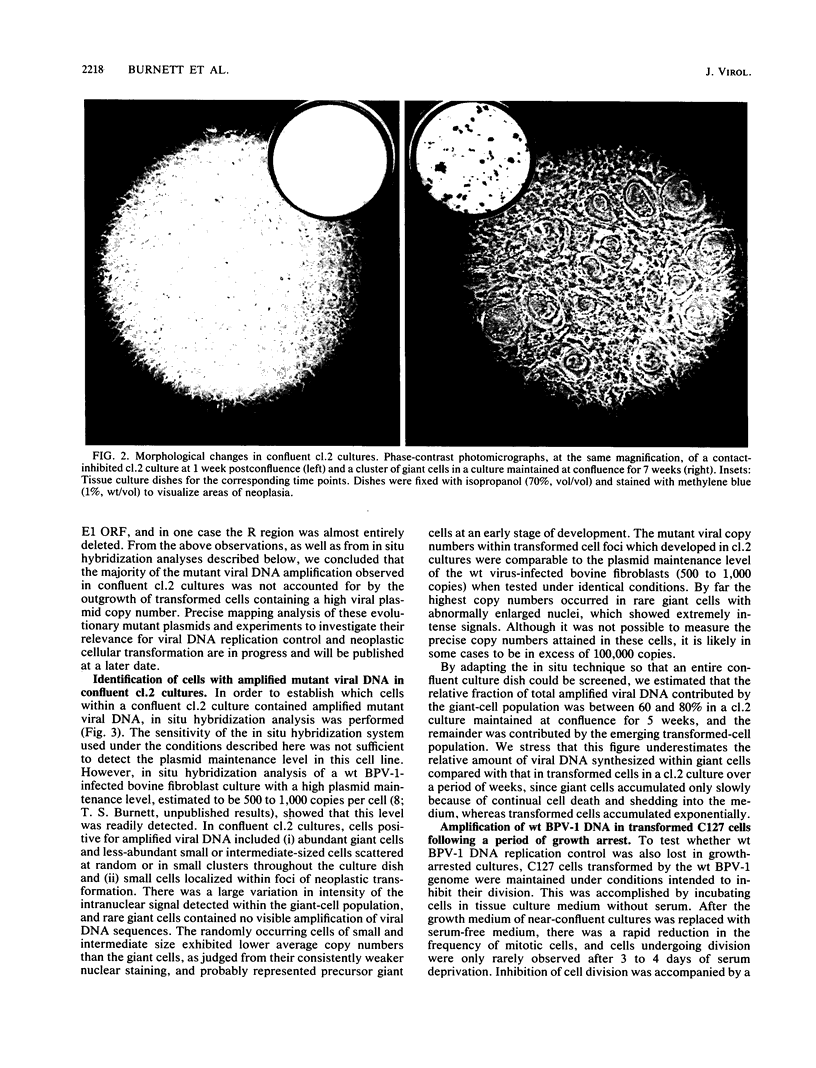
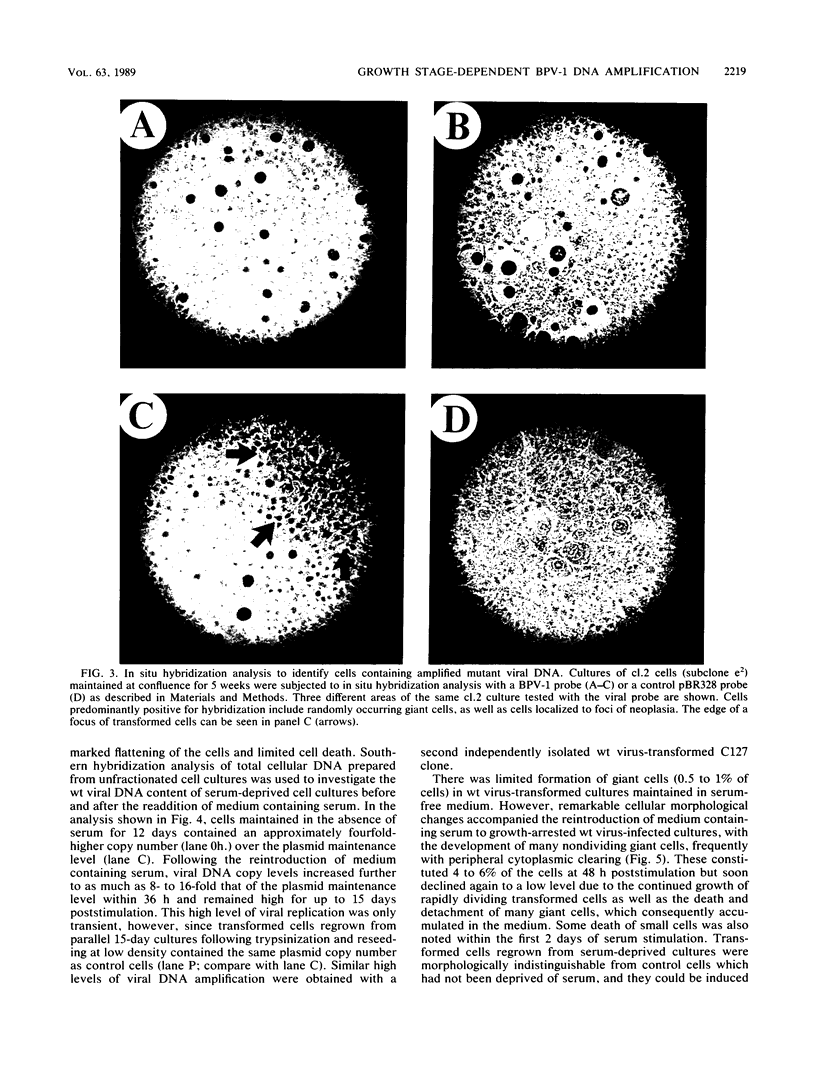
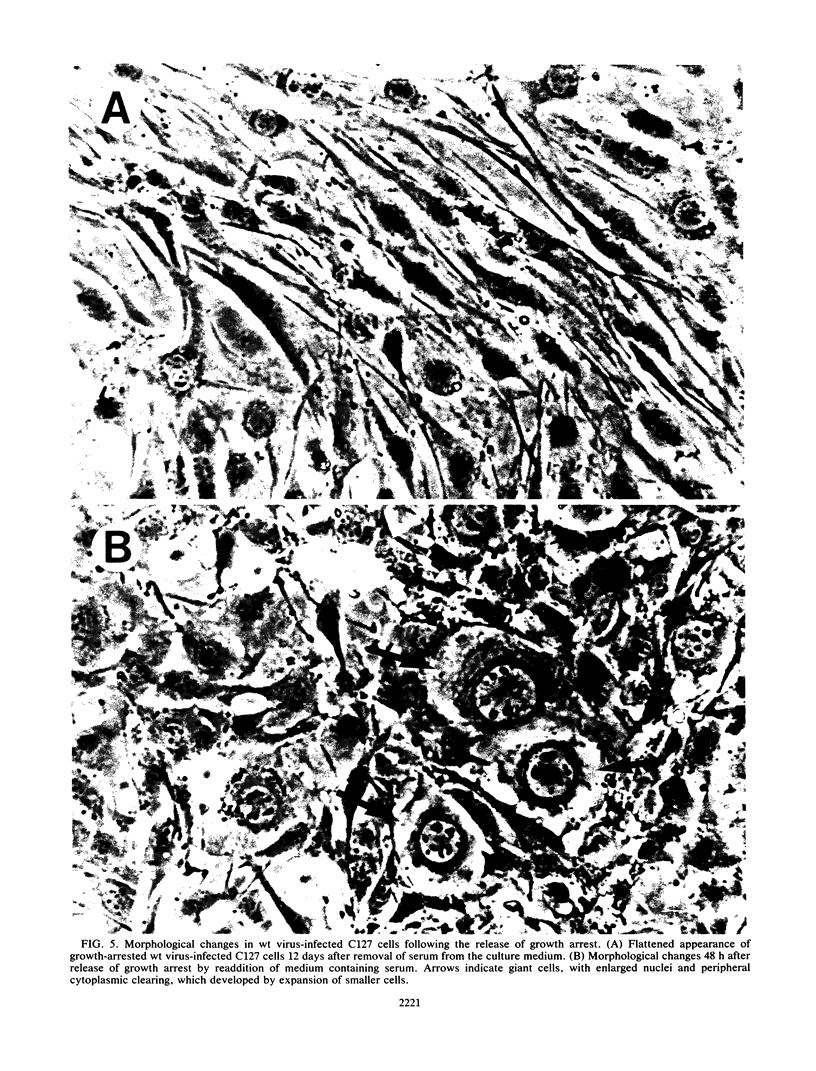
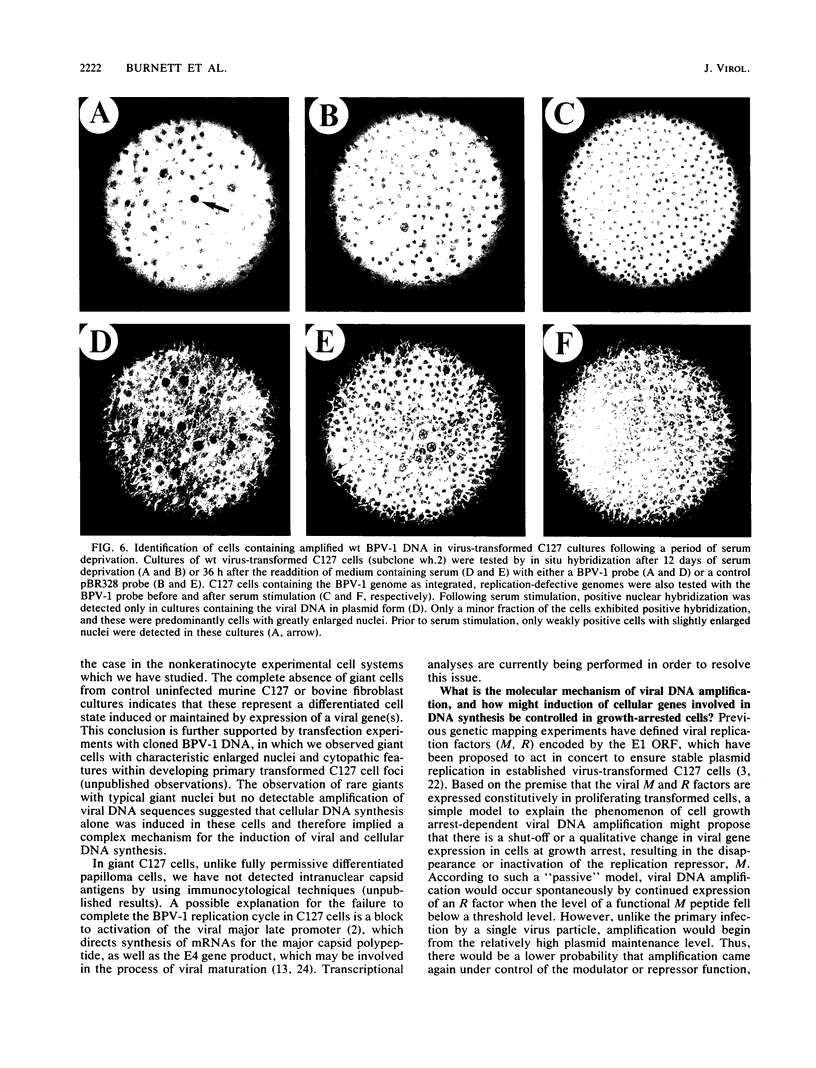
The bovine papillomavirus type 1 (BPV-1) genome replicates as a plasmid within the nuclei of BPV-1-transformed murine C127 cells at a constant multiple copy number, and spontaneous amplification of the viral DNA is rarely observed. We report here that a mutant BPV-1 plasmid within a contact-inhibited C127 cell line replicated as a stable multicopy plasmid in exponentially growing cells but amplified to a high level in confluent cell culture. In situ hybridization analysis revealed that most of the mutant viral DNA amplification occurred in a minor subpopulation of cells within the culture. These consisted of giant nondividing cells with greatly enlarged nuclei, a cell form which was specifically induced in stationary-phase cultures. These observations indicated that expression of a viral DNA replication factor was cell growth stage specific. Consistent with this hypothesis, considerable amplification of wild-type BPV-1 DNA associated with characteristic giant cell formation was observed in typical wild-type virus-transformed C127 cultures following a period of growth arrest achieved by serum deprivation. Further observations indicated that induction of the giant-cell phenotype was dependent on BPV-1 gene expression and implicated a viral E1 replication factor in this process. Moreover, heterogeneity in virus genome copy numbers within the giant-cell population suggested a complex regulation of induction of DNA synthesis in these cells. It appears that this process represents a mechanism employed by the virus to ensure maximal viral DNA synthesis within a growth-arrested cell. Fundamental questions concerning the integration of the virus-cell control circuitry in proliferating and resting cells are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amtmann E., Sauer G. Activation of non-expressed bovine papilloma virus genomes by tumour promoters. Nature. 1982 Apr 15;296(5858):675–677. doi: 10.1038/296675a0. [DOI] [PubMed] [Google Scholar]
- Baker C. C., Howley P. M. Differential promoter utilization by the bovine papillomavirus in transformed cells and productively infected wart tissues. EMBO J. 1987 Apr;6(4):1027–1035. doi: 10.1002/j.1460-2075.1987.tb04855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg L. J., Singh K., Botchan M. Complementation of a bovine papilloma virus low-copy-number mutant: evidence for a temporal requirement of the complementing gene. Mol Cell Biol. 1986 Mar;6(3):859–869. doi: 10.1128/mcb.6.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg L., Lusky M., Stenlund A., Botchan M. R. Repression of bovine papilloma virus replication is mediated by a virally encoded trans-acting factor. Cell. 1986 Aug 29;46(5):753–762. doi: 10.1016/0092-8674(86)90351-x. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Botchan M., Berg L., Reynolds J., Lusky M. The bovine papillomavirus replicon. Ciba Found Symp. 1986;120:53–67. doi: 10.1002/9780470513309.ch5. [DOI] [PubMed] [Google Scholar]
- Burnett S., Moreno-Lopez J., Pettersson U. Messenger RNAs from the E1 region of bovine papillomavirus type 1 detected in virus-infected bovine cells. Nucleic Acids Res. 1987 Nov 11;15(21):8607–8620. doi: 10.1093/nar/15.21.8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett S., Zabielski J., Moreno-Lopez J., Pettersson U. Evidence for multiple vegetative DNA replication origins and alternative replication mechanisms of bovine papillomavirus type 1. J Mol Biol. 1989 Mar 5;206(1):239–244. doi: 10.1016/0022-2836(89)90537-8. [DOI] [PubMed] [Google Scholar]
- Burnett T. S., Gallimore P. H. Establishment of a human keratinocyte cell line carrying complete human papillomavirus type 1 genomes: lack of vegetative viral DNA synthesis upon keratinization. J Gen Virol. 1983 Jul;64(Pt 7):1509–1520. doi: 10.1099/0022-1317-64-7-1509. [DOI] [PubMed] [Google Scholar]
- Butel J. S. Studies with human papilloma virus modeled after known papovavirus systems. J Natl Cancer Inst. 1972 Feb;48(2):285–299. [PubMed] [Google Scholar]
- Chen E. Y., Howley P. M., Levinson A. D., Seeburg P. H. The primary structure and genetic organization of the bovine papillomavirus type 1 genome. Nature. 1982 Oct 7;299(5883):529–534. doi: 10.1038/299529a0. [DOI] [PubMed] [Google Scholar]
- Doorbar J., Campbell D., Grand R. J., Gallimore P. H. Identification of the human papilloma virus-1a E4 gene products. EMBO J. 1986 Feb;5(2):355–362. doi: 10.1002/j.1460-2075.1986.tb04219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Green M., Loewenstein P. M. Demonstration that a chemically synthesized BPV1 oncoprotein and its C-terminal domain function to induce cellular DNA synthesis. Cell. 1987 Dec 4;51(5):795–802. doi: 10.1016/0092-8674(87)90102-4. [DOI] [PubMed] [Google Scholar]
- LaPorta R. F., Taichman L. B. Human papilloma viral DNA replicates as a stable episome in cultured epidermal keratinocytes. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3393–3397. doi: 10.1073/pnas.79.11.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Byrne J. C., Howley P. M. A stable bovine papillomavirus hybrid plasmid that expresses a dominant selective trait. Mol Cell Biol. 1983 Nov;3(11):2110–2115. doi: 10.1128/mcb.3.11.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Lowy D. R., Dvoretzky I., Howley P. M. Mouse cells transformed by bovine papillomavirus contain only extrachromosomal viral DNA sequences. Proc Natl Acad Sci U S A. 1981 May;78(5):2727–2731. doi: 10.1073/pnas.78.5.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. A bovine papillomavirus type 1-encoded modulator function is dispensable for transient viral replication but is required for establishment of the stable plasmid state. J Virol. 1986 Nov;60(2):729–742. doi: 10.1128/jvi.60.2.729-742.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Characterization of the bovine papilloma virus plasmid maintenance sequences. Cell. 1984 Feb;36(2):391–401. doi: 10.1016/0092-8674(84)90232-0. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Genetic analysis of bovine papillomavirus type 1 trans-acting replication factors. J Virol. 1985 Mar;53(3):955–965. doi: 10.1128/jvi.53.3.955-965.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Transient replication of bovine papilloma virus type 1 plasmids: cis and trans requirements. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3609–3613. doi: 10.1073/pnas.83.11.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneguzzi G., Binétruy B., Grisoni M., Cuzin F. Plasmidial maintenance in rodent fibroblasts of a BPV1-pBR322 shuttle vector without immediately apparent oncogenic transformation of the recipient cells. EMBO J. 1984 Feb;3(2):365–371. doi: 10.1002/j.1460-2075.1984.tb01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary K., Horwitz B. H., DiMaio D. Mutational analysis of open reading frame E4 of bovine papillomavirus type 1. J Virol. 1987 Apr;61(4):1248–1252. doi: 10.1128/jvi.61.4.1248-1252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth G., Jeanteur P., Croissant O. Evidence for and localization of vegetative viral DNA replication by autoradiographic detection of RNA-DNA hybrids in sections of tumors induced by Shope papilloma virus. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1876–1880. doi: 10.1073/pnas.68.8.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashad A. L., Evans C. A. A difference in sites of DNA synthesis in virus-induced (Shope) and in chemically induced epidermal tumors of rabbit skin. Cancer Res. 1967 Sep;27(9):1639–1647. [PubMed] [Google Scholar]
- Roberts J. M., Weintraub H. Cis-acting negative control of DNA replication in eukaryotic cells. Cell. 1988 Feb 12;52(3):397–404. doi: 10.1016/s0092-8674(88)80032-1. [DOI] [PubMed] [Google Scholar]
- Roberts J. M., Weintraub H. Negative control of DNA replication in composite SV40-bovine papilloma virus plasmids. Cell. 1986 Aug 29;46(5):741–752. doi: 10.1016/0092-8674(86)90350-8. [DOI] [PubMed] [Google Scholar]
- Schneider C., King R. M., Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988 Sep 9;54(6):787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Eng C. Y., Berk A. J. An adenovirus early region 1A protein is required for maximal viral DNA replication in growth-arrested human cells. J Virol. 1985 Mar;53(3):742–750. doi: 10.1128/jvi.53.3.742-750.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenlund A., Bream G. L., Botchan M. R. A promoter with an internal regulatory domain is part of the origin of replication in BPV-1. Science. 1987 Jun 26;236(4809):1666–1671. doi: 10.1126/science.3037693. [DOI] [PubMed] [Google Scholar]
- Thorner L., Bucay N., Choe J., Botchan M. The product of the bovine papillomavirus type 1 modulator gene (M) is a phosphoprotein. J Virol. 1988 Jul;62(7):2474–2482. doi: 10.1128/jvi.62.7.2474-2482.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck W., Rösl F., Zentgraf H. Origin of replication in episomal bovine papilloma virus type 1 DNA isolated from transformed cells. EMBO J. 1984 Sep;3(9):2173–2178. doi: 10.1002/j.1460-2075.1984.tb02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]